In East Asian medicine, the cold-heat pattern differentiation system has long been used in clinical diagnosis as well as in the treatment of patients suffering from various diseases, including menopausal disorders, chronic rhinitis, dyspepsia, diarrhea, dysmenorrhea, headache, inflammation in the digestive tract, atopic dermatitis (Bae et al., Reference Bae, Lee, Park, Yoon, Mun and Lee2017), and rheumatoid arthritis (Ferreira & Lopes, Reference Ferreira and Lopes2011). The cold pattern (CP) is characterized by a preference for warm temperature, intolerance of cold (high cold susceptibility), hypothermia, paleness, diarrhea, peripheral chills, and spasms, whereas the heat pattern (HP) is characterized by diaphoresis, rapid pulse, flushed face, vexation, constipation, thirst, aversion to hot temperature (high heat susceptibility), and deep-colored urine (Jian, Reference Jian2005). It has been suggested that incorporation of cold-heat patterns in diagnosis gives clinicians information about the nature and the location of physiological imbalances of the patient, which can improve diagnostic accuracy and the efficacy of the treatment (WHO Regional Office for the Western Pacific, 2007).
Several studies have consistently shown that body mass index (BMI) is positively correlated with the HP and negatively correlated with the CP (Anderson, Reference Anderson1999; Lee et al., Reference Lee, Lee, Nam and Kim2018; Mun et al., Reference Mun, Kim, Bae and Lee2017; Pham et al., Reference Pham, Lee, Kim, Song, Kim and Leem2016). However, shared etiologies of these three traits remain unknown. Twin studies have demonstrated that BMI is highly heritable (Hur et al., Reference Hur, Kaprio, Iacono, Boomsma, McGue, Silventoinen and Mitchell2008; Silventoinen et al., Reference Silventoinen, Jelenkovic, Sund, Hur, Yokoyama and Honda2016). In support of the findings from twin studies, gene identification studies have successfully detected several FTO gene variants related to BMI (e.g., Qi et al., Reference Qi, Kilpeläinen, Downer, Tanaka, Smith, Sluijs and Qi2014). Using the sample employed in the present study, we recently showed that the CP and the HP are heritable traits, with heritability estimates being 40% (95% CI [38, 42]) for the former and 33% (95% CI [25, 42) for the latter (Hur et al., Reference Hur, Yu, Lee and Jin2018). As with many other complex traits, shared environmental influences on the CP and the HP were negligible, and environmental factors that influenced the CP and the HP were mainly individual specific. These results suggest that cold-heat patterns are complex, multi-factorial phenotypes caused by a combination of genetic and environmental factors. In the present study, we investigated whether the relationships between cold-heat patterns and BMI arise due to shared genetic and/or environmental etiology.
Material and Methods
Sample
The sample consisted of 1,752 (1,047 monozygotic (MZ) and 705 dizygotic (DZ)) twins (mean age = 19.1 yrs; SD of age = 3.1 yrs; range = 12–29 yrs) drawn from the South Korean Twin Registry (Hur et al., Reference Hur, Jeong, Chung, Shin and Song2013). A detailed description of the present sample can be found in Hur et al. (Reference Hur, Jeong, Chung, Shin and Song2013, Reference Hur, Yu, Lee and Jin2018).
three-item zygosity questionnaire was used to determine twins’ zygosity (Ooki et al., Reference Ooki, Yamada and Asaka1993). When compared to DNA analysis, this approach has been shown to achieve over 90% accuracy (Ooki et al., Reference Ooki, Yamada and Asaka1993). The mean (SD) of BMI in our sample was 21.3 (3.1), which was very close to that of adolescents and young adults in South Korea (Park, Reference Park2011), suggesting that our sample is fairly representative of South Korean adolescents and young adults in terms of BMI. Means and standard deviations of the CP and the HP in our sample were reported elsewhere (Hur et al., Reference Hur, Yu, Lee and Jin2018).
Measures
A telephone interview was undertaken with twins to complete a questionnaire of cold-heat patterns (Yeo et al., Reference Yeo, Park, Bae, Jang and Lee2016) and report their current height and weight. BMI was calculated as weight (kg) divided by height (m2). The cold-heat patterns questionnaire used in the present study was comprised of eight items for typical symptoms of the CP and seven items for typical symptoms of the HP (see Table 2 in Hur et al., Reference Hur, Yu, Lee and Jin2018 for the items). Twins were instructed to respond to five-point Likert scale (1 = not at all true, 5 = very true) for each item. Responses to the HP and the CP items were summed so that higher scores on the HP indicate higher HP and higher scores on the CP indicate higher CP. This questionnaire was developed for the general population through factor analysis and clinicians’ diagnostic consensus. The items in the questionnaire have been demonstrated to have adequate psychometric properties (Yeo et al., Reference Yeo, Park, Bae, Jang and Lee2016). Cronbach alpha reliabilities in the present sample were 0.66 for the CP and 0.70 for the HP.
