Carotenoids can be divided into two subgroups. One is carotenes containing only hydrocarbons (such as β-carotene and lycopene) and the other is xanthophylls containing oxygenated substituent(s) (such as lutein, zeaxanthin and β-cryptoxanthin). In animals, as in plants, xanthophyll lutein is believed to function in three important ways: (1) as a filter of high-energy blue light, (2) as an antioxidant that quenches and scavenges photo-induced reactive oxygen species(Reference Demmig-Adams and Adams1, Reference Alves-Rodrigues and Shao2) and (3) as an important immune response modulator(Reference Jin, Ohgami and Shiratori3, Reference Rafi and Shafaie4). There is evidence that carotenoids modify the activities of antioxidants and lipid peroxidation in vivo in humans and rodents. Dietary lutein could inhibit erythrocyte phospholipid hydroperoxide formation(Reference Nakagawa, Kiko and Hatade5), increase blood reduced glutathione (GSH) level and protect against DNA damage and chromosome instability(Reference Serpeloni, Grotto and Mercadante6), as well as decrease reactive oxygen species generation, inflammation and immunosuppression(Reference Lee, Faulhaber and Hanson7). Xanthophylls (mix of lutein and zeaxanthin) could provide antioxidant protection for human skin as measured by photoprotective activity and the lipid peroxidation product malondialdehyde (MDA)(Reference Palombo, Fabrizi and Ruocco8), and protected mice against UVB-induced epidermal hyperproliferation and acute inflammation(Reference Gonzalez, Astner and An9). The same antioxidant and protective effects were observed in human subjects and rodents supplemented with β-carotene(Reference Dixon, Burri and Clifford10), lycopene(Reference Bhuvaneswari, Velmurugan and Balasenthil11, Reference Matos, Capelozzi and Gomes12), canthaxanthin(Reference Palozza, Calviello and Emilia De Leo13) and mixed carotenoid administration(Reference Dixon, Shie and Warden14, Reference Zhao, Aldini and Johnson15).
In addition, the antioxidant effects of carotenoids were also revealed in vitro or ex vivo. The protective effects of lutein and zeaxanthin against oxidative damage of egg-yolk lecithin liposomal membranes induced by exposure to UV radiation and incubation with 2,2′-azobis (2-methylpropionamidine) dihydrochloride have been reported(Reference Sujak, Gabrielska and Grudzinski16). Furthermore, zeaxanthin and β-cryptoxanthin were more effective in protecting egg-yolk phosphatidylcholine liposomes against oxidation than β-carotene, astaxanthin, canthaxanthin and lycopene in vitro (Reference Woodall, Britton and Jackson17). The in vitro or ex vivo antioxidant role of lycopene(Reference Fuhrman, Volkova and Rosenblat18), astaxanthin(Reference Kozuki, Miura and Yagasaki19) and mixed carotenoid(Reference Kiokias and Gordon20) has also been reported.
Maize–soyabean basal diets are used in some places (such as USA and China) and lutein is added to these diets to pigment skin and eggs only to satisfy consumer acceptance, but it has been neglected for a long time whether dietary xanthophylls play an antioxidant role in the chicken. Furthermore, there have been many studies on the activities of antioxidant enzymes and lipid peroxidation of carotenoids in humans and rodents in vivo, but few experiments about the antioxidant capacity of carotenoids in vivo have been carried out in chickens. In addition, most yolk-derived carotenoids are deposited into the embryonic liver(Reference Speake, Murray and Noble21), but dietary carotenoids were more broadly distributed in the tissues of the post-hatch chick(Reference Ganguly, Mehl and Deuel22), so there may be different functions between in ovo and dietary carotenoids. Besides, data are sparse regarding the effects of in ovo and dietary xanthophylls on antioxidant capacity of the progeny, so the objectives of the present study were to investigate the effects of xanthophylls on antioxidant capacity and lipid peroxidation in hens and chicks.
Materials and methods
Institutional and national guidelines for the care and use of animals were followed and all experimental procedures involving animals were approved by the Committee of Animal Experiments of South China Agricultural University (approval ID 201004152). All efforts were made to minimise suffering.
