Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), is a global health threat, with 8 million active new cases and 2–3 million deaths annually. The majority of TB cases reside in developing countries, among others in Indonesia, which harbours more than 10 % of TB cases worldwide1. MTB is an intracellular pathogen that targets host phagocytes, and consequently an effective host defence is required to coordinate cellular immune responseReference Flynn and Chan2. Following phagocytosis, MTB lives within phagosomes of host macrophages and competes with the host to acquire Fe in order to survive and replicateReference Schaible and Kaufmann3. For the host, Fe is an essential component of Hb, as Fe binds and transports O2. Fe is also needed for electron transport, DNA synthesis and immune function, for example, for the formation of oxygen radicalsReference Marx4.
It remains unclear how MTB accumulates Fe in macrophages. An excess of Fe supply will result in MTB growth, and Fe overload is a known risk factor for infections, as this may worsen the disease. Fe overload, for example from dietary Fe, causes individuals to be more susceptible to TBReference Gangaidzo, Moyo and Mvundura5. Interestingly, the MTB growth within macrophages from individuals with hereditary haemochromatosis, a genetic disease which leads to Fe overload, is reducedReference Olakanmi, Schlesinger and Britigan6. This can be explained by the fact that, even in severe Fe overload, macrophages in hereditary haemochromatosis provide an Fe-deficient environment due to increased export of Fe from the labile Fe pool to plasma by the export protein ferroportinReference Swinkels, Janssen, Bergmans and Marx7, Reference Moura, Noordermeer, Verhoeven, Verheul and Marx8.
On the other hand, Fe deficiency can increase susceptibility to various infectious diseases, since macrophages require Fe to function wellReference Schaible and Kaufmann3. Even mild Fe deficiency causes a significant impairment in the immune status and reduces the capacity to control infections. Fe-deficiency anaemia is the most common cause of nutritional deficiency anaemia in developing countries, affecting mostly children and pregnant and lactating womenReference Dijkhuizen, Wieringa, West, Muherdiyantiningsih and Muhilal9. Therefore, Fe supplementation is often prescribed in developing countriesReference Bharti10.
Several susceptibility-associated genetic polymorphisms have been proposed to explain differential susceptibility to TB. Various studies have reported that subtle variations in the natural resistance-associated macrophage protein gene (NRAMP1) result in a higher risk for having TBReference Bellamy, Ruwende, Corrah, McAdam, Whittle and Hill11–Reference Soborg, Andersen, Madsen, Kok-Jensen, Skinhoj and Garred13. NRAMP1 is a metal transporter protein localised in late endosomal and lysosomal compartments, and probably plays an important role in transferring diferric Fe across the phagosome membraneReference Canonne-Hergaux, Gruenheid, Govoni and Gros14, Reference McDermid and Prentice15.
In the present study we conducted a case–control study in Indonesia, a country with a high prevalence of both TB and Fe-deficiency anaemia. Fe status of pulmonary TB patients and controls was explored to determine the prevalence of anaemia with or without Fe deficiency in active TB patients. Also, the effect of TB infection on Fe status indicators during the course of TB therapy was investigated, as inflammation affects many indicators of Fe statusReference Wieringa, Dijkhuizen, West, Northrop-Clewes and Muhilal16. Distribution of NRAMP1 alleles and genotypes in Indonesia has not been reported before. In the present study, three commonly investigated polymorphisms in the NRAMP1 gene, INT4, D543N and 3′UTR, were examined to explore whether such polymorphisms are associated with susceptibility to TB or TB severity.
Materials and methods
Subject recruitment
After written informed consent was obtained from all subjects, 494 newly detected sputum smear-positive pulmonary TB patients aged over 15 years were recruited in a poor setting area at an out-patient TB control clinic, Perkumpulan Pemberantasan Tuberkulosis Indonesia (PPTI), in Jakarta from January 2002 until December 2005. This case–control study was part of a larger TB study in Indonesia. The study design was approved by the Medical Faculty University of Indonesia and the Eijkman Institute Jakarta ethical committees. Pulmonary TB was diagnosed based on the clinical presentation, chest X-ray radiography (CXR), and confirmed by two consecutive acid-fast bacilli-positive sputa. All patients were provided with free anti-TB therapy according to the national TB programme (2HRZE/4H3R3). Patients with seropositive HIV (n 7; 1·4 %), diabetes mellitus (n 96; 19·4 %), CHD (n 3; 0·6 %) or incomplete data (n 10; 2·0 %) were excluded from the statistical analyses. For analysis after therapy, only patients with complete medical records after TB therapy (n 153) were included.