Statistical Analysis
To determine the causes of covariance among BMI, the HP, and the CP, twin correlations and cross-twin, cross-trait correlations for MZ and DZ twins were computed and model-fitting analyses were carried out. As our previous study has shown no significant sex or age differences in genetic and environmental influences on the HP and the CP (Hur et al., Reference Hur, Yu, Lee and Jin2018), prior to twin correlation and model-fitting analyses, we combined both sexes and corrected the data for age, age2, sex, and age × sex, using a regression method (McGue & Bouchard, Reference McGue and Bouchard1984).
A trivariate Cholesky model (Neale & Cardon, Reference Neale and Cardon1992) was applied to the data. In the full trivariate Cholesky model, each of the three Cholesky factors for the CP, the HP, and BMI are decomposed into additive genetic factors (A, the sum of the average effect of all alleles that influence a phenotype), shared environmental factors (C, those environmental factors shared between the two members of a twin pair) and individual specific environmental factors (E, those environmental factors specific to each member of a twin pair and measurement error). The first Cholesky factors (i.e., A1, C1, and E1) exert influences on all three traits, that is, the CP, the HP, and BMI, although they mainly influence the CP. The second Cholesky factors (i.e., A2, C2, and E2) have effects mainly on the HP, although they also influence BMI. The third Cholesky factors (i.e., A3, C3, and E3) are those unique to BMI. The A, C, and E covariance matrices were computed by the product of their respective Cholesky factor-loading matrix and its transpose. The genetic and environmental correlations among the three scales were also derived from the A, C, and E variances and covariance. The raw data option in Mx (Neale et al., Reference Neale, Boker and Xie2003) was used to conduct model-fitting analyses. To select the best-fitting, most parsimonious model, submodels of the full trivariate Cholesky model were constructed, and the fits of these submodels were compared with the fit of the full model by using the criteria of Akaike information criterion (AIC = χ2 – 2df; Akaike, Reference Akaike1987) and the likelihood-ratio test (LRT).
Results
Phenotypic Correlations and Twin Correlations
Table 1 presents phenotypic correlations and MZ and DZ cross-twin, cross-trait correlations for the CP, the HP, and BMI. Although the magnitudes of the phenotypic correlations between BMI and the cold-heat patterns were moderate, they were all significant and consistent with those found in other studies (e.g., Anderson, Reference Anderson1999; Mun et al., Reference Mun, Kim, Bae and Lee2017; Pham et al., Reference Pham, Lee, Kim, Song, Kim and Leem2016). Negative but low correlation between the CP and the HP found in the present sample suggested that although some of the symptoms of the two patterns were opposite to each other (e.g., aversion to cold vs. heat), the two patterns could not be considered a single phenotype.
TABLE 1 Age- and Sex-Corrected Phenotypic Correlations (Below the Diagonal) and MZ and DZ Cross-Twin, Cross-Trait Correlations (Above the Diagonal) for the Cold Pattern (CP), the Heat Pattern (HP), and body mass index (BMI).
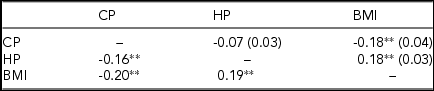
Cross-twin, cross-trait correlations for DZ twins are in parenthesis. **p < 0.01.
Neither MZ nor DZ cross-twin cross-trait correlation between the CP and the HP was significant. However, MZ cross-twin, cross-trait correlations between the CP and BMI and between the HP and BMI were significant and greater than the corresponding DZ cross-twin, cross-trait correlations. None of the DZ cross-twin, cross-trait correlations attained statistical significance. These results suggest that the relationships between the HP and BMI and the CP and BMI might be due in part to common genes. Note, however, that MZ cross-twin cross-trait correlations were much less than 1.0, suggesting that individual specific environment also plays an important role in the phenotypic relationships.