Expt 1
Animals and diets
To examine the effects of dietary xanthophylls (containing 40 % lutein and 60 % zeaxanthin following analysis; Juyuan Biochemical Company Limited) on antioxidant capacity and lipid peroxidation in breeding hens, 432 hens at 34 weeks of age with similar weight (2·5 kg) and genetic background were randomly assigned to three treatments. Hens were obtained from the College of Animal Science, South China Agricultural University and were raised in cages in a temperature-controlled room (25°C). Every cage was an experimental unit. Each treatment was replicated six times with twenty-four breeding hens each. Hens were fed a control diet not supplemented with xanthophylls (as the control group; containing 0·05 mg xanthophyll/kg following analysis) or a xanthophyll-supplemented diet with 20 or 40 mg xanthophyll/kg (containing 20·07 and 39·94 mg xanthophyll/kg following analysis, respectively). Dietary xanthophyll contents were chosen to be similar to those fed to commercial poultry. The diets were formulated according to the Chinese Feeding Standard of Chicken (2004) and the National Research Council (1994). Details of the ingredient composition and calculated nutrient content of diets for hens were provided as described previously(Reference Gao, Xie and Jin23), including 66 % rice, 20·18 % soyabean meal, 2 % fishmeal, 7·35 % limestone powder, 1·54 % calcium monohydrogen phosphate, 1·585 % wheat bran, 0·095 % dl-methionine, 1 % premix compound and 0·25 % salt. The experiment lasted for 35 d, and water and diet were provided ad libitum. Production performance (egg number, total egg weight, feed intake, broken eggs, qualified eggs and hen mortality) of each replicate was recorded daily. Blood of hens (two hens for each replicate) was sampled at 7, 14, 21, 28 and 35 d after xanthophyll supplementation. Serum was collected after centrifugation at 1000 g for 15 min at 4°C to determine xanthophyll content, antioxidant capacity and lipid peroxidation. The method to determine xanthophyll content was described by Koutsos et al. (Reference Koutsos, Lopez and Klasing24). From 29 to 35 d of the trial, 510 eggs (n 85 from each replicate) were collected from the control group or the 40 mg xanthophyll/kg diet group, and hatched artificially to determine fertilisation rate, the hatchability of fertilised eggs, chick birth weight and healthy chick rate. The incubation temperature was 38°C from 1 to 2 d, 37·9°C from 3 to 6 d, 37·8°C from 7 to 10 d, 37·6°C from 11 to 15 d, 37·4°C from 16 to 18 d and 37°C from 19 to 21 d. The incubation humidity was 55 % from 1 to 18 d and 60 % from 19 to 21 d. The eggs were collected from 29 d of the trial because yolk carotenoid concentration has been shown to reach a new steady state after about 3 weeks of supplementation in the hens' diet, as we and other researchers have determined(Reference Gao, Xie and Jin23, Reference Karadas, Pappas and Surai25, Reference Surai and Speake26). On the 35 d of trial, two hens from each replicate (twelve hens for each treatment) were weighed and slaughtered. Liver and jejunum samples were collected immediately after slaughter. Liver was frozen in liquid N2 and then stored at − 80°C for further analysis.
Preparation of intestinal mucosal and liver samples
The method for preparing intestinal mucosal and liver samples has been described previously(Reference Gao, Jiang and Lin27). The first half of the jejunum was cut into several 5 cm pieces, which were opened longitudinally and cleaned with PBS containing 137 mm-NaCl, 2·7 mm-KCl, 10 mm-Na2HPO4 and 2 mm-KH2PO4, and a pH of 7·4. Jejunal mucosa was collected by scratching with a glass slide for the next step and frozen in liquid N2. In brief, 9 ml PBS at 4°C was added to 1 g of intestinal mucosa or liver, followed by homogenisation (Ultra-Turrax T8 homogeniser (3000–5000 rpm for 1–2 min); IKA Labortechnik). The homogenates were centrifuged (4000 g for 5 min at 4°C) and the supernatant fluid was used for determining antioxidant capacity and lipid peroxidation. Antioxidant capacity was assessed by glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), catalase (CAT), total antioxidant capacity (T-AOC) and the GSH:oxidised glutathione (GSSG) ratio (GSH:GSSG). Lipid peroxidation was measured by MDA. Antioxidant capacity and lipid peroxidation in the serum, liver and intestinal mucosal supernatant were determined by using commercially available kits (Nanjing Jiancheng Bioengineering Institute). The method and principle to determine antioxidant indicators and lipid peroxidation using these kits have been described elsewhere(Reference Sun, Li and Li28), and activity was normalised to protein concentration as determined by the Coomassie Blue assay. Briefly, CAT activity was determined by incubating in the presence of a known concentration of H2O2 and then the reaction was quenched with ammonium molybdate. The amount of H2O2 remaining in the reaction mixture forms a stable coloured complex with ammonium molybdate and the complex is measured at 405 nm using a UV–visible spectrophotometer (BioMate 5; Thermo). SOD activity was measured at 550 nm using a UV–visible spectrophotometer following the reduction of nitrite by a xanthine–xanthine oxidase system which is a superoxide anion generator. GSH-Px was assayed by the decrease in GSH, which was reflected by a change in absorbance at 412 nm using a UV–visible spectrophotometer. T-AOC was determined by antioxidants existed in the body which can reduce Fe3+ to Fe2+. Fe2+ combines with phenanthrene and forms a coloured compound which can be measured at 520 nm using a UV–visible spectrophotometer. GSH and GSSG were determined at 405 nm with an automated ELISA reader (MODEL550; Bio-Rad) by using the 3-carboxy-4-nitrophenyl disulphide circular response. MDA was determined at 532 nm with a UV–visible spectrophotometer by thiobarbituric acid reaction.