In the same period, 519 healthy controls from the neighbourhood where the cases lived, with the same socio-economic status, were randomly selected and matched for sex and age ( ± 10 %). Controls were interviewed using the same standardised questionnaire and underwent the same physical and blood examination and CXR as cases. Control subjects with CXR suspective of TB (n 17; 3·3 %) or history of prior anti-TB treatment (n 7; 1·4 %), diabetes mellitus (n 25; 4·8 %) and incomplete data (n 34; 6·5 %) were excluded. Although not all control subjects were tested for HIV status, since informed consent for HIV testing in the control group could only be obtained later in the study, HIV seropositivity was only found in two of 115 (1·7 %) tested controls. Indonesia has a low HIV prevalence in the general population, which was similar to the number found in TB patients and in accordance with earlier reports for Indonesia1.
Blood samples were obtained by venepuncture. Full blood counts were performed routinely in the clinic for all patients before therapy and all controls using an automated blood analyser (Cell-Dyn 3200, Abbott Laboratories, Abbott Park, IL, USA). Haematology data could be obtained only in sixty-five of 153 patients after therapy since full blood counts are not routinely performed in the clinical setting.
Plasma from heparinised blood was collected and stored at − 80°C for further analysis. Fe status indicators including plasma Fe, plasma Fe-binding capacity, Fe saturation and plasma ferritin were measured from patients for whom haematology data were available (n 65) and in a set of randomised controls (n 76). Total plasma Fe was measured using an ascorbate/FerroZine colorimetric method (Abbott Laboratories, Abbott Park, IL, USA). The plasma ferritin was measured by a solid-phase, two-site chemiluminescent immunometric assay (Immulite 2000; Diagnostic Product Corporation, Los Angeles, CA, USA). Fe status indicators could, however, only be measured in the plasma of thirty-three patients after therapy due to limited plasma availability. Erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP) examination were measured as indicators of the inflammatory response.
Genotyping of NRAMP1 single nucleotide polymorphisms
Genomic DNA was isolated from EDTA blood of patients and controls. Two single nucleotide polymorphisms in the gene NRAMP1, D543N (1703G>A in exon 15 leading to an aspartate to asparagine substitution at codon 543) and INT4 (a single nucleotide change in intron 4; 469+14G>C), were analysedReference Bellamy, Ruwende, Corrah, McAdam, Whittle and Hill11. Multiplex assays were designed using Assay Design software (Sequenom Inc., San Diego, CA, USA). Genotyping was performed using the MassArray platform according to the manufacturer's protocols (Sequenom Inc.). In brief, after PCR on 2·5 ng DNA a primer extension reaction was performed to introduce mass differences between alleles and, after removing salts by adding a resin, about 15 nl of the product was spotted onto a target chip with 384 patches containing matrix. Mass differences were detected using a Bruker Autoflex matrix-assisted laser desorption ionisation time-of-flight (MALDI-TOF) mass spectrometer (Sequenom Inc., San Diego, CA, USA) and genotypes were assigned real-time using Typer 3.1 software (Sequenom Inc.). As quality control, 10 % of samples were genotyped in duplicate and no inconsistencies were observed. Primer sequences are available upon request.
Genotyping using fragment length analyses
PCR for a TGTG deletion polymorphism in the 3′ untranslated region (1729+55del4), denoted as 3′UTR, were performed using 100 ng genomic DNA, 200 μm of each dNTP, 10 pmol of each primer, 50 mm-KCl, 10 mm-tri(hydroxymethyl)-aminomethane-HCl (pH 9·0), 0·1 % Triton X-100, 1·5 mm-MgCl2 and 0·5 U Taq DNA polymerase (Biolabs, Beverly, MA, USA) in a total volume of 25 μl. Forward primers were 5′-labelled with tetrachloro-6-carboxy-fluorescein (TET); primer sequences and cycle conditions are available on request. PCR products and a 400 HD-ROX size standard (Applied Biosystems, Foster City, CA, USA) in HiDi formamide were run on an ABI Prism 3700 DNA Analyzer (Applied Biosystems), and results were analysed using GeneScan Analysis and Genotyper software (Applied Biosystems). Several homozygous alleles were sequenced to verify allele lengths.
Statistical analysis
Data from the questionnaires, physical examinations, laboratory analyses and genotypings were analysed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). Data were checked for normality using the Kolmogorov–Smirnov test. Independent and paired t tests were used to compare means. Ferritin concentrations were transformed to natural logarithms to obtain normality. Analysis of covariance was used to compare indicators of Fe status.