Model Fitting
Table 2 presents the results of trivariate Cholesky model-fitting analysis. Model 2 removed genetic variances and covariance from the full model, which resulted in a significant change in chi-square. In contrast, when shared environmental variances and covariance were eliminated from the full model, the difference in chi-square was not significant (model 3). These results suggest that shared environmental factors were not significant for the relationships among the CP, the HP, and BMI. In model 4, we further dropped genetic covariance between the CP and the HP from model 3, whereas in model 5 we dropped non-shared environmental covariance between the two traits. The resulting chi-square change was significant in model 5 but not in model 4, suggesting that non-shared environmental variance alone explains the relationship between the CP and the HP. Similarly, models 6 and 7 tested significance of genetic versus non-shared environmental covariance between the HP and BMI. As change in chi-square was significant in model 6 but not in model 7, it was concluded that genetic covariance alone mediated the relationship between the HP and BMI. In model 8, genetic covariance between the CP and BMI was removed from model 3, whereas in model 9, non-shared environmental covariance between the two traits was removed. Both models showed a significant change in chi-square, indicating that both genetic and non-shared environmental covariances are necessary to explain the relationship between the CP and BMI. Model 10 eliminated genetic covariance between the CP and the HP and non-shared environmental covariance between the HP and BMI simultaneously from model 3, which produced a non-significant change in chi-square. From these model comparisons based on the LRT, we concluded that model 10 was the best fit. In agreement with the conclusion from the LRT, AIC was the lowest in model 10 among the models tested.
TABLE 2 Model-Fitting Results

Best-fitting model is indicated in bold type. LL = log-likelihood. CP = cold pattern, HP = heat pattern.
Figure 1 presents the parameter estimates in the best-fitting trivariate Cholesky model. Additive genetic influences derived from the best-fitting model were 0.38 (95% CI [0.31, 0.45]) for the CP, 0.32 (95% CI [0.24, 0.39]) for the HP, and 0.84 (95% CI [0.82, 0.86]) for BMI. Non-shared environmental influences for corresponding traits were 0.62 (95% CI [0.55, 0.69]), 0.68 (95% CI [0.61, 0.76]), and 0.16 (95% CI [0.14, 0.18]), respectively. These estimates were very close to the estimates found in our previous studies based on univariate biometrical models (Hur et al., Reference Hur, Yu, Lee and Jin2018).
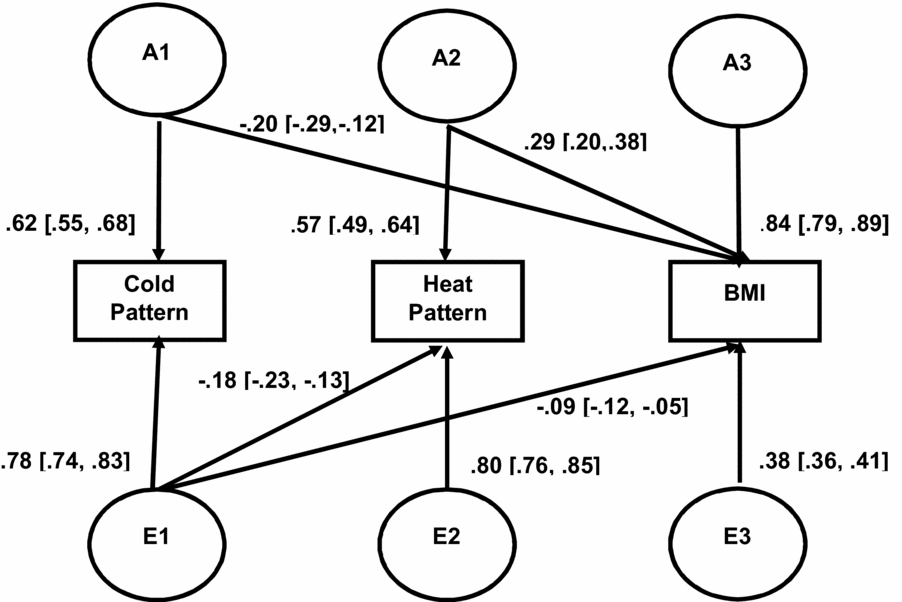
FIGURE 1 Additive genetic (A1, A2, A3) and individual specific environmental (E1, E2, E3) path coefficients in the best-fitting trivariate Cholesky factor model for cold-heat patterns and BMI. Path estimates should be squared to obtain variance due to each factor. Note: 95% CIs are in square brackets.