Expt 2
Animals and diets
To examine the effects of xanthophylls supplied either in ovo or directly from the diet (containing 40 % lutein and 60 % zeaxanthin) on antioxidant capacity and lipid peroxidation in chicks, a 2 × 2 factorial arrangement of treatments consisting of two in ovo xanthophyll levels and two dietary xanthophyll levels was designed. To perform the analysis, 510 eggs were collected from hens fed 0 or 40 mg xanthophyll/kg in Expt 1. On the day of hatching, 180 healthy male chicks from each in ovo xanthophyll treatment were chosen randomly and assigned to one of two dietary xanthophyll levels: a basal diet supplemented with 0 or 40 mg xanthophyll/kg (containing 0·07 and 40·02 mg xanthophyll/kg following analysis, respectively). Within 12 h after hatching, twelve chicks (0 d chicks, two for each replicate) without feeding from each in ovo xanthophyll treatment were weighed and slaughtered, and then liver samples were removed immediately after slaughter and frozen in liquid N2. There were four groups of progeny designed as follows: parents and chicks fed 40 mg xanthophyll/kg (HH group), parents fed 40 mg xanthophyll/kg and chicks fed 0 mg xanthophyll/kg (HL group), parents fed 0 mg xanthophyll/kg and chicks fed 40 mg xanthophyll/kg (LH group), parents and chicks fed 0 mg xanthophyll/kg (LL group). Each of the four progeny groups contained six replicate pens with fifteen male chicks each. The diets were formulated according to the Chinese Feeding Standard of Chicken (2004) and the National Research Council (1994). Details of the ingredient composition and calculated nutrient content of diets for chicks were provided as described previously(Reference Gao, Xie and Jin23), including 58·69 % rice, 32·93 % soyabean meal, 2 % fishmeal, 1·23 % limestone powder, 1·50 % calcium monohydrogen phosphate, 2·1 % soyabean oil, 0·25 % dl-methionine, 1 % premix compound and 0·3 % salt. Dietary xanthophyll levels were chosen to be similar to those fed to commercial poultry. Chicks were housed in battery cages, and water and diet were provided ad libitum. The experiment lasted for 21 d, and growth performance (average daily feed intake, average daily gain and gain:feed ratio) of chicks was analysed after the experiment. From each of the four groups, six chicks (one for each replicate) were slaughtered and liver samples were collected at 7, 14 and 21 d after hatching. In addition, blood samples were also collected at 21 d after hatching from six chicks from each of the four groups. Blood samples were not collected at 0, 7 and 14 d after hatching because the chicks were too little to collect enough blood for determination of antioxidant capacity and lipid peroxidation.
Preparation of blood and liver samples
Preparation of liver and blood samples and determination of antioxidant capacity and lipid peroxidation in serum and liver homogenate supernatant were the same as in Expt 1.
