Introduction
Urban ecology, which is concerned with the study of urban ecosystems, includes core city environments, suburban surroundings, isolated towns, villages and agricultural lands as components of the ‘urbanization gradient’ (Pickett et al. Reference Pickett, Cadenasso, Grove, Boone, Groffman and Irwin2011). Urban ecology is therefore inclusive in that it allows for the study of rural habitats surrounding urban centres, but it is notable that urban ecological theory has historically assumed that wildland habitats are unavailable to core urban-dwelling species. However, many avian species have the locomotive advantage of efficient flight, which allows them to maintain large home ranges. As such, these individuals may be able to fluidly utilize resources across the urbanization gradient. Even species with smaller ranges may be able to move readily between urban and non-urban habitats as the urban–wildland interface expands (Radeloff et al. Reference Radeloff, Helmers, Kramer, Mockrin, Alexandre and Bar-Massada2018).
Studies in urban bird ecology have focused on species-level tolerances of urbanization (Blair Reference Blair1996, Kettel et al. Reference Kettel, Gentle, Quinn and Yarnell2018, Seress et al. Reference Seress, Sándor, Evans and Liker2020) and community assemblages along urbanization gradients (Crooks et al. Reference Crooks, Suarez and Bolger2004, Shochat et al. Reference Shochat, Lerman, Katti and Lewis2004, McKinney Reference McKinney2006, Fraissinet et al. Reference Fraissinet, Ancillotto, Migliozzi, Capasso, Bosso and Chamberlain2022). The three most widely used labels classify species as ‘urban avoiders’, ‘urban exploiters’ or ‘suburban adaptable’ based on whether the species is uncommon/absent from urban areas, more abundant in urban areas than non-urban areas or most abundant in mildly disturbed (namely, suburban) areas, respectively (Blair Reference Blair1996, Pennington & Blair Reference Pennington, Blair, Lepczyk and Warren2012; variations on these labels are discussed in McKinney Reference McKinney2006, Evans et al. Reference Evans, Hatchwell, Parnell and Gaston2010). Far fewer studies have explored species in which individuals rely on resources from both urban and non-urban habitat types as part of their daily routine. Due to the lack of attention to this response to urbanization in the literature, the extent to which concurrent use of urban and wildland resources may improve individual fitness, facilitate population persistence and affect species conservation status is unknown.
Waterbirds represent the avian clade that is most able to persist in urban environments by adaptation (Callaghan et al. Reference Callaghan, Major, Wilshire, Martin, Kingsford and Cornwell2019). Many species in this group (e.g., orders Anseriformes, Pelecaniformes and Ciconiiformes) have large foraging ranges during the nesting season and may therefore readily access both wildland and urban resources. Concurrent use of wildland and urban resources by individuals of a species – colloquially, ‘urban commuting’ – may translate into a higher-order response to urbanization, whereby a population or species is most stable at urban/natural borders or within urban matrices such as the urban–wildland interface.
One Ciconiiform, the wood stork (Mycteria americana; hereafter, ‘stork’), is an ideal subject for examining this potential commuter response. Individuals of this species make daily foraging flights up to 74 km away from their nest location (Herring et al. Reference Herring, Herring and Gawlik2015) and nest in both marsh (wildland) and urban habitats. In south Florida (USA), urban/wildland land-cover types are sharply contrasted because protected land in the Everglades wetland system is adjacent to the Miami metropolitan area. Hence, we investigated the effect of concurrent urban/wildland resource use on nest-level productivity by monitoring six stork colonies located along the Everglades–Miami metropolitan interface.
While urbanization was underway across this region, two important behavioural changes in the stork were observed: the novel use of urban habitats for foraging and nesting and the novel consumption of non-native prey species. Urban nesting colonies started forming in the late 1990s (Gawlik Reference Gawlik2000), and they continue to grow (Cook & Baranski Reference Cook and Baranski2021). In years when the hydrological condition of the Everglades marsh is poor for foraging (Frederick & Ogden Reference Frederick and Ogden2001, Herring & Gawlik Reference Herring and Gawlik2011, Evans & Gawlik Reference Evans and Gawlik2020), the number of storks nesting in urban colonies has even exceeded the number of nests in nearby natural colonies (Cook & Baranski Reference Cook and Baranski2021). Urban-nesting storks may benefit from the ability to access foraging areas outside the natural system (Evans & Gawlik Reference Evans and Gawlik2020, Evans et al. Reference Evans, Klassen and Gawlik2022) because storks are a food-limited species (Frederick et al. Reference Frederick, Gawlik, Ogden, Cook and Lusk2009).