The best-fitting model also yielded genetic and individual specific environmental correlations, that is, the degree of genetic and environmental overlaps among the three traits (see Table 3). The genetic correlation was 0.31 (95% CI [0.22, 0.41]) between BMI and the HP, while the individual specific environmental correlation was not significant, suggesting that the phenotypic association between BMI and the HP can be explained entirely by the shared genetic basis. The genetic and individual specific environmental correlations between the CP and BMI were both -0.22, which suggested that 65% of the phenotypic correlation between the CP and BMI was attributable to common genes, with the remaining 35% being due to common individual specific environmental factors. While the genetic correlation between the CP and the HP was not significant, the individual specific environmental correlation was -0.22 (95% CI [-0.28, -0.15]). These results suggested that the CP and the HP may be genetically independent and that common environmental factors alone can explain the phenotypic correlation between the CP and the HP.
TABLE 3 Genetic and Individual Specific Environmental Correlations and Their 95% Confidence Interval for the Cold Pattern, the Heat Pattern, and BMI Derived from the Best-Fitting Trivariate Cholesky Model

Genetic correlations (r g) are above the diagonal; Individual-specific environmental correlations (r e) are below the diagonal. 95% CI are in square brackets. ns = non-significant.
Discussion
The present study explored shared etiologies of the relationships among cold-heat patterns and BMI. The results showed that the phenotypic relationships of these traits were mediated in part by common genetic influences. That is, many of the genes affecting BMI also influence the HP and the CP, perhaps as a result of some general mechanisms such as the metabolic rate. The metabolic rate has been shown to be a correlate of BMI (Hasson et al., Reference Hasson, Howe, Jones and Freedson2011) and heritable (Bouchard et al., Reference Bouchard, Perusse, Deriaz, Despres and Tremblay1993), and negatively associated with the CP and positively associated with the HP (Mun et al., Reference Mun, Kim, Bae and Lee2017; Yoshino et al., Reference Yoshino, Katayama, Munakata, Horiba, Yamaguchi, Imoto and Watanabe2013).
The genetic correlations were positive between the HP and BMI and negative between the CP and BMI. The signs of these genetic correlations agreed with those of the phenotypic correlations. Thus, our findings suggest that genes that increase the HP can increase BMI, whereas genes that increase the CP can lower BMI.
Although the phenotypic relationship between the HP and BMI was explained entirely by common genes, the relationship between the CP and BMI was due in part to the common individual-specific environmental influence as well. As with the genetic correlation, the sign of the individual-specific environmental correlation between the CP and BMI was negative, suggesting that certain environmental factors that increase the CP can lower BMI. Candidate environmental factors for this relationship may include smoking and diet.
Interestingly, the present study showed that the HP and the CP were genetically independent. In support of our findings, Li et al. (Reference Li, Zhang, Wu, Zhang, Li and Wang2007) in a systems biology study found that immune factors were related to the HP network, while hormones were mainly related to the CP network, suggesting that the biological pathways of the two phenotypes may be largely distinguishable. In a small Norwegian twin sample, Nielsen et al. (Reference Nielsen, Stubhaug, Price, Vassend, Czajkowski and Harris2008) examined genetic and environmental relationships between the cold-pressure and the contact heat pain sensitivities, the phenotypes related to the CP and the HP in our study. The authors also found very modest genetic and environmental overlaps between the two phenotypes. Taken together, these findings imply that information gained from gene identification studies from one pattern may not be generalized to the other pattern, and that separate genetic association studies should be carried out for each pattern.
The present study suggests that although the phenotypic relationship between the CP and the HP was modest, it was explained mainly by common individual-specific environmental influences. Note, however, the individual-specific environmental correlation between the CP and the HP contains correlated measurement error as twins in our study completed both the CP and the HP via a telephone interview using a questionnaire with the same Likert scale.
There were a few limitations in the present study. First, we noted that the correlations between BMI and the two patterns were higher in males than in females. However, to increase the sample size, we combined both sexes prior to model-fitting analysis. Future studies should increase sample size and examine whether genetic and environmental etiologies of the relationships between BMI and the two patterns differ across two sex groups. Second, in our study, only the questionnaire method was employed to determine the CP and the HP. It would be of interest for future studies to use clinician's diagnosis of the CP and the HP. Finally, our model was based on the assumption that the relationship between the CP and BMI is linear as we did not find any non-linearity in our sample. However, some (e.g., Anderson, Reference Anderson1999) have maintained that the CP should be more common in the lowest and the highest groups of BMI. More studies should be carried out to determine the linearity of the relationship between the CP and BMI.
Acknowledgments
This work was supported by the ‘Development of Health Prediction Technology based on Big Data’ (K18092) funded by the Ministry of Science and ICT(MSIT) of Korea given to the Korea Institute of Oriental Medicine (KIOM).