The Hardy–Weinberg equilibrium of each polymorphism was checked using the program HWEReference Ott17. The program CONTING was used to calculate χ2 and associated values for a contingency tableReference Ott17. All statistical analyses were two-sided and P values < 0·05 were considered as statistically significant.
Results
A total number of 378 newly detected sputum-positive pulmonary TB patients (median age 29 (range 15–67) years) and 436 community healthy controls (median age 33 (range 15–70) years) were included. Clinical characteristics of all included TB patients and controls are presented in Table 1. In active TB, CRP concentrations and ESR were both elevated and correlated highly in the active TB patients at recruitment (Spearman's rank r 0·52; P < 0·001). Both CRP concentrations and ESR returned to normal levels after successful TB therapy (paired t test).
Table 1 Clinical characteristics of pulmonary tuberculosis (TB) patients before and after TB therapy compared with healthy community controls* (Mean values and standard deviations)

ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; MCV, mean corpuscular volume; MCH, mean corpuscular Hb.
* All values before TB therapy were significantly different compared with controls (t test). All values before TB therapy were also increased or decreased significantly (P < 0·05) at the end of the therapy (paired t test, tested only in individuals with two time points).
† Data were collected from 240 individuals.
‡ Data were collected from 295 individuals.
§ Criteria for anaemia: males Hb < 130, females Hb < 120 g/l.
‖ Data were collected from sixty-five individuals.
¶ Male and female values were taken together.
** Data were collected from thirty-three individuals.
†† Data were collected from seventy-six individuals.
Anaemia in active tuberculosis and its association with clinical presentation
Active TB was a strong predictor of lower Hb concentrations. In the patients, Hb concentrations were significantly correlated to CRP concentrations (P = 0·013), but not to ferritin concentration (P>0·2; analysis of covariance controlling for age and sex). Anaemia was found in 239 active TB patients (63·2 %) compared with only thirty controls (6·8 %). Females were more often affected by anaemia than males, both among TB patients (74·5 v. 55·6 %) and among controls (10·9 v. 3·6 %). At the end of successful TB therapy, anaemia had been corrected without any Fe therapy or dietary supplementation in almost all patients. Hb concentrations were 20 g/l higher after treatment (paired t test; P < 0·001). The increase of Hb concentrations between recruitment and end of TB treatment was associated with a decrease of the inflammatory indicators such as ESR and CRP. Also mean corpuscular volume (MCV) of erythrocytes and mean corpuscular Hb (MCH) levels increased (Table 1). Only five patients (7·6 % of sixty-five measured post-therapy) remained anaemic but their Hb concentrations increased significantly.
TB patients had coughing (98 %) as their main complaint. As TB is a chronic disease, the duration of the main complaint before TB patients presented themselves in the clinic might be of importance. Anaemia was more prevalent in TB patients with coughing for more than 1 month as compared with patients with a recent complaint ( < 1 month) (P = 0·041; data not shown), probably reflecting long-term effects of immune activation on Hb concentrationsReference Wieringa, Dijkhuizen, West, Northrop-Clewes and Muhilal16. Blood coughing was present in 46 % of the cases. Although a trend could be observed, there was no significant correlation between occurrence of anaemia and blood coughing (P = 0·054) (Table 2). Furthermore, anaemia was present more frequently in advanced TB (ninety-two of 118 known CXR results; 77·9 %) compared with mild or moderate TB (forty-eight of eighty-eight cases; 54·5 %) (P < 0·001) as assessed by CXR abnormalities (Table 2). CXR abnormalities were classified as mild or moderate TB (n 88) or advanced TB (n 118), based on the extent of lesions on CXR as described elsewhereReference Falk, O'Connor, Pratt, Webb, Wier and Wolinsky18. Anaemia was also negatively associated with smoking habits. TB patients who were currently smoking or had ever smoked in the past (designated as ‘ever’) were surprisingly less frequently anaemic compared with those who never smoked (P = 0·003) (Table 2). In a more extended study in our group we found that smoking was not associated with TB (OR 0·99 (95 % CI 0·76, 1·31); data not shown)
Table 2 Anaemia status of active pulmonary tuberculosis (TB) patients in relation to clinical signs or symptoms (Frequency and percentage frequency)
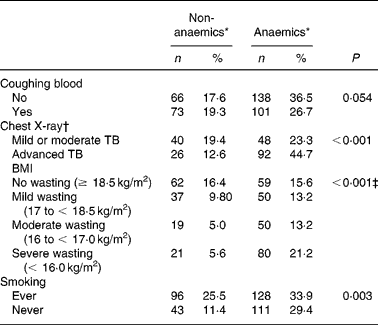
* Criteria for anaemia: males Hb < 130, females Hb < 120 g/l.