Statistical analysis
Statistical analysis of data was performed using SAS 8.1 (SAS Institute Inc.). Fertilisation rate, hatchability of fertilised eggs, chick birth weight, healthy chick rate, antioxidant capacity and lipid peroxidation in 0 d chicks were analysed by the t test between the two treatments. For analysing production performance, antioxidant capacity and lipid peroxidation in breeding hens, one-way ANOVA were performed to test for the effect of dietary xanthophylls in hens. For growth performance at 21 d of chicks, and antioxidant capacity and lipid peroxidation at 7, 14 and 21 d of chicks, data were analysed by using two-way ANOVA. The model included the main effects of in ovo, diet and their interaction. Replicate was used as the experimental unit. Results are presented as means and pooled standard errors. When the main effect(s) or interaction was significant, differences among means were determined using Tukey's honestly significant difference. Differences between means were considered significant at P≤ 0·05.
Results
Effects of xanthophylls on antioxidant capacity and lipid peroxidation in hens
The addition of 20 or 40 mg xanthophyll/kg had no effect on the production performance of hens, and maternal xanthophyll addition did not affect fertilisation rate, hatchability of fertilised eggs, chick birth weight and healthy chick rate (data not shown). Supplementation of 20 or 40 mg xanthophyll/kg increased serum SOD activity at 21 and 28 d compared with the control, and serum T-AOC at 21 d and liver T-AOC were elevated by both 20 and 40 mg xanthophyll/kg supplementation (Table 1). In addition, supplementation of 20 or 40 mg xanthophyll/kg also increased serum GSH:GSSG at 21, 28 and 35 d and liver GSH:GSSG. Serum MDA level at 21 d was decreased in both xanthophyll groups compared with the control. Supplementation of 40 mg xanthophyll/kg also reduced serum MDA at 28 and 35 d and jejunal mucosal MDA. There was no difference in CAT and GSH-Px activities among the treatments (data not shown).
Table 1 Effects of xanthophylls on superoxide dismutase (SOD), total antioxidant capacity (T-AOC), reduced glutathione:oxidised glutathione ratio (GSH:GSSG) and malondialdehyde (MDA) in the serum, liver and jejunal mucosa of hens* (Mean values with their standard errors, n 6)
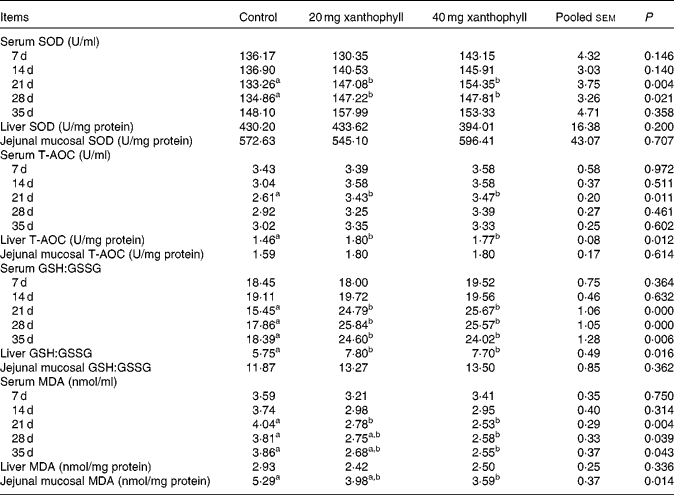
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05). * 1 unit of SOD is defined as the amount of enzyme that inhibits the rate of hydroxylamine oxidation by 50% in the reaction system. 1 unit of T-AOC is defined as the optical density value that increases 0·01 per min in the reaction system.
Effects of xanthophylls on antioxidant capacity and lipid peroxidation in chicks
Xanthophylls from in ovo, diet or their interaction had no effect on the growth performance of chicks (data not shown). In ovo xanthophylls enhanced liver GSH-Px activity at 0 d, liver T-AOC at 0 d and serum T-AOC, and liver GSH:GSSG at 7 and 14 d (Table 2). Liver MDA levels at 0 and 7 d were decreased by in ovo xanthophyll addition. Dietary xanthophyll supplementation promoted liver GSH-Px activity at 14 d, serum T-AOC, and liver GSH:GSSG at 14 and 21 d and serum GSH:GSSG. The addition of dietary xanthophylls also reduced liver MDA at 21 d and serum MDA. There was no difference in CAT and SOD activities with in ovo or dietary xanthophyll supplementation (data not shown). In addition, liver GSH-Px activity at 14 d, serum T-AOC, liver GSH:GSSG at 7 and 14 d were enhanced in the HH group compared with the LL group. Liver GSH:GSSG at 7 d was increased in the HH group compared with the LH group.
