α-Lipoic acid is a natural compound widely distributed in plants and animals, and is synthesized through a reaction catalysed by lipoic acid synthase within the mitochondria(Reference Wollin and Jones1). Lipoic acid functions as a cofactor within mitochondrial pyruvate dehydrogenase and α-ketoglutarate dehydrogenase(Reference Wollin and Jones1, Reference Reed2). Aside from its role in the mitochondrial metabolic pathway, lipoic acid when supplemented in diets exerts various physiological activities in experimental animals. Lipoic acid is enzymatically reduced to form dihydrolipoic acid in the cytosol and mitochodria(Reference Wollin and Jones1). Lipoic acid and dihydrolipoic acid are powerful antioxidants. Therefore, dietary lipoic acid is effective in attenuating oxidative stress induced by drugs(Reference Amudha, Josephine and Varalakshmi3), a high-fructose diet(Reference Thirunavukkarasu and Anuradha4), ageing(Reference Arivazhagan, Thilakavathy, Ramanathan, Kumaran and Panneerselvam5) and physical exercise(Reference Khanna, Atalay, Laaksonen, Gul, Roy and Sen6). Also, lipoic acid modulates glucose metabolism and is effective in ameliorating insulin resistance and type 2 diabetes(Reference Henriksen7).
In addition, a hypolipidaemic effect of lipoic acid has been reported by many investigators. Yi & Maeda(Reference Yi and Maeda8) reported that a diet containing 1·65 g lipoic acid/kg reduced plasma cholesterol concentrations in streptozotocin-induced diabetic apoE-deficient mice. Thirunavukkarasu & Anuradha(Reference Thirunavukkarasu and Anuradha4) reported that the intraperitoneal injection of lipoic acid (35 and 70 mg/kg body weight per d) reduced plasma cholesterol, TAG, phospholipid and NEFA levels in fructose-fed rats. Also, it has been reported that the administration of lipoic acid (35 mg/kg body weight per d) intraperitoneally once a week prevented adriamycin-induced hyperlipidaemia in rats(Reference Malarkodi, Balachandar and Varlakshmi9). Ford et al. (Reference Ford, Cotter, Cameron and Greaves10) observed that dietary supplementation of lipoic acid to give a dose of approximately 300 mg/kg body weight per d, reduced serum TAG concentrations in rats with streptozotocin-induced diabetes. Song et al. (Reference Song, Lee and Koh11) reported that a diet containing 5 g lipoic acid/kg significantly decreased serum concentrations of TAG and NEFA, and TAG concentrations in pancreatic islets and soleus muscles in an animal model of obesity and diabetes mellitus (Otsuka Long–Evans Tokushima Fatty (OLETF) rats). However, the mechanism underlying the hypolipidaemic effect of α-lipoic acid is poorly understood. In this context, Lee et al. (Reference Lee, Naseem, Park, Garry, Richardson, Schaffer and Unger12) recently reported that a diet containing 10 g lipoic acid/kg reduced mRNA levels of fatty acid synthase and sterol regulatory element binding protein (SREBP)-1, a transcription factor that regulates the gene expression of lipogenic enzyme(Reference Horton, Goldstein and Brown13), in acyl-CoA synthase transgenic mice after 4 d and 6 weeks of feeding. However, it is still difficult to conclude that lipoic acid has a specific physiological role in reducing lipogenesis, because it considerably reduced food intake in their study. Also, there is the possibility that the reduction by dietary lipoic acid of the mRNA expression of fatty acid synthase is a particular phenomenon observable only in acyl-CoA synthase transgenic mice. Moreover, it should be stated that alterations in the mRNA level may not necessarily accompany parallel changes in the amount of enzyme protein and hence its activity. In this context, we examined the effect of diets containing varying amounts of α-lipoic acid on the activity and mRNA levels of hepatic enzymes involved in lipogenesis in Sprague–Dawley rats in the present study.
Materials and methods
Animals and diets
Male Sprague–Dawley rats obtained from Charles River Japan (Kanagawa, Japan), were housed individually in animal cages in a room with controlled temperature (20–22°C), humidity (55–65 %) and lighting (lights on from 07.00 to 19.00 hours), and fed a commercial non-purified diet (Type NMF, Oriental Yeast Co., Tokyo, Japan). After 7 d of acclimatization, rats were fed purified experimental diets containing 0, 1, 2·5 and 5 g lipoic acid/kg for 21 d. The basal composition of the experimental diet was (g/kg): palm oil, 100; casein, 200; maize starch, 150; cellulose, 20; mineral mixture(Reference Reeves, Nielsen and Fahey14), 35; vitamin mixture(Reference Reeves, Nielsen and Fahey14), 1·0; l-cystine, 3; choline bitartrate, 2·5 and saccharose to 1 kg. Chemically synthesized dl-α-lipoic acid with a purity exceeding 990 g/kg (maker's statement) was purchased from Tokyo Chemical Industry (Tokyo, Japan). Varying amounts of lipoic acid were added to the diet in lieu of saccharose. Previous animal experiments(Reference Ford, Cotter, Cameron and Greaves10–Reference Lee, Naseem, Park, Garry, Richardson, Schaffer and Unger12, Reference Kim, Park and Namkoong15–Reference Lee, Song, Koh, Won, Kim, Park, Kim, Kim, Lee and Park17) using rats or mice employed dietary levels of lipoic acid of 0·5–10 g/kg, which are similar to the levels employed in the present study. Many previous human studies employed a dose of lipoic acid of 600–2400 mg/d per person(Reference Ziegler, Ametov and Barinov18, Reference Yadav, Marracci, Lovera, Woodward, Bogardus, Marquardt, Shinto, Morris and Bourdette19). A nutritional survey conducted in Japan in 2005 (http://www.mhlw.go.jp/houdou/2006/05/h0508-1.html) indicated the average food intake of adults to be 423 g/d. A similar value (403 g/d) was reported in a nutritional survey conducted in the UK in 2001 (http://www.statistics.gov.uk/downloads/theme_health/NDNS_V2.pdf). Therefore, the intake of diets containing 1, 2·5 and 5 g lipoic acid/kg employed in the present animal study correspond to the daily consumption of approximately 400, 1000 and 2000 mg/person of this compound when extrapolated in man. These values are comparable to those employed in previous human studies. The present study was approved by the review board of animal ethics of our institute and we followed the institute's guidelines in the care and use of laboratory animals.