Non-native fish, which are prey for storks, have been expanding their ranges in south Florida since the 1950s, but they notably increased after changes to water management regimes in the 1990s (Kline et al. Reference Kline, Loftus, Kotun, Trexler, Rehage, Lorenz and Robinson2014). Storks nesting in both urban and marsh colony types now consume non-native fishes, which is a change from the stork’s diet prior to the 1980s (Klassen & Gawlik Reference Klassen and Gawlik2018). Diet is a key mechanism of demographic change for food-limited species, so changes in diet such as the ones observed by Klassen and Gawlik (Reference Klassen and Gawlik2018) and Evans and Gawlik (Reference Evans and Gawlik2020) could have significant impacts on productivity and survival. The presence of non-native fish may affect storks positively or negatively, possibly in a complex manner.
We proposed two testable hypotheses regarding the urbanization of storks breeding in south Florida: first, that urban resource use affects nest-level productivity; and second, that the consumption of non-native prey affects nest-level productivity. If the use of urban colony sites, urban prey items and/or the amount of non-native fish biomass found in the diet were found to be associated with increased or decreased productivity metrics, we would have concluded that one or more of these documented behavioural shifts have demographic consequences. Identifying whether these are mechanisms of demographic change could help wildlife managers predict future trends in the south Florida stork population. Therefore, this study is foundational to assessing the health and longevity of the US breeding population, which has been protected under the Endangered Species Act since 1984 but is under review for delisting based on the species’ flexible habitat use, including of urban areas (US Fish and Wildlife Service 2023). More broadly, storks serve as a case study to explore the dynamics of species that have individuals concurrently utilizing urban and non-urban resources along urban–wildland interfaces.
Methods
Colony monitoring
The Miami metropolitan area stretches from Miami to West Palm Beach in south-eastern Florida and is positioned between the Atlantic Ocean to the east and the protected Everglades wetlands to the west. The current human population in this coastal corridor is c. 6 million people (South Florida Regional Planning Council 2021) and is expected to continue growing through the remainder of the century (Carr & Zwick Reference Carr and Zwick2016). This metropolis is also relatively young, having grown to its current size from a population of under 100 000 people in 1940 (South Florida Regional Planning Council 2021).
Six stork nesting colonies in south-eastern Florida – three located in the natural Everglades system (Tamiami West 25.75784, –80.54484; Paurotis Pond 25.2815, –80.803; and Jetport South 25.8051, –80.84902) and three located in urban areas (Griffin 26.0636333, –80.3664916; BallenIsles 26.830142, –80.109086; and Sawgrass 26.149802, –80.337681) – were each visited during the cool morning or evening hours one to two times per week during the breeding season (January–June) 2015–2020 (2014 excluded from analysis due to methodological differences; Fig. S1). After the mid-point of incubation (i.e., 2–3 weeks after incubation behaviour was observed from a distance in the majority of nesters), each colony was entered and a short strip of numbered flagging tape was tied beneath nest bowls for identification. Clutch size, estimated hatch dates and the number, estimated age and keel score of chicks were recorded during every subsequent visit until the fate of all monitored nests in each colony was determined. The chick age used to define fledging success in storks varies from 4 to 7 weeks, but our study favoured the 4-week cut-off used by Evans et al. (Reference Evans and Gawlik2020) because of logistical and ethical constraints imposed by our study locations. Keel score, a proxy for body condition, ranged from 1 to 5, where 1 represented a protruding keel bone with no overlaid muscle and 5 represented a muscle layer thick enough to hide the shape of the keel bone entirely (after Evans & Gawlik Reference Evans and Gawlik2020).
Diet sampling
During the chick-rearing period of our annual colony monitoring effort (generally March–June, 2014–2020), one or more chicks of any age per nest were handled to stimulate voluntary regurgitation of a food bolus, which was collected for subsequent diet analysis. If a chick did not voluntarily regurgitate but food could be felt in the crop, the throat of the bird was gently massaged to induce regurgitation. The chick was then returned to the nest and provided with purchased bait fish (Atlantic silversides, Menidia menidia), which the storks readily consumed, to compensate for their lost meal. Boluses were stored in plastic bags on ice in the field and frozen upon return. In the laboratory, boluses were thawed, rinsed, weighed, measured (standard length, mm) and identified to the lowest possible taxonomic level, which was usually the species level. In the database, prey species were grouped into native, non-native and trash (discarded human food and other refuse) categories.