† A chest X-ray was assessed in 206 patients.
‡ Subjects with BMI < 18·5 kg/m2 were pooled as the wasting group and compared with the no wasting group (χ2 test).
Anaemia also affected thirty-one control individuals, consisting of twenty-two females (10·0 %) and nine males (5·7 %). Of the female controls with low Hb (69–118 g/l), eleven individuals had a very low MCV (48·6–76·0 fl), and in the male controls with low Hb (103–128 g/l), seven individuals had a very low MCV (59·1–69·3 fl), suggesting that these individuals may have anaemia with Fe deficiency. Furthermore, nine males of the control group had a normal Hb (133–174 g/l) with a very low MCV (58·5–75·7 fl) and six females had a normal Hb (124–140 g/l) with a low MCV (60·4–74·1 fl), which could be related to thalassaemia minor. Thalassaemia (especially minor) must be considered in the differential diagnosis of normal Hb with a low MCV value.
Follow up of thirty-three tuberculosis patients: iron status indicators and anaemia
The Fe status indicators were measured in plasma for the baseline data in a random set of active TB patients before therapy (n 65) and after therapy (n 33), and compared with controls, matched for sex and age (n 76). Many indicators of Fe status were influenced by the inflammatory response. In patients with active TB, plasma Fe concentrations and Fe saturation were lower, whereas ferritin concentration was increased as compared with controls. As was to be expected, there was a strong correlation between these indicators of Fe status and CRP concentrations. Linear regression analysis showed a correlation between ferritin and CRP concentrations (R 2 0·19; P < 0·001) with an unadjusted coefficient of 2·9, meaning that for every 10 g/l increase in CRP concentration, ferritin concentration had increased by almost 30 μg/l. Moreover, at the end of TB therapy, all Fe indicators had returned to normal values with disappearance of the inflammatory response as indicated by normal concentrations of CRP and a normal ESR (Table 1). At the end of successful TB therapy, anaemia without Fe deficiency (ferritin concentrations >12 μg/l) was found in three patients (all males, Hb range 119–129 g/l, CRP all < 5 g/l). One of those anaemic patients had a very low MCV and MCH value (Hb 128 g/l, MCV 60·2 fl, MCH 20·8 pg), which may suggest thalassaemia minor. On the other hand, Fe deficiency was observed in three female patients who were, however, not anaemic.
In the control group, three anaemic individuals (all female) showed a normal plasma ferritin level (35–130 μg/l). One of these controls had very low MCV and MCH values (Hb 118 g/l, MCV 63·4 fl, MCH 22·1 pg), again suggesting thalassaemia minor. A plasma ferritin value ≤ 12 μg/l was observed in one female control with a normal Hb value (122 g/l).
NRAMP1 gene polymorphisms and susceptibility to tuberculosis
In the Indonesian population, the INT4 polymorphism proved to be rare and was not further analysed. The genotypes of the NRAMP1 D543N and the 3′UTR polymorphisms were in Hardy–Weinberg equilibrium in the total group of individuals as well as in the healthy controls and patients. No significant differences could be observed between healthy controls and TB patients, suggesting that NRAMP1 polymorphisms in our population are not associated with TB susceptibility. The distribution of the alleles and genotypes of the NRAMP1 polymorphisms in the Indonesian population is presented in Table 3. Furthermore, NRAMP1 polymorphisms are not associated with TB severity, as evidenced by CXR, or by anaemia in active TB (data not shown).
Table 3 Distribution of NRAMP1 alleles and genotypes* (Frequency and percentage frequency)
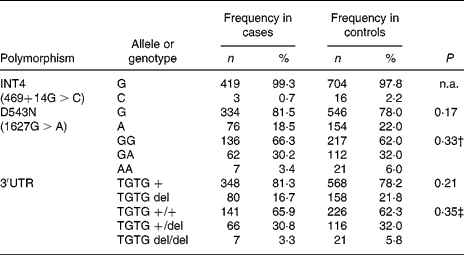
n.a., Not analysed.
* No significant differences were observed in the distribution of NRAMP1 single nucleotide polymorphism alleles or genotypes between tuberculosis patients and healthy controls (χ2 tests).