Enzyme assays
Upon termination of the experimental period, animals were anaesthetized using diethyl ether and killed by bleeding from the abdominal aorta, after which livers were excised immediately. About 1·5 g of each liver was homogenized with 10 ml 0·25 m-sucrose containing 1 mm-EDTA and 3 mm-Tris–HCl (pH 7·2) and the homogenate was centrifuged at 200 000 g for 30 min. The activities of enzymes involved in fatty acid synthesis were measured spectrophotometrically using the 200 000 g supernatant of the liver homogenate. Fatty acid synthase activity was measured as malonyl-CoA-dependent oxidation of NADPH in the presence of acetyl-CoA(Reference Kelley, Nelson and Hunt20). ATP-citrate lyase activity represented the rate of CoA-dependent oxidation of NADH in the presence of citrate, ATP and malate dehydrogenase (Oriental Yeast Co.)(Reference Takeda, Suzuki and Inoue21). The rate of NADP reduction following the addition of malic acid was analysed to measure malic enzyme activity(Reference Hsu and Lardy22). The rate of glucose 6-phosphate-dependent reduction of NADP in the presence of excess amounts of 6-phosphogluconate dehydrogenase (Oriental Yeast Co.) represented glucose 6-phosphate dehydrogenase activity(Reference Kelley and Kletzien23). Pyruvate kinase activity was measured as the rate of phosphoenolpyruvate-dependent oxidation of NADH in the presence of ADP and lactate dehydrogenase (Roche Diagnostics, Manheim, Germany)(Reference Tanaka, Harano, Sue and Morimura24).
RNA analysis
Hepatic RNA was extracted using the acid guanidium thiocyanate–phenol–chloroform method(Reference Chomczynski and Sacchi25) and mRNA abundance was analysed by a quantitative real-time PCR using an Applied Biosystems Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA). Total RNA was reverse-transcribed with random hexamers using MultiScribe reverse transcriptase (Applied Biosystems) to generate cDNA. The PCR mixture in a final volume of 20 μl contained 20 ng cDNA, 900 nm each of the forward and reverse primers, 250 nm TaqMan probe and 10 μl × 2 PCR master mix (Applied Biosystems). mRNA abundance was calculated as a ratio to the mRNA abundance of β-actin in each cDNA sample and expressed as a percentage, assigning a value of 100 for rats fed a control diet free of lipoic acid. Nucleotide sequences of forward and reverse primers and probes to detect the respective mRNA were the same as described previously(Reference Lim, Adachi, Takahashi and Ide26).
Analyses of serum and liver lipids, and serum glucose, leptin, adiponectin and insulin
Liver lipids were extracted and purified(Reference Folch, Lees and Sloane-Stanley27). Amounts of TAG(Reference Fletcher28) and phospholipid(Reference Rouser, Fkeischer and Yamamoto29) in the liver lipid extracts were analysed colorimetrically and the amount of cholesterol was measured enzymatically(Reference Ide, Oku and Sugano30). Serum TAG, cholesterol, phospholipid and NEFA concentrations were measured using commercial enzyme kits (Wako Pure Chemical, Osaka, Japan). Serum leptin (Morinaga Co., Tokyo, Japan), adiponectin (Otsuka Pharmaceutical Co., Tokushima, Japan) and insulin (Morinaga Co.) concentrations were analysed with commercial ELISA kits.
Statistical analysis
Data are expressed as means and their standard errors. StatView for Macintosh (SAS Institute Inc., Cary, NC, USA) was used for the statistical analysis. The data were subjected to a one-way ANOVA, followed by a Tukey–Kramer post-hoc analysis to detect significant differences of the means at the level of P < 0·05.
Results
Serum and liver lipid concentrations and serum concentrations of glucose, insulin and adipokines
Lipoic acid at a dietary level of up to 2·5 g/kg did not affect the food intake (20·5 (sem 0·7), 18·9 (sem 0·8) and 19·4 (sem 0·8) g/d for rats fed diets containing 0, 1 and 2·5 g/kg lipoic acid, respectively) or growth (169 (sem 8), 157 (sem 9) and 149 (sem 9) g/21 d, respectively) of the animals. However, a diet containing 5 g lipoic acid/kg compared to a diet free of this compound significantly (P < 0·01) reduced these parameters (13·6 (sem 0·4) g/d and 84·1 (sem 5·4) g/21 d, respectively). Liver weights were the same among rats fed diets containing 0, 1 and 2·5 g lipoic acid/kg (4·92 (sem 0·14), 5·08 (sem 0·14) and 5·26 (sem 0·11) g/100 g body weight, respectively). However, this parameter was significantly higher in rats fed a 5 g lipoic acid/kg diet (5·61 (sem 0·12) g/100 g body weight) than in the animals fed a lipoic acid-free diet. Epididymal and perirenal adipose tissue weights were significantly lower in rats fed a diet containing 5 g lipoic acid/kg (0·97 (sem 0·08) and 0·92 (sem 0·12) g/100 g body weight, respectively) than in the animals fed a lipoic acid-free diet (1·51 (sem 0·19) and 1·62 (sem 0·25) g/100 g body weight, respectively). However, these values in rats fed 1 (1·38 (sem 0·08) and 1·61 (sem 0·13) g/100 g body weight, for epididymal and perirenal depots, respectively) and 2·5 (1·61 (sem 0·15) g/100 g body weight, respectively) g lipoic acid/kg were the same as those in the animals fed a lipoic acid-free diet.