Statistical analysis
Land-cover types available to storks within core foraging areas (CFAs; i.e., all land within a 30-km radius of each monitored colony site; Brooks & Dean Reference Brooks and Dean2008) were compared. First, a land-cover classification system (Kawula & Redner Reference Kawula and Redner2018) was imported into ArcGlobe 10.8 as a raster, clipped to the buffer edges, summed by land-cover type based on the number of pixels per CFA and then exported to a text file. Then, in Excel, land-cover types were manually reclassified as ‘urban’, ‘rural’ or ‘marsh’ based on their descriptions (Kawula & Redner Reference Kawula and Redner2018). Finally, the proportions of urban, rural and marsh habitats found within the CFA were calculated for each colony (Fig. S1).
Stork productivity (i.e., nest success) was investigated using R version 4.0.2 (R Core Development Team 2020). Productivity (response variable) and diet (predictor variables), which were recorded at the nest level in the field, were summarized as the mean ± standard deviation (SD) at the levels of colony and colony type (urban or marsh). Nest success (%) was based on the proportion of nests that successfully fledged at least one chick. Keel score was reported as the average score of chicks at fledge (age = 4 weeks). For diet, the biomass (g) of non-native prey and trash were calculated and then divided by the total biomass of each nest to be represented as a proportion of the diet (%).
In preparation for a generalized linear mixed model (GLMM), the proportions of non-native prey and trash at the nest level were binned into nests that consumed relatively low, medium or high amounts of each. For non-native prey, most nests (130 of 234) consumed <1.0%, so these were binned into the ‘Low’ category. Of the nests that consumed ≥1.0% non-native prey by biomass, the number of nests was divided in half to preserve sample size in the analysis, and the percentage of non-native biomass at that divide was used as the cut-off. In doing so, 1.0–22.0% non-native prey was considered a ‘Medium’ level of consumption (n = 51) and 22.1–100% non-native prey was considered a ‘High’ level of consumption (n = 53). Similarly, most storks (213 of 234 nests) consumed no trash, so 0.0% was considered the ‘Low’ consumption level. The numbers of nests that did eat trash were divided in half, so that ‘Medium’ consumption ranged from 0.1% to 20.0% (n = 10) and ‘High’ consumption included those nests that consumed >20.0% trash (n = 11).
We used Akaike’s information criterion for small sample sizes (AICc; Anderson Reference Anderson2008) to compare competing GLMMs explaining the number of fledglings per nest and then averaged the top model set following the method described in Grueber et al. (Reference Grueber, Nakagawa, Laws and Jamieson2011). Biologically relevant variables and interaction terms were selected (Table S1) and standardized to a mean of 0 and a SD of 0.5 using the package arm. In addition to the diet and colony type (urban or marsh) parameters related to our hypotheses, we included a variable summarizing the hydrological condition of the natural marsh during the breeding season (suboptimal, moderate, optimal), as that is known to have a strong influence on breeding success via prey availability (Frederick et al. Reference Frederick, Gawlik, Ogden, Cook and Lusk2009, Evans & Gawlik Reference Evans and Gawlik2020). Twenty-six candidate models were analysed using the package lme4 based on the following global model:
 $$\matrix{ {{\rm{Productivity}} \sim {\rm{Nonnatives}} + {\rm{Trash}} + {\rm{Colony}}\;{\rm{Type}}} \hfill \cr
{ + {\rm{Hydro}}\;{\rm{Year}} + {\rm{Nonnatives}} \times {\rm{Colony}}\;{\rm{Type}}} \hfill \cr
{ + {\rm{Nonnatives}} \times {\rm{Hydro}}\;{\rm{Year}} + {\rm{Colony}}\;{\rm{Type}}} \hfill \cr
{ \times {\rm{Hydro}}\;{\rm{Year}} + \left( {{\rm{1|ColonyID}}} \right)} \hfill \cr } $$
$$\matrix{ {{\rm{Productivity}} \sim {\rm{Nonnatives}} + {\rm{Trash}} + {\rm{Colony}}\;{\rm{Type}}} \hfill \cr
{ + {\rm{Hydro}}\;{\rm{Year}} + {\rm{Nonnatives}} \times {\rm{Colony}}\;{\rm{Type}}} \hfill \cr
{ + {\rm{Nonnatives}} \times {\rm{Hydro}}\;{\rm{Year}} + {\rm{Colony}}\;{\rm{Type}}} \hfill \cr
{ \times {\rm{Hydro}}\;{\rm{Year}} + \left( {{\rm{1|ColonyID}}} \right)} \hfill \cr } $$
Models within 4 ΔAICc of the top model were deemed informative. To determine the relative influence of each predictor variable on nest success, we calculated model-averaged parameter estimates and 95% confidence intervals (CIs) for these estimates using the top model sets in the package MuMIn (Bartoń Reference Bartoń2021).