Table 2 Effects of in ovo and dietary xanthophylls on glutathione peroxidase (GSH-Px), total antioxidant capacity (T-AOC), reduced glutathione:oxidised glutathione ratio (GSH:GSSG) and malondialdehyde (MDA) in the serum and liver of chicks* (Mean values with their standard errors, n 6)

a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05). * 1 unit of GSH-Px is defined as the amount of enzyme that catalyses the conversion of 1 μmol GSH per min in the reaction system. 1 unit of T-AOC is defined as the optical density value increases 0·01 per min in the reaction system.
Discussion
The health benefits of lutein and zeaxanthin have attracted public attention because they may protect against the development of cataract, macular degeneration, cancer and heart disease(Reference Mares-Perlman, Millen and Ficek29). Evidence suggests that the action of carotenoids on immunity and diseases may be mediated, at least in part, by their ability to quench reactive oxygen species(Reference Chew and Park30). In Expt 1, a novel and important finding of the present study was that xanthophylls could influence antioxidant capacity and lipid peroxidation in breeding hens in vivo. Generally speaking, antioxidant capacity of hens was enhanced after xanthophyll supplementation for 21 d. This is consistent with changes in serum carotenoid concentrations in hens following dietary supplementation that we observed in a previous study(Reference Gao, Xie and Jin23), which indicated that serum carotenoids reach a new steady state after 21 d of xanthophyll supplementation. Higher SOD activity at 21 and 28 d was observed in the present study, and the same in vivo results have been reported in mice supplemented with canthaxanthin(Reference Palozza, Calviello and Emilia De Leo13), rats supplemented with astaxanthin, lutein or β-carotene(Reference Sangeetha and Baskaran31), and human subjects supplemented with β-carotene(Reference Dixon, Burri and Clifford10). The present findings on MDA agree with several carotenoid researches on human subjects and rodents(Reference Palombo, Fabrizi and Ruocco8, Reference Dixon, Burri and Clifford10, Reference Matos, Capelozzi and Gomes12), which have reported that carotenoid supplementation decreased MDA in plasma, liver and skin. CAT activity was not affected by xanthophylls in hens and chicks, as revealed by the present results, and in mice added with lutein(Reference Serpeloni, Grotto and Mercadante6), but some papers have also reported that CAT activity was affected by carotenoids in rodents(Reference Palozza, Calviello and Emilia De Leo13, Reference Sangeetha and Baskaran31, Reference He, Root and Parker32). The conflict was also observed with GSH-Px activity in the present study and other experiments(Reference Bhuvaneswari, Velmurugan and Balasenthil11, Reference Palozza, Calviello and Emilia De Leo13). It is difficult to compare the change in antioxidant enzymes between studies because of the discordance of methodological conditions, such as animal species, environment (housing condition and density), dosage and type of carotenoids used, the interaction with other antioxidants (vitamin C and vitamin E), and the oxidant stress challenge. The major determinant of redox status in mammalian cells is GSH, a tripeptide thiol that couples with its disulphide form (GSSG)(Reference Haddad33). Redox status depends on the relative amounts of reduced and oxidised partners of these major redox molecules, and GSH:GSSG reflects the redox status within the cell. The glutathione system acts as a homeostatic redox buffer and the oxidised partner predominates under oxidative conditions(Reference Morel and Barouki34). The present results showed that xanthophylls increased serum GSH:GSSG, strongly suggesting that addition of xanthophylls enhanced the antioxidant capacity of the body. Similar results were also reported in mice fed a diet added with lutein(Reference Serpeloni, Grotto and Mercadante6) and in hamsters fed a diet added with lycopene(Reference Bhuvaneswari, Velmurugan and Balasenthil11).