A diet containing 1 g lipoic acid/kg reduced the serum TAG concentration to a level one-half that observed with a diet free of this compound (Table 1). The value obtained with a diet containing 2·5 g lipoic acid/kg was indistinguishable from the level observed with a diet containing 1 g lipoic acid/kg, but a diet containing 5 g/kg of this compound further decreased this parameter to one-third that observed with a lipoic acid-free diet. Diets containing varying amounts of lipoic acid compared to a lipoic acid-free diet also significantly reduced serum concentrations of phospholipid and NEFA. However, lipoic acid was rather ineffective in reducing serum concentrations of cholesterol. A diet containing 5 g lipoic acid/kg relative to a diet free of this compound caused a significant 26 % decrease in this parameter, but the values obtained with diets containing 1 and 2·5 g lipoic acid/kg were the same as those observed with a lipoic acid-free diet. Lipoic acid dose-dependently decreased serum concentrations of glucose. The values observed with diets containing 1, 2·5 and 5 g lipoic acid/kg were 11, 20 and 30 % lower, respectively, than the level observed with a lipoic acid-free diet. Dietary lipoic acid greatly decreased serum concentrations of insulin. The diets containing 1 and 2·5 g lipoic acid/kg caused 44 and 57 % decreases, respectively, in this parameter. The level observed with a diet containing 5 g lipoic acid/kg was very low (94 % decrease). Lipoic acid also affected serum concentrations of adipokines that modify lipid, energy and glucose metabolism. This compound dose-dependently decreased serum concentrations of leptin. The values observed with 1, 2·5 and 5 g lipoic acid/kg were 43, 53 and 66 % lower than the level obtained with a lipoic acid-free diet. In contrast, lipoic acid increased serum concentrations of adiponectin. A diet containing 1 g lipoic acid/kg relative to a diet free of this compound caused a 75 % increase in this parameter, and the level was doubled with a diet containing 2·5 g lipoic acid/kg . However, the levels obtained with diets containing 2·5 and 5 g lipoic acid/kg were indistinguishable. A significant positive correlation was observed between the serum concentrations of insulin and leptin (r 0·759, P < 0·001). In contrast, a significant negative correlation was observed between the serum concentrations of insulin and adiponectin (r 0·508, P = 0·005).
Table 1 Effect of dietary lipoic acid on serum and liver lipid concentrations, and serum concentrations of glucose, insulin and adipocytokines
(Mean values with their standard errors, n 7–8)

Mean values were significantly different from those of rats fed a lipoic acid-free diet: *P < 0·05, **P < 0·01.
Lipoic acid greatly and dose-dependently decreased hepatic concentrations of TAG. Diets containing 1, 2·5 and 5 g lipoic acid/kg compared to a diet free of this compound caused 58, 75 and 82 % decreases, respectively, in this parameter. Although the extent of the reduction was attenuated, lipoic acid also dose-dependently decreased hepatic concentrations of cholesterol (20, 29 and 37 % decreases for rats fed diets containing 1, 2·5 and 5 g lipoic acid/kg, respectively). However, diets containing varying amounts of lipoic acid slightly but significantly increased hepatic concentrations of phospholipid (8, 15 and 15 % increases for rats fed diets containing 1, 2·5 and 5 g lipoic acid/kg, respectively).
Activity of enzymes involved in hepatic fatty acid synthesis
Dietary lipoic acid dose-dependently decreased the activity of lipogenic enzymes and pyruvate kinase (Fig. 1). Diets containing 1, 2·5 and 5 g lipoic acid/kg caused 35, 66 and 86 % decreases in the activity of fatty acid synthase, respectively. The magnitude of the changes was similar for the activity of ATP-citrate lyase (43, 64 and 83 % decreases for rats fed diets containing 1, 2·5 and 5 g lipoic acid/kg, respectively). Lipoic acid-dependent decreases were considerably attenuated for the activities of enzymes needed to generate NADPH in the cytosol (glucose 6-phosphate dehydrogenase and malic enzyme). A diet containing 1 g lipoic acid/kg caused 24 and 11 % decreases in the activity of glucose 6-phosphate dehydrogenase and malic enzyme, respectively, and the reduction was not significant in the latter case. Diets containing 2·5 and 5 g lipoic acid/kg caused 34 and 38 %, and 24 and 43 % decreases in the activity of glucose 6-phosphate dehydrogenase and malic enzyme, respectively. Pyruvate kinase, an enzyme involved in the glycolytic pathway in the liver, is co-ordinately regulated with other enzymes involved in lipogenesis, and is considered to participate in the regulation of fatty acid synthesis(Reference Noguchi, Inoue and Tanaka31). Dietary lipoic acid also dose-dependently decreased the activity of this enzyme (26, 40 and 61 % decreases in rats fed diets containing 1, 2·5 and 5 g lipoic acid/kg, respectively).