We were specifically concerned that ‘trash’ might be an uninformative parameter since no marsh-nesting storks consumed any amount of trash, and therefore ‘trash’ aligned in pattern with ‘colony type’. To test this, we followed the protocol described by Leroux (Reference Leroux2019), re-running the models in the top model set that contained ‘trash’ without that variable. Next, we compared the results of the paired models to determine whether ‘trash’ contributed information to the model in the top set by comparing the paired models’ log likelihood and AICc values (Table S2). One of five of the models in the top model set that contained ‘trash’ probably contains an uninformative parameter, so this is labelled as such in the results.
Results
Over our sampling years (2014–2020), storks experienced a broad range of hydrological conditions in the natural marsh system, relatedly experiencing very productive breeding years as well as years of high nest failure (Cook & Baranski Reference Cook and Baranski2021). Storks nested in all urban colonies in all years of record (2015–2020), but not in all marsh colonies in all years (2014–2020). Specifically, storks did not nest in Tamiami West in 2016 or 2018 or Jetport South in 2015, 2016 or 2019, and they initiated very late and in low numbers in Tamiami West in 2019. The number of nests in each urban colony was small and consistent between years (mean ± SD: 55 ± 39 nests/colony/year, range: 10–150 nests/colony/year; Tables 1 & S3), while the number of nests in each marsh colony was ‘boom or bust’ (mean ± SD excluding non-nesting events: 328 ± 274 nests/colony/year, range: 0–953 nests/colony/year; Tables 1 & S3). Over our six years of study, in all known stork colonies in south Florida, nesting in seven urban colonies accounted for 26% ± 7% (mean ± SD; range: 18–37%) of all nesting in south Florida each year (Table S3).
Table 1. Summary of nest-level wood stork (Mycteria americana) productivity in urban and marsh colonies in south Florida, 2014–2020. A nest is considered successful if at least one hatched chick survived to fledging age (4 weeks old). Keel score, a proxy for body condition at the age of fledging, ranges from 1 to 5, where 1 is a protruding keel bone with no overlaid muscle and 5 is a muscle layer thick enough to hide the shape of the keel bone entirely.
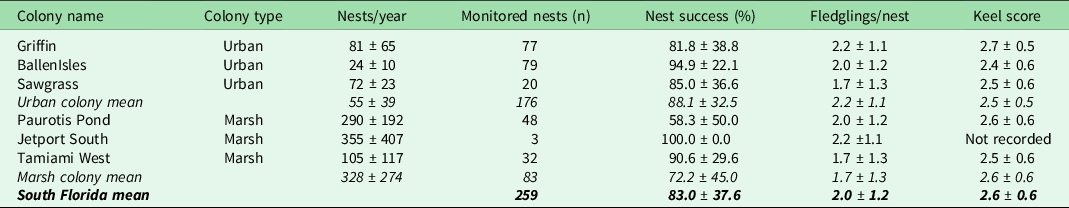
Storks nesting in urban colonies could readily access both urban (mean 46.9%, range 41.5–51.6%) and marsh (mean 49.4%, range 48.3–51.4%) land-cover types within their CFAs, while storks nesting in marsh colonies had little access to urban habitats (mean 9.2%, range 0.2–26.8%) and increased access to marsh (mean 88.3%, range 66.2–99.6%) land-cover types within their CFAs (Table 2 & Fig. S1).