† Genotype GA and AA combined for analysis.
‡ Genotype TGTG +/del and TGTG del/del combined for analysis.
Discussion
Fe-deficiency anaemia has been reported in many developing countries19, Reference Ramakrishnan20. In these countries many chronic infectious diseases are present at high rates, including pulmonary TB. The prevalence of anaemia in our healthy control group, living in a poor and TB-endemic area, was surprisingly low (6·7 %), females being more affected than males. In the present study children aged less than 15 years and pregnant women, individuals at high risk of anaemia, were not included. Like others we observed that in developing countries Fe deficiency is becoming a less important cause of anaemia compared with infectionReference Das, Thurnham and Das21, Reference Devi, Mohan, Srivastava, Rath and Das22.
It is well known that most patients with active pulmonary TB have anaemiaReference Lienhardt, Fielding and Sillah23–Reference Goldenberg, Rome and Garay25, but the precise mechanism remains unclear. Blood loss in the sputum (haemoptysis) has been mentioned in textbooks as one of the causes. However, original studies were never performed and haemoptysis was not associated with anaemia in the present study population. Furthermore, deficiencies of Fe and other nutrients, caused by loss of appetite and fever, are associated with a low BMIReference Karyadi, Schultink, Nelwan, Gross, Amin, Dolmans, van der Meer, Hautvast and West24, Reference van Lettow, Kumwenda, Harries, Whalen, Taha, Kumwenda, Kang'ombe and Semba26, Reference van Lettow, Harries, Kumwenda, Zijlstra, Clark, Taha and Semba27.
Anaemia of chronic disease (ACD), also in active TB, is associated with a low serum Fe, Fe saturation and Fe-binding capacity, and with a high serum ferritinReference Gangaidzo, Moyo and Mvundura5, Reference Kassu, Yabutani and Mahmud28, while in uncomplicated Fe deficiency serum ferritin is always lowReference Mei, Cogswell, Parvanta, Lynch, Beard, Stoltzfus and Grummer-Strawn29. During inflammation ferritin, being an acute-phase reactant, is increased. Hence, the presence of inflammation in ACD can be estimated by increased concentrations of acute-phase protein such as CRP or by ESRReference Wieringa, Dijkhuizen, West, Northrop-Clewes and Muhilal16, Reference Karyadi, Schultink, Nelwan, Gross, Amin, Dolmans, van der Meer, Hautvast and West24. In our population of TB patients, anaemia was mostly due to ACD and not to Fe deficiency, as shown by the comparison of haematological and Fe parameters within the same patients before and at 6 months after TB therapy, as these subjects received no Fe treatment. In ACD the decrease of Fe in the plasma compartment and the increase of ferritin, mainly in macrophages, is due to cytokine-mediated up regulation of ferritin, and reduced Fe export due to increased hepcidin productionReference Weiss and Goodnough30. All such modifications in Fe status may be a protective response against the invading microbesReference Marx4, Reference Jurado31.
As the patients in the present study did not receive Fe supplementation, an increase of the Hb concentration over 6 months is mainly due to the normalisation of the inflammatory response. Alternatively, the increase of Hb concentration could be influenced by a better nutrition and a better appetite; patients gained weight as evidenced by a higher BMI after successful TB therapyReference van Lettow, Kumwenda, Harries, Whalen, Taha, Kumwenda, Kang'ombe and Semba26. Fe supplementation in developing countries, where Fe deficiency is highly prevalent, should therefore not be routinely prescribed when Fe status is unknown, as this may exacerbate infection, not only TBReference Lounis, Truffot-Pernot, Grosset, Gordeuk and Boelaert32, but also malariaReference Richard, Zavaleta, Caulfield, Black, Witzig and Shankar33 or helminth infectionReference Held, Bungiro, Harrison, Hamza and Cappello34. In children living in malaria endemic areas, Fe supplementation appears to have beneficial effects in Fe-deficient children, but harmful effects in Fe-replete childrenReference Sazawal, Black and Ramsan35. In contrast, a study in Malawi reported that Fe supplementation in developing countries with a high prevalence of both HIV infection and Fe deficiency is not contraindicatedReference Clark and Semba36. Fe is believed to enhance the activity of the pyrazionic acid-containing first-line TB drug pyrazinamideReference Somoskovi, Wade, Sun and Zhang37. The conclusion that some patients with pulmonary TB and mild to moderate anaemia may benefit from Fe supplementationReference Devi, Mohan, Srivastava, Rath and Das22 has no practical implication since Fe treatment of TB patients only caused a minimal increase of Hb after the first month, which disappeared after the second month of treatment. In contrast, in Fe-overload patients, Fe chelators may become important drugs for treatment of malaria, TB and HIV, to deprive micro-organisms from Fe as an essential nutrientReference Marx4, Reference Cronje, Edmondson, Eisenach and Bornman38. Fe chelators added to TB therapy should only be given to known overload patients as it may impair the host defence, and reliable investigations are not availableReference Meyer39.