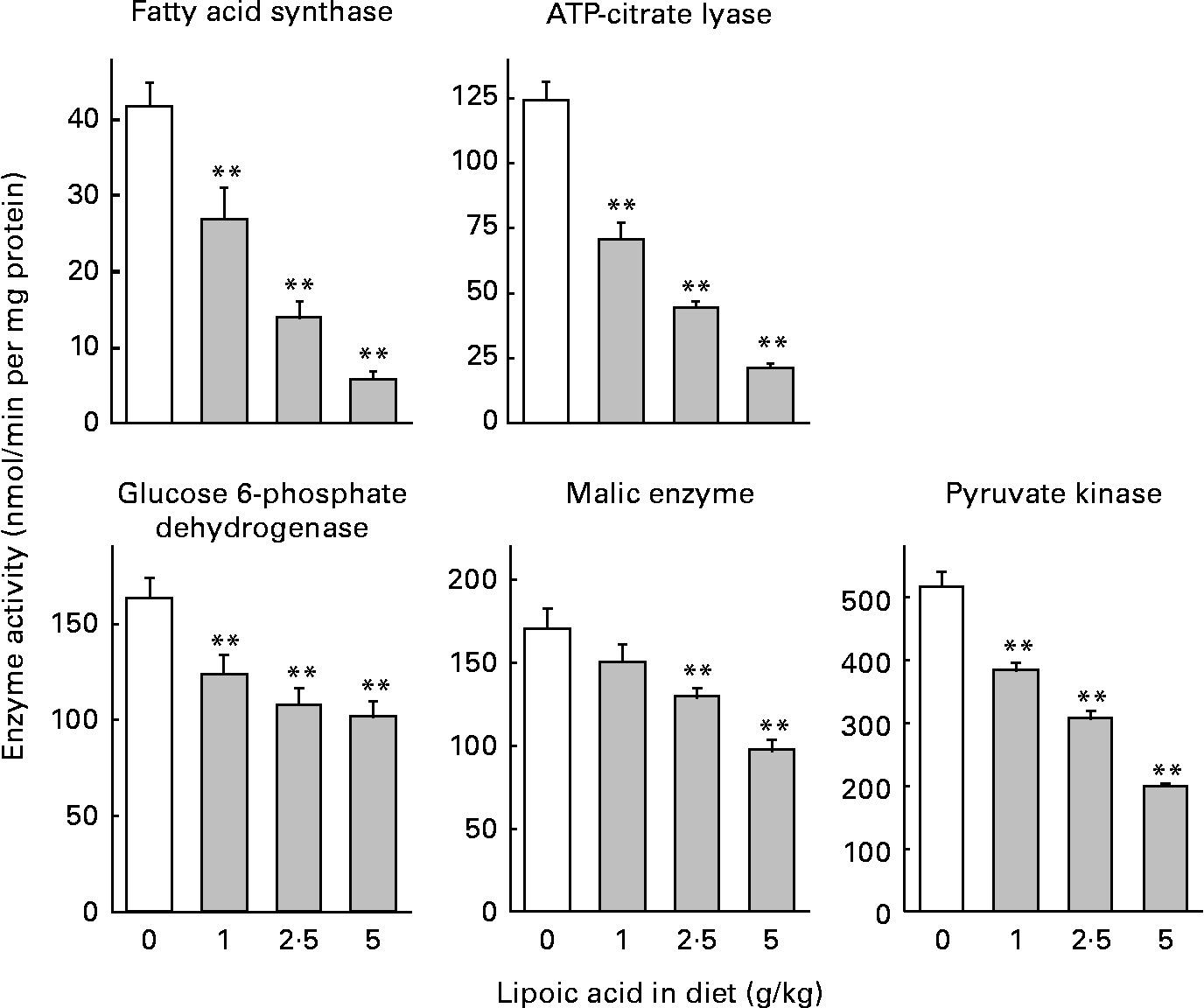
Fig. 1 Effect of dietary lipoic acid on the activity of enzymes involved in hepatic fatty acid synthesis. Values are means with their standard errors depicted by vertical bars (n 7–8). Mean values were significantly different from those of rats fed a lipoic acid-free diet: *P < 0·05, **P < 0·01.
mRNA levels of hepatic proteins involved in lipogenesis and fatty acid desaturation
mRNA abundance was calculated as a ratio to the mRNA abundance of β-actin and expressed as a percentage, assigning a value of 100 for rats fed a control diet free of lipoic acid (Fig. 2). Mammals have four types of pyruvate kinase named L, M1, M2 and R(Reference Inoue, Noguchi and Tanaka32). Type L is the major isozyme in the liver. Lipoic acid at a dietary level as low as 1 g/kg caused significant (25–43 %) decreases in the mRNA levels of acetyl-CoA carboxylase, fatty acid synthase, ATP-citrate lyase and l-pyruvate kinase. The diets containing 2·5 and 5 g lipoic acid/kg further decreased these parameters, and the values obtained with a diet containing 5 g lipoic acid/kg were less than one-half those observed with a lipoic acid-free diet. Consistent with the findings regarding the enzyme activities, lipoic acid-dependent decreases in mRNA levels of glucose 6-phophate dehydrogenase and malic enzyme were attenuated. Decreases were only 12–17 % with diets containing 1 and 2·5 g lipoic acid/kg, and were 25 and 34 % lower in rats fed a diet containing 5 g lipoic acid/kg than in those fed a diet free of this compound.