Table 2. Percentage of the core foraging area (CFA; 30-km radius surrounding colony site) by land-cover type for each of the wood stork (Mycteria americana) colonies monitored for this study.

Averaged over all years, storks in urban colonies experienced a nest success rate of 88.1% ± 32.5% compared to the marsh colony average of 83.0% ± 37.6%. The number of fledglings produced per successful nest and the average keel score of those fledglings did not differ by colony type (Table 1). Diet was highly variable at the nest level, although storks from marsh colonies tended to provision more non-native prey (24.7% ± 55.3% biomass) and ate no trash in comparison to urban-nesting storks that ate 15.7% ± 37.8% non-native prey and 7.0% ± 24.0% trash as proportions of their dietary biomass (Figs 1 & S2 & Table S4).

Figure 1. Histograms of the number of nests by the proportion of diet that was non-native prey (% biomass) in (a) urban colonies, (b) marsh colonies and (c) all colonies. The thresholds that were used to define ‘Low’, ‘Medium’ and ‘High’ consumption of non-native prey in subsequent models are also displayed (see text for details).
Of the 26 GLMMs in the candidate model set, 10 were considered informative, having scored within 4 ΔAICc of the top-ranked model (top model w i = 0.263, cumulative w i = 1.0; Table S2). All parameters except for Colony Type × Hydro Year appeared in this top model set, and the Hydro Year parameter appeared in all 10 models. Three parameters – Hydro Year (Optimal), Trash (High) and Colony Type × Nonnatives (High) – had coefficient estimates with 95% CIs that did not bound 0, indicating that these had the most influence on stork productivity (Table 3 & Figs S3–S5).
Table 3. Average parameter estimates from the top model set (n = 10 models) identified using an information theoretic approach (Akaike’s information criterion for small sample sizes; AICc). Bolded parameters are those with 95% confidence intervals that do not overlap 0.

a Strong evidence that Trash_Med was a non-informative parameter in one of these top models. See text and Table S2 for details.
NA = not applicable.
Discussion
Our results aligned with previous Everglades’ research showing the importance of marsh hydrological conditions on wading bird productivity (Frederick & Ogden Reference Frederick and Ogden2001, Herring & Gawlik Reference Herring and Gawlik2011, Evans & Gawlik Reference Evans and Gawlik2020), but they extend the pattern to urban-nesting storks in addition to those that nest in natural marsh colonies. This is noteworthy because it suggests that urban storks, which have ample access to both urban and marsh habitats within their CFAs, are still at their most productive when foraging conditions are good in the natural system. Across all years, storks in urban colonies had higher nesting success (i.e., higher proportion of nests that fledged at least one chick) but produced a similar number of fledglings per successful nest compared to storks in marsh colonies. This indicates that early nest failures were common in marsh colonies, at least in our years of study. Urban nesters may be less susceptible to abandonment because they have access to alternative food types and foraging habitat types in the urban environment (Evans et al. Reference Evans, Klassen, Gawlik and Gottlieb2023), benefitting urban breeders in years when the marsh hydrological condition was poor. Prey switching, as may be happening in urban storks, has been noted in the white ibis (Eudocimus albus; Kushlan Reference Kushlan1979, Dorn et al. Reference Dorn, Cook, Herring, Boyle, Nelson and Gawlik2011), another south Florida wading bird, and in waterbirds globally (e.g., Australian white ibis Threskiornis molucca (Chard et al. Reference Chard, French, Martin and Major2018), grey heron Ardea cinerea (Jakubas & Manikowska Reference Jakubas and Manikowska2011), Larus spp. (Hostetter et al. Reference Hostetter, Payton, Roby, Collis and Evans2022, Serré et al. Reference Serré, Irvine, Williams and Hebert2022), Phalacrocoracidae (Lehikoinen Reference Lehikoinen2005, Hostetter et al. Reference Hostetter, Payton, Roby, Collis and Evans2022)). In our models, colony type had only a moderate influence on productivity in favour of urban breeders (average estimate: –0.38, 95% CI: –1.01 to 0.25), but high trash consumption was associated with high productivity of storks at the nest level as one of the three most influential parameters (average estimate: 0.69, 95% CI: 0.02–1.46). In combination, these findings lend strong support to our first hypothesis: urban habitat use increases productivity at the nest level.