The only effective treatment for ACD is, thus, to cure the underlying disease. Individuals in the present study with a very low MCV and only mild anaemia may have had thalassaemia minor. Haemoglobinopathies, present in 0·1–10 % of individuals of various ethnic groups in IndonesiaReference Setianingsih, Williamson, Marzuki, Harahap, Tamam and Forrest40, Reference Lie-Injo, Cai, Wahidijat, Moeslichan, Lim, Evangelista, Doherty and Kan41, may be underdiagnosedReference Weatherall and Clegg42. The risk of these individuals developing TB, however, needs to be further investigated.
Macrophages have several strategies to acquire Fe from their specific environment, including erythrophagocytosis and uptake of transferrin-bound Fe, non-transferrin-bound Fe, haem and Hb. Fe is needed for both host defence and survival of the pathogenReference Marx4. There is a constant competition between host and microbes for this essential but toxic element. It remains unclear which role NRAMP1 plays in the pathogenesis of TB, being a metal transporter that resides in the phagosome membraneReference Canonne-Hergaux, Gruenheid, Govoni and Gros14. Atkinson & BartonReference Atkinson and Barton43 showed that NRAMP1 has a function in Fe efflux from phagosome to cyotosolReference Atkinson and Barton43, while others found that it promotes the influx of Fe into the phagosomeReference Kuhn, Baker, Lafuse and Zwilling44. In a recently published meta-analysis of the influence on TB susceptibility of the four most frequently studied NRAMP1 polymorphismsReference Li, Zhang, Zhou, Huang and Huang45 including INT4, D543N and the 3′UTR, it was shown that a large difference between populations can be observed. In Europeans none of the polymorphisms were associated with TBReference Soborg, Andersen, Madsen, Kok-Jensen, Skinhoj and Garred13. In Africans three of the four polymorphisms (not the 3′UTR variant) were associated with TBReference Bellamy, Ruwende, Corrah, McAdam, Whittle and Hill11 and in Asians also three out of four polymorphisms (not the INT4 variant) were associated with TBReference Gao, Fujishima and Mao46, Reference Liu, Cao and Zhang47. A striking difference between the present study and the other studies in Asian populations is that the allele frequencies of the polymorphisms are very different. We find the C allele of the INT4 polymorphism in only 2 % of our controls while this is found in 14 % of Japanese and 21 % of Chinese controls. We find the 3′UTR deletion allele in 22 % of our controls while this is found in 8–19 % in five other Asian populations. Similarly we find the A allele of the D543N polymorphism in 22 % of our controls while this is found in 2–15 % of six other Asian populations. It appears that not only the association of TB with NRAMP1 polymorphisms is different between continents but also the distribution of alleles is also very different between Asian populations, which may reflect a difference in selective pressure in the past. If the NRAMP1 polymorphisms studied here are functional polymorphisms influencing TB susceptibility directly, we should have observed a similar association. If these polymorphisms are, however, merely in linkage disequilibrium with a functional variant elsewhere in NRAMP1 (or a neighbouring gene) the association between certain alleles and TB susceptibility can vary greatly between populations as has been shown by Li et al. Reference Li, Zhang, Zhou, Huang and Huang45. Further studies into other variations in NRAMP1 or in other genes involved in susceptibility to TB are needed.
To conclude, the present results support earlier observations that anaemia in active TB is mainly the result of ACD, more than of Fe deficiency. As the Hb concentration increased after successful TB therapy, Fe supplementation was not necessary. Fe supplementation in developing countries should be restricted to children and women of reproductive age, who have the highest prevalence of Fe deficiency.
Acknowledgements
The present study is part of the project ‘Immunogenetic basis of susceptibility to and disease manifestations of mycobacterial infections’, financially supported by the Royal Academy of Arts and Sciences (KNAW), The Netherlands. We thank Maya Anugrah and Erita Istriana for their assistance in the clinical work in PPTI Jakarta under supervision of Dr Halim Danusantoso. We thank Dr Dorine Swinkels for her support and fruitful discussion.