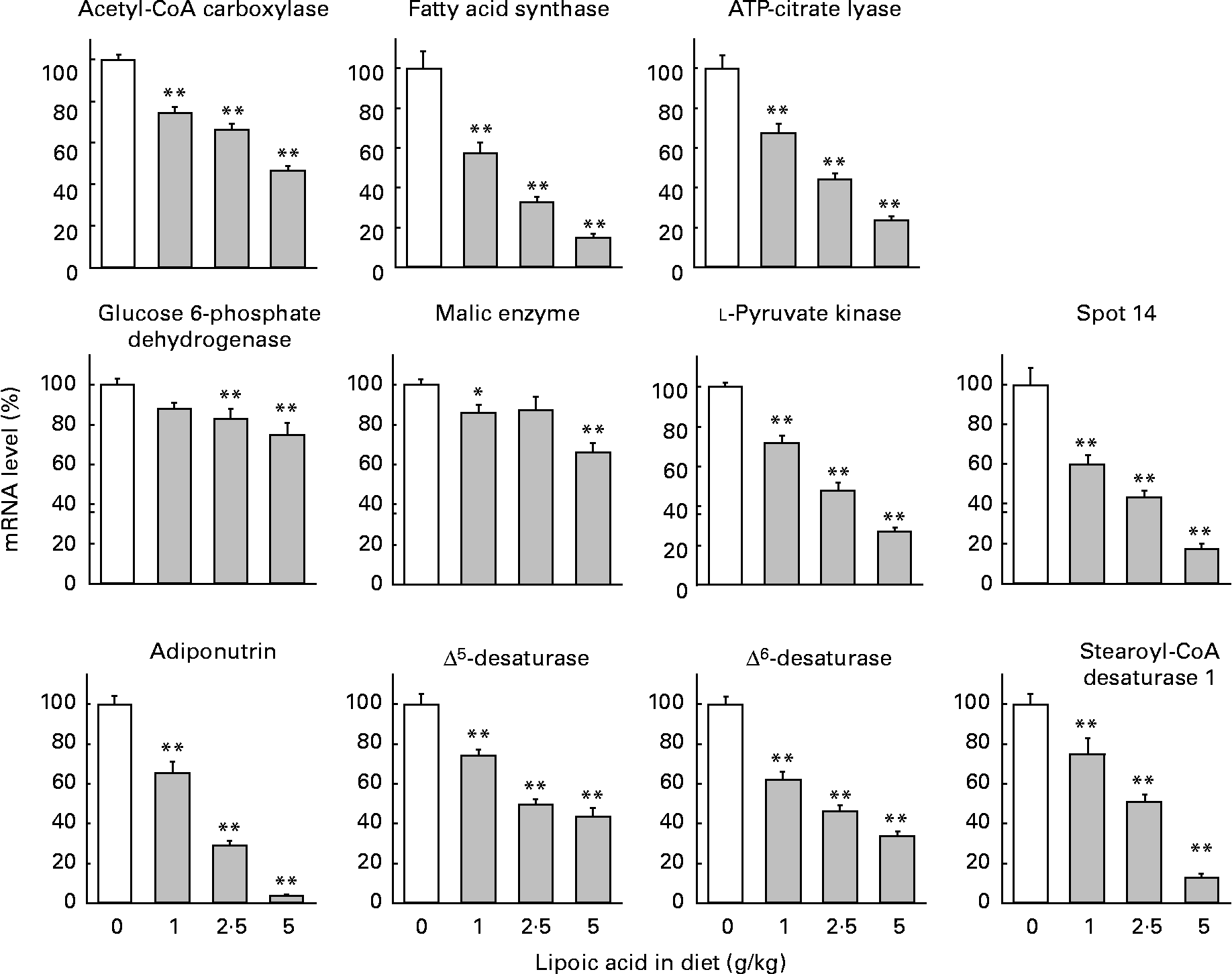
Fig. 2 Effect of dietary lipoic acid on mRNA levels of hepatic proteins involved in lipogenesis and fatty acid desaturation. Values are means with their standard errors depicted by vertical bars (n 7–8). Mean values were significantly different from those of rats fed a lipoic acid-free diet: *P < 0·05, **P < 0·01.
Spot 14 and adiponutrin are proteins presumed to be involved in the regulation of lipogenesis(Reference Ide33–Reference Jenkins, Mancuso, Yan, Sims, Gibson and Gross35). Dietary lipoic acid at a dietary level as low as 1 g/kg greatly decreased mRNA levels of these proteins (40 and 35 % decreases for spot 14 and adiponutrin, respectively). Diets containing 2·5 and 5 g lipoic acid/kg further decreased these parameters. It has been well demonstrated that SREBP-1 is a transcription factor involved in the regulation of the gene expression of many lipogenic enzymes(Reference Horton, Goldstein and Brown13). In addition, this transcription factor up regulates the gene expression of enzymes involved in the desaturation of SFA (stearoyl-CoA desaturase 1)(Reference Ntambi36) and PUFA (Δ5- and Δ6-desaturases)(Reference Matsuzaka, Shimano and Yahagi37). We therefore measured the mRNA expression of these desaturases. Dietary lipoic acid also dose-dependently decreased mRNA levels of these desaturases. This compound at a dietary level of 1 g/kg caused 26–38 % decreases in these parameters. The values in rats given a diet containing 2·5 g lipoic acid/kg were approximately one-half those in the animals given a diet free of this compound. A diet containing 5 g lipoic acid/kg further decreased these parameters, and the reduction was most prominent with stearoyl-CoA desaturase 1. The observations in the present study therefore indicated that lipoic acid decreased hepatic lipogenesis through a SREBP-1-dependent mechanism. However, lipoic acid did not significantly modify mRNA levels of two isoforms of this transcription factor. mRNA levels of SREBP-1a in rats fed diets containing 0, 1, 2·5 and 5·0 g/kg were 100 (sem 6), 92·5 (sem 3·7), 113 (sem 4) and 108 % (sem 6), respectively. Those of SREBP-1c were 100 (sem 15), 83·7 (sem 6·3), 98·2 (sem 7·4) and 87·7 % (sem 9·5), respectively.