Our second hypothesis on the effect of non-native prey consumption on productivity is not well supported except in relation to colony type. In the models, medium and high levels of non-native prey in the diet had a moderate influence on productivity, with moderate levels of consumption being associated with increased productivity (average estimate: 0.22, 95% CI: –0.15 to 0.59) and high levels of consumption being associated with low productivity (average estimate: –0.21, 95% CI: –0.55 to 0.13). More notably, the interaction term Nonnatives × Colony Type (High) had a strong influence (average estimate: 0.74, 95% CI: 0.02–1.46), although it only appeared in two models in the top model set. Here, urban nesters that consumed high levels of non-native prey paid a bigger penalty in terms of productivity than marsh-nesting storks with a similar level of non-native prey consumption. This interaction was not previously known. The increased penalty to urban breeders could be due to the prey itself (e.g., accessing non-native prey that are low in nutrients and high in parasites or contaminants) or it could be because urban nesters are flying further to access foraging patches where the non-natives are present, thereby gaining less net energy per trip and having less time available for nest defence. Further research would be necessary to distinguish between these possible causes.
Our study found a positive association between trash consumption and productivity. This association has been debated in the literature because consumption of trash and other anthropogenic foods is common in Ciconiiformes globally (marabou stork Leptoptilos crumenifer (Francis et al. Reference Francis, Kingsford, Murray-Hudson and Brandis2021), white stork Ciconia ciconia (Peris Reference Peris2003, Chenchouni Reference Chenchouni2017), woolly-necked stork Ciconia episcopus (Thabethe et al. Reference Thabethe, McPherson and Downs2021)), but it is also known to have some negative nutritional consequences (Peris Reference Peris2003, Urfi Reference Urfi2011, Francis et al. Reference Francis, Kingsford, Murray-Hudson and Brandis2021). In south Florida, no marsh-nesting wood storks consumed trash and only some urban-nesting storks did, primarily in years when the hydrological condition of the marsh was moderate or poor. Hydrological patterns are known to have an extreme impact on stork productivity in south Florida, and productivity is generally very low in years with poor hydrological conditions (Frederick & Ogden Reference Frederick and Ogden2001). Therefore, we believe that the positive impact of trash on the birds we observed is due to birds utilizing alternative food resources when preferred food types were extremely scarce, as has been found in other prey-switching studies (Dorn et al. Reference Dorn, Cook, Herring, Boyle, Nelson and Gawlik2011, Chard et al. Reference Chard, French, Martin and Major2018, Serré et al. Reference Serré, Irvine, Williams and Hebert2022).
The urbanization of wading birds, including Ciconiiformes, is a global phenomenon (Sundar et al. Reference Sundar, Chauhan and Kittur2015, Rawal et al. Reference Rawal, Kittur, Chatakonda and Sundar2021, Gula et al. Reference Gula, Sundar, Willows-Munro and Downs2023). White storks in Algeria (Chenchouni Reference Chenchouni2017), painted storks (Mycteria leucocephala) in India (Suryawanshi & Sundar Reference Suryawanshi and Sundar2019), marabou storks in Botswana (Francis et al. Reference Francis, Kingsford, Murray-Hudson and Brandis2021) and woolly-necked storks in South Africa (Thabethe et al. Reference Thabethe, McPherson and Downs2021) are known to nest in urban or suburban habitats and eat anthropogenic foods. In south Florida, urban-nesting wood storks not only used urban areas for foraging and breeding, but in some years do better than those storks that nested in the natural system. Urban colonies may therefore be key to the future longevity of stork nesting in south Florida. However, we also note that storks from both urban and marsh colonies foraged in the marsh when hydrological conditions were optimal, and optimal hydrological years are key to the high-productivity years of both urban and marsh nesters. Only in suboptimal hydrological years did urban-nesting storks take advantage of urban food resources to benefit productivity. These findings are in accordance with Evans and Gawlik (Reference Evans and Gawlik2020), and together they suggest that the concurrent use of resources from the urban and non-urban environments contributes to the success of storks in south Florida.