Serum SOD activity at 21 and 28 d, and serum T-AOC at 21 d of hens were increased in the 40 mg xanthophyll/kg group compared with the control, but all these effects were not observed at 35 d, which indicated that the control group, to some extent, also elevated the body antioxidant capacity after 35 d on a non-xanthophyll-supplemented diet. We assumed that the body in a carotenoid-depleted state for a long time (35 d in the present experiment) may modulate the activities of antioxidant enzymes through utilising other antioxidants (such as tocopherol). This could counteract the disadvantage of decreased antioxidant enzymes and lead to a reduction of the concentration of other antioxidants. The speculation was supported by evidence that β+γ-tocopherol was lower in eggs, and liver and yolk sac membrane of newly hatched chicks from hens on a wheat-based diet compared with a maize-based diet(Reference Surai and Sparks35) and that xanthophylls (lutein and zeaxanthin) could protect tocopherol from the oxidative loss(Reference Ojima, Sakamoto and Ishiguro36). The same protective effects against tocopherol decrease in liver and plasma have also been reported for canthaxanthin(Reference Surai, Surai and Steinberg37), lycopene and β-carotene(Reference Ojima, Sakamoto and Ishiguro36). In addition, lower MDA of jejunal mucosa was observed by xanthophyll supplementation, the same increased effects were also noted in liver T-AOC and GSH:GSSG when adding dietary xanthophylls. These data demonstrate that xanthophylls also play an important role in antioxidant capacity and lipid peroxidation in the liver and jejunum of hens. Besides, the mean values of CAT varied widely between the serum, liver and intestinal mucosa of hens (2·01 U/ml, 15·77 U/mg protein and 4·17 U/mg protein, respectively), implying different antioxidant enzymes and agents may play a leading role in the antioxidant defence of different tissues. (One unit of CAT is defined as the amount of enzyme that catalyses the conversion of 1 μmol hydrogen peroxide per S in the reaction system.) The same results can be observed for SOD.
In Expt 2, in ovo or dietary xanthophylls significantly increased antioxidant capacity and decreased lipid peroxidation in chicks. For a precocial species such as the chicken, metabolic rate and oxygen consumption increase rapidly(Reference Hohtola, Visser, Starck and Ricklefs38), which may cause oxidative stress, so it is helpful and meaningful if an effective antioxidant system is built. Generally speaking, maternal xanthophylls enhanced antioxidant capacity and reduced lipid peroxidation mainly at 0–7 d after hatching. During 7–14 d after hatching, the maternal antioxidant effects gradually disappeared and the progeny's diet began to take over. Dietary xanthophylls increased antioxidant capacity and decreased lipid peroxidation of the body mainly from 2 weeks onwards. The antioxidant results were consistent with liver carotenoid change of chicks, as we and other researchers have determined(Reference Gao, Xie and Jin23, Reference Karadas, Pappas and Surai25). Furthermore, liver GSH-Px activity at 14 d of chicks was increased by dietary xanthophylls, but this effect was not observed at 21 d, indicating that chicks in a carotenoid-depleted state may also up-regulate antioxidant enzymes through utilising other antioxidants (such as tocopherol) as discussed above. Moreover, the liver MDA level and GSH-Px activity were higher in all four groups on the day of hatching than in 7-d-old chicks. Therefore, we inferred that the most serious oxidative stress happened immediately after hatching as proved by the MDA level, and the body needs to raise antioxidant enzymes (GSH-Px) to counteract the negative effects produced by serious oxidative stress. According to our knowledge, the present study is the most extensive paper measuring the effects of xanthophylls on antioxidant capacity and lipid peroxidation in parents and progeny.
In conclusion, xanthophyll supplementation in the diet enhanced antioxidant capacity in the serum and liver and decreased lipid peroxidation in the serum and intestinal mucosa of breeding hens. The antioxidant role of in ovo-supplied xanthophylls mainly lasted for at least the first week after hatching in the liver and serum of chicks, whereas dietary xanthophylls played an important antioxidant role mainly from 2 weeks onwards. In addition, the different responses of antioxidant enzymes, antioxidant agent and MDA to xanthophyll supplementation between breeding hens and chicks may be due to their different roles in oxidative stress. The present results also showed that maternal xanthophyll nutrition plays an important antioxidant role for progeny, which may have a significant implication for animals and humans.
Acknowledgements
This study was supported by the Guangdong Natural Science Foundation of China (9151064201000052) and the Guangdong Major Science and Technology Special Projects of China (2009B020201008). Y.-Y. G., Q.-M. X., J.-Y. M. and Y.-Z. B. designed the research; X.-B. Z., J.-M. Z., D.-M. S., B.-L. S., L. J., and Y.-Z. B. conducted the research; Y.-Y. G. and Y.-Z. B. analysed the data; Y.-Y. G., Q.-M. X., J.-Y. M. and Y.-Z. B. wrote the paper. The authors declare that there are no conflicts of interest.