Lipoic acid was rather ineffective in modulating serum cholesterol levels. However, this compound decreased not only the concentration of TAG, but also that of cholesterol in the liver. We therefore measured mRNA levels of enzymes involved in the cholesterogenic pathway. Although a diet containing 1 g lipoic acid/kg compared to a diet free of this compound did not affect the mRNA level of cytoplasmic 3-hydroxy-3-methylglutaryl-CoA synthase (100 (sem 6) and 94·7 (sem 5·6) % for rats given diets containing 0 and 1 g lipoic acid/kg, respectively), diets containing 2·5 and 5 g lipoic acid/kg significantly decreased this parameter (73·2 (sem 3·8) and 62·8 (sem 5·2) %, respectively). Diets containing 1 and 2·5 g lipoic acid/kg, however, did not affect mRNA levels of 3-hydroxy-3-methylglutaryl-CoA reductase and farnesyl diphosphate synthase, and a diet containing 5 g lipoic acid/kg slightly but significantly increased these values (120 (sem 7) and 121 (sem 9) % for 3-hydroxy-3-methylglutaryl-CoA reductase and farnesyl diphosphate synthase, respectively). SREBP-2 is a transcription factor involved in the regulation of the gene expression of these cholesterogenic enzymes and the LDL receptor(Reference Horton, Goldstein and Brown13). A diet containing 5 g, but not 1 or 2·5 g lipoic acid/kg, relative to a lipoic acid-free diet significantly decreased mRNA levels of the LDL receptor (100 (sem 4) and 71·2 (sem 2·5) % in rats fed diets containing 0 and 5 g lipoic acid/kg, respectively). Dietary lipoic acid did not affect mRNA levels of SREBP-2 (data not shown). Therefore, the impact of lipoic acid on the mRNA expression of cholesterogenic enzymes was rather moderate and inconsistent.
Dietary lipoic acid considerably modified serum concentrations of adipokines (adiponectin and leptin). However, lipoic acid at various dietary levels was totally ineffective in modifying mRNA levels of these adipokines in epididymal white adipose tissue (data not shown). We also measured mRNA levels of various proteins (glucose transporter 4, adiponutrin, PPARγ2, lipoprotein lipase, adipocyte lipid-binding protein and fatty acid translocase/CD36) in epididymal fat depot. However, lipoic acid was rather ineffective in altering these mRNA levels and only slight changes (16–20 %) were observed on some occasions.
Discussion
In the present study, we confirmed previous findings that dietary lipoic acid reduces serum and tissue lipid levels(Reference Thirunavukkarasu and Anuradha4, Reference Yi and Maeda8–Reference Song, Lee and Koh11) despite that this compound was rather ineffective in reducing serum cholesterol levels, and increased the hepatic concentration of phospholipid. Moreover, we showed that this compound dose-dependently reduced hepatic activity and mRNA levels of lipogenic enzymes, and circulating insulin and leptin levels, but increased the serum adiponectin concentration. As the diet containing 5 g but not 1 or 2·5 g lipoic acid/kg considerably decreased the food intake of animals, the largest effect seen with the highest dose may be related to side-effects or toxic effects of the compound. Generally, diets containing high levels of lipoic acid reduce the intake of food in rats(Reference Ford, Cotter, Cameron and Greaves10–Reference Lee, Naseem, Park, Garry, Richardson, Schaffer and Unger12, Reference Kim, Park and Namkoong15). It is currently unknown if this represents toxicity or a problem with the palatability of the compound. A study suggested that lipoic acid decreased the activity of AMP-activated protein kinase (AMPK) in the hypothalamus and hence reduced food intake(Reference Kim, Park and Namkoong15). Significant clinical toxicity of lipoic acid assessed by changes in serum ammonia and amino acid concentrations has been observed in cats(Reference Hill, Werner, Rogers, O'Neill and Christopher38). However, no sign of the toxicity of lipoic acid in rats was observed in a 2-year study even at a high dose that reduces food intake(Reference Cremer, Rabeler, Roberts and Lynch39). Therefore, it is unlikely that the large effects seen with a diet containing 5 g lipoic acid/kg are related to the toxicity of lipoic acid. However, it is possible that the large reductions in hepatic lipogenesis, serum and liver lipid levels, and circulating levels of insulin and leptin seen with a diet containing 5 g lipoic acid/kg represent not only the physiological activity of lipoic acid but also a consequence of the reduction of food intake, because it is well demonstrated that food deprivation causes profound decreases in these parameters(Reference Margetic, Gazzola, Pegg and Hill40, Reference Desvergne, Michalik and Wahli41).