Classic categorizations of species-level responses to urbanization do not adequately describe storks and, we suspect, many other species whose home ranges could include both natural and urban habitat types. The stork is not an ‘urban exploiter’ as it is not a purely urban bird, relying heavily on optimal hydrological conditions of the natural marsh for peak productivity. In most years, the stork breeds more abundantly in marsh colonies than in urban colonies, and storks from both colony types utilize marsh foraging habitats. Similarly, the stork is not an ‘urban avoider’ as it does use urban habitats for both nesting and foraging and relies on urban habitats for foraging when marsh conditions are suboptimal. It is also not a ‘suburban adaptor’ as storks seek resources from truly urban and truly wild habitats within the course of a day. As such, we believe a fourth category, what we call ‘urban commuters’, is most appropriate to describe storks and other species whose populations are most stable at urban/natural borders and within urban matrices such as the urban–wildland interface.
Commuter behaviour has been documented but not labelled within the theoretical framework of urban ecology in other species. Breeding white storks in southern Portugal forage on both landfill waste and natural food resources; however, foraging at landfills was found to save the birds time and energy despite their greater distance from colony sites (Soriano-Redondo et al. Reference Soriano-Redondo, Franco, Acácio, Martins, Moreira and Catry2021). Similarly, great egrets (Ardea alba) nesting in Narragansett Bay (Rhode Island, USA) nest in colonies at rural coastal locations but experience greater net caloric gain from foraging in urban habitats compared to rural habitats (McKinney & Raposa Reference McKinney and Raposa2013). Several species of gull (Larus spp.) across the North Atlantic nest predominantly on natural, offshore islands that are relatively undisturbed by humans and predators, yet they opt to forage for anthropogenic food, often within urban areas (Isaksson et al. Reference Isaksson, Evans, Shamoun-Baranes and Åkesson2015, Shlepr et al. Reference Shlepr, Ronconi, Hayden, Allard and Diamond2021). All of these are examples of species that depend on non-urban habitats for reproduction yet quantifiably benefit in terms of caloric intake, survival and/or productivity when opting to utilize urban resources as part of their daily activity.
Outside of the waterbird clade, which has been recognized for its extraordinary tolerance of urban habitats (Callaghan et al. Reference Callaghan, Major, Wilshire, Martin, Kingsford and Cornwell2019), commuter patterns are evidenced in other vertebrates that share waterbirds’ abilities to efficiently travel long distances and maintain large home ranges, including raptors (Falco spp. (Kettel et al. Reference Kettel, Gentle, Quinn and Yarnell2018), Stephanoaetus coronatus (Muller et al. Reference Muller, Amar, Sumasgutner, McPherson and Downs2020)), monkeys (Chlorocebus pygerythrus (Patterson et al. Reference Patterson, Kalle and Downs2019, Thatcher et al. Reference Thatcher, Downs and Koyama2019)), bears (Urus spp. (Beckmann & Berger Reference Beckmann and Berger2003, Bateman & Fleming Reference Bateman and Fleming2012)) and wolves and coyotes (Lupus spp. (Bateman & Fleming Reference Bateman and Fleming2012, Poessel et al. Reference Poessel, Breck and Gese2016)). Additionally, the responses of animals with smaller home ranges that are populous at the urban–wildland interface (e.g., porcupine Hystrix africaeaustralis (Ngcobo et al. Reference Ngcobo, Wilson and Downs2019), mongoose Atilax paludinosus (Streicher et al. Reference Streicher, Ramesh and Downs2021)) should be reconsidered in light of the commuter concept.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0376892923000152.
Acknowledgements
We thank BallenIsles Country Club and the City of Weston for granting land access permissions, especially in 2020 at the beginning of the COVID-19 pandemic, and two anonymous reviewers for their constructive comments on this manuscript.
Financial support
This project was supported by the Florida Department of Transportation (BDV 27-922-02), the Waterbird Society’s Kushlan Award (2019) and the Harte Research Institute’s Crutchfield Fellowship Endowment (2021–2022).
Competing interests
The authors declare none.
Ethical standard
The authors assert that all procedures contributing to this work comply with applicable national and institutional ethical guidelines on the care and use of laboratory or otherwise regulated animals. Data collection was conducted in accordance with the conditions stated in the following permits: FAU IACUC A14-11, A14-28, A17-33; USFWS TE65550A, TE65550A-2; NPS EVER-2014/2016-SCI-0021, EVER-2018-SCI-0017, BICY-2014-SCI-0014; FFWCC S-15-02, LSSC-18-00027; and the Miccosukee Tribe of Indians of Florida.