Dietary lipoic acid reduced hepatic concentrations of TAG and cholesterol, but increased the value for phospholipid. This may imply the proliferation of hepatic organelles like the endoplasmic reticulum, lysosomes, mitochondria and peroxisomes. In fact, increases in hepatic phospholipid levels are reported in rats treated with drugs and food components that increase the activity and expression of hepatic lysosomal acid phosphatase(Reference Matsuzawa and Hostetler42), microsomal drug-metabolizing enzymes(Reference Orrenius, Ericsson and Ernster43), and mitochondrial and peroxisomal fatty acid oxidation enzymes(Reference Ashakumary, Rouyer, Takahashi, Ide, Fukuda, Aoyama, Hashimoto, Mizugaki and Sugano44). As we did not observe lipoic acid-dependent increases in the activities of mitochondrial carnitine palmitoyltransferase and peroxisomal palmitoyl-CoA oxidase in the present study (data not shown), an increase in the hepatic phospholipid concentration may not represent the proliferation of mitochondria and peroxisomes.
We observed that lipoic acid reduced the activity and mRNA levels of enzymes involved in hepatic fatty acid synthesis and pyruvate kinase, and mRNA levels of proteins presumed to be involved in the regulation of lipogenesis (spot 14 and adiponutrin)(Reference Ide33–Reference Jenkins, Mancuso, Yan, Sims, Gibson and Gross35). Therefore, it is highly possible that reduced hepatic lipogenesis is a principal mechanism for the lipid-lowering effect of this compound. However, the lipoic acid-dependent decreases in the activity and mRNA expression of NADPH-producing enzymes (glucose 6-phosphate dehydrogenase and malic enzyme) were rather moderate. It has been suggested that NADPH-producing enzymes not only play a role in the regulation of lipogenesis, but also are involved in the attenuation of oxidative stress in tissues(Reference Taniguchi, Yasutake, Takedomi and Inoue45). Therefore, the responses different from those of other lipogenic enzymes may in some way be related to the anti-oxidative activity of lipoic acid(Reference Amudha, Josephine and Varalakshmi3–Reference Khanna, Atalay, Laaksonen, Gul, Roy and Sen6). Lipoic acid reduced mRNA levels not only of enzymes and proteins involved in lipogenesis, but also of stearoyl-CoA desaturase 1, and Δ5- and Δ6-desaturases. This observation supports the notion that the SREBP-1 signalling pathway is involved in the regulation of lipogenic enzymes, because this transcription factor up regulates the gene expression not only of many lipogenic enzymes, but also of the enzymes involved in the desaturation of fatty acids(Reference Ntambi36, Reference Matsuzaka, Shimano and Yahagi37). Although lipoic acid was not effective in reducing mRNA levels of two isoforms of this transcription factor in the present study, it is still possible that this compound impairs the proteolytic cleavage of the membrane-bound immature form of this transcription factor to convert it to the mature form located in the nucleus without altering its gene expression and hence affects hepatic lipogenesis. An analysis of the nuclear levels of the mature form of SREBP-1 is required to clarify this point.
In relation to the physiological activity of lipoic acid in reducing hepatic lipogenesis, we observed that lipoic acid dose-dependently reduced serum concentrations of insulin. It is well documented that insulin up regulates hepatic lipogenic enzymes through a SERBP-1-dependent mechanism(Reference Horton, Goldstein and Brown13, Reference Desvergne, Michalik and Wahli41). Therefore, the lipoic acid-dependent decrease in the activity and mRNA levels may be caused by the reduction in circulating insulin levels. An insulin-lowering action of lipoic acid in rats has also been reported(Reference Thirunavukkarasu and Anuradha4, Reference Song, Lee and Koh11, Reference Midaoui, Elimadi, Wu, Haddad and de Champlain16). In relation to this, Song et al. (Reference Song, Lee and Koh11) examined the effect of lipoic acid on pancreatic morphology in obese OLETF rats. They observed that OLETF rats, compared with control Long–Evans Tokushima Otsuka rats, showed a dispersion and reduction in the number of the insulin-positive cells in pancreatic islets. The abnormalities observed with OLETF rats were ameliorated by the treatment with lipoic acid even though this compound significantly reduced the serum concentration of insulin. Therefore, it is unlikely that lipoic acid impairs the function of the pancreas and hence reduces the circulating insulin level. Many studies have indicated that lipoic acid promotes the insulin-mediated transport of glucose or mimics the action of insulin to stimulate the utilization of glucose in muscle(Reference Saengsirisuwan, Perez, Sloniger, Maier and Henriksen46, Reference Streeper, Henriksen, Jacob, Hokama, Fogt and Tritschler47) and 3T3-L1 adipocytes(Reference Moini, Tirosh, Park, Cho and Packer48, Reference Estrada, Ewart, Tsakiridis, Volchuk, Ramlal, Tritschler and Klip49). Therefore, it is likely that dietary lipoic acid spares insulin that is required to metabolize glucose and hence reduces the pancreatic secretion and serum concentration of this hormone.
We also observed that lipoic acid significantly reduced serum concentrations of leptin, but increased those of adiponectin without altering mRNA levels of these adipokines in epididymal adipose tissue in the present study. Therefore, lipoic acid may affect factors other than the transcription of genes and mRNA stability and hence modulate serum concentrations of these adipokines. Song et al. (Reference Song, Lee and Koh11) observed that a diet containing 5 g lipoic acid/kg caused about a 65 % decrease in serum leptin concentrations in OLETF rats. This observation is well consistent with the result obtained in the present study. As many studies have demonstrated that insulin stimulates the secretion of leptin from adipocytes(Reference Huerta50), alterations by lipoic acid of the serum concentration of this adipokine may represent a consequence of the alteration in insulin status of the animals fed this compound. In fact, a strong positive correlation between serum concentrations of insulin and leptin was observed in the present study. Studies have indicated that overexpression of leptin reduces hepatic lipogenesis(Reference Kakuma, Lee, Higa, Wang, Pan, Shimomura and Unger51) while a deficiency of this adipokine increases it(Reference Iizuka, Miller and Uyeda52). In the present study, lipoic acid reduced both serum leptin concentrations and the activity and mRNA levels of hepatic lipogenic enzymes. Therefore, alterations in the serum leptin concentration cannot account for lipoic acid-dependent changes in hepatic lipogenesis.
A hypoglycaemic effect of lipoic acid has been observed by many investigators(Reference Henriksen7), and was confirmed in the present study. Surprisingly, however, no information regarding the effect of lipoic acid on serum adiponectin concentrations has hitherto been available despite that this adipokine exerts a potent insulin-sensitizing effect and hence lowers blood glucose levels(Reference Huerta50, Reference Kadowaki and Yamauchi53). The present finding that lipoic acid increased serum adiponectin concentrations indicated that this adipokine may be involved in the hypoglycaemic effect of this compound. With regard to the physiological activity of adiponectin in regulating hepatic lipogenesis, this adipokine increases the amount of the phosphorylated form of AMPK in the liver(Reference Huerta50, Reference Kadowaki and Yamauchi53). AMPK plays a crucial role in regulating fatty acid and glucose metabolism. Activation through phosphorylation of this enzyme in turn stimulates phosphorylation of acetyl-CoA carboxylase and decreases the enzyme activity. This results not only in a diminished lipogenesis, but also in an enhancement of fatty acid oxidation through a decrease in the hepatic concentration of malonyl-CoA, an inhibitor of carnitine palmitoyltransferase I. Moreover, information suggests that the activation of AMPK results in the down-regulation of the expression of fatty acid synthase, spot 14, l-pyruvate kinase and SREBP-1(Reference Leclerc, Kahn and Doiron54, Reference Zhou, Myers and Li55). Recent studies have indicated that dietary lipoic acid activates AMPK in the aortic endothelium(Reference Lee, Lee and Kim56), and skeletal muscle(Reference Lee, Song, Koh, Won, Kim, Park, Kim, Kim, Lee and Park17), but decreases the enzyme activity in the hypothalamus(Reference Kim, Park and Namkoong15). There is the possibility that lipoic acid also affects the activity of AMPK in the liver through an adiponectin-mediated mechanism and hence decreases the gene expression of lipogenic enzymes. In this context, Lee et al. (Reference Lee, Naseem, Park, Garry, Richardson, Schaffer and Unger12) observed an increase in the hepatic content of the active phosphorylated form of AMPK after 4 d on a diet containing 10 g lipoic acid/kg, but not after 42 d of feeding in acyl-CoA synthase transgenic mice. Therefore, the role of the AMPK-signalling pathway and of adiponectin in lipoic acid-dependent changes in the gene expression of hepatic lipogenic enzymes is not clear at present. There is the possibility that the metabolic responses to lipoic acid observed in acyl-CoA synthase transgenic mice are considerably different from those in Sprague–Dawley rats employed as experimental animals in the present study. Therefore, an analysis of hepatic AMPK activity is required to clarify the role of the AMPK-cascade in the lipoic acid-dependent changes in the hepatic lipogenesis in rats observed currently.
It has been reported that orally administered lipoic acid in rats is well absorbed from the intestine(Reference Peter and Borbe57). Pharmacokinetic analyses of the metabolic fate of orally and intravenously administered [7,8-14C]lipoic acid indicated that 66 % of orally administered lipoic acid is absorbed from the intestine. An alternative evaluation based on measurements of urinary excretion of 14C gave a higher value for the absorption (93 %). However, information on tissue or serum levels of lipoic acid in animals treated with this compound is lacking except for a report by Khanna et al. (Reference Khanna, Atalay, Laaksonen, Gul, Roy and Sen6) who intragastrically administered lipoic acid to rats at a dose of 150 mg/kg body weight per d for 8 weeks. However, the treatment caused a mere 35 % insignificant increase in the hepatic concentration of this compound. The dose employed by Khanna et al. (Reference Khanna, Atalay, Laaksonen, Gul, Roy and Sen6) approximates that observed in the present study in rats fed the diet containing 2·5 g lipoic acid/kg (168 (sem 3) mg/kg body weight per d) that exerts strong activity in altering parameters of lipid metabolism. Therefore, there is the possibility that metabolite(s) of lipoic acid(Reference Khanna, Atalay, Laaksonen, Gul, Roy and Sen6) rather than lipoic acid per se is responsible for the physiological activity of dietary lipoic acid observed currently.
In conclusion, we demonstrated that dietary lipoic acid strongly decreased hepatic activity and mRNA levels of lipogenic enzymes. This may account for the lipid-lowering effect of lipoic acid reported to date. Alterations in serum concentrations of insulin and (or) adiponectin may trigger this consequence.
Acknowledgements
This study was supported by a grant from the Ministry of Agriculture, Forestry and Fisheries (MAFF) research project “Development of Evaluation and Management Methods for the Supply of Safe, Reliable and Functional Food and Farm Produce” and the United Nation University-Kirin Fellowship Program awarded to D. T. T. H. There are no conflicts of interest for all sources of funding and the contribution of each author to the manuscript.





