Peripheral arterial disease (PAD) is associated with increased cardiovascular (CV) morbidity and mortality(Reference Criqui and Aboyans1). Even in patients receiving optimal management of CV risk factors including antiplatelet therapy, statins, smoking cessation, control of hypertension and diabetes, the prognosis remains poor due to multiple CV events indicating progressive atherosclerosis. Thus, novel approaches to tackle this excess risk are needed.
Deterioration of endothelial function is an early sign of functional impairments of the vascular system during the development of clinically manifest atherosclerosis. This parameter can be measured by flow-mediated vasodilation (FMD), which is related to severity and prognosis in many CV disorders(Reference Brunner, Cockcroft and Deanfield2, Reference Mordi and Tzemos3) including PAD(Reference Gokce, Keaney and Hunter4–Reference Brevetti, Silvestro and Schiano6). n-3 PUFA have multiple effects on the CV system that can be beneficial also in PAD patients(Reference Mozaffarian and Wu7). n-3 PUFA have been shown to improve endothelial function in healthy volunteers(Reference Shah, Ichiuji and Han8, Reference Rizza, Tesauro and Cardillo9) and also in subjects at high CV risk or suffering from manifest atherosclerosis or heart failure(Reference Cicero, Ertek and Borghi10–Reference Tousoulis, Plastiras and Siasos14). However, some studies did not show such a benefit(Reference Siniarski, Haberka and Mostowik15, Reference Grenon, Owens and Nosova16). Furthermore, n-3 PUFA have been shown to reduce inflammatory cytokines(Reference Borow, Nelson and Mason17, Reference Calder18), which play an important role in the development of endothelial dysfunction and atherosclerosis. Moreover, they are used as TAG-lowering treatment in patients with hypertriacylglycerolaemia, a situation that also deteriorates endothelial function and worsen prognosis in CVD(Reference Ganda, Bhatt and Mason19). Further potential benefits comprise a reduction in blood pressure, decrease in blood viscosity, promotion of fibrinolysis and diminished platelet activation(Reference Tousoulis, Plastiras and Siasos14, Reference Moertl, Berger and Hammer20–Reference McEwen, Morel-Kopp and Tofler22). Importantly, high doses were required for most of the pharmacological actions relevant for the effects described above. Similarly, for the treatment of hypertriacylglycerolaemia 4 g daily are currently recommended.
n-3 PUFA do not only have proven effects on surrogate markers of CVD, but could also have the potential to reduce clinical endpoints in heart disease. Several trials have shown that moderate fish oil intake (by eating fish rich in n-3 fatty acids or consuming respective supplements) decreases the risk of major CV events, while other studies including a recent meta-analysis did not find such a beneficial effect(Reference Elagizi, Lavie and Marshall23–Reference O’Keefe, Jacob and Lavie26). The recently published REDUCE-IT trial showed that high doses (4 g daily) of the n-3 oil EPA had a large benefit on CV events after a median follow-up of 4·9 years in patients with hypertriacylglycerolaemia(Reference Bhatt, Steg and Miller27).
In contrast to CHD, there are only sparse data on the effects of n-3 PUFA in PAD patients. While one study reported improved endothelial function in response to n-3 PUFA in PAD patients(Reference Schiano, Laurenzano and Brevetti12), another trial did not show such a benefit after a short-term intake of 1 month(Reference Grenon, Owens and Nosova16).
In the present study, we aimed to determine the effects of 4 g n-3 PUFA daily on top of standard therapy on surrogate markers of disease severity and prognosis in patients with PAD after 3 months of treatment. Primary outcome parameter was endothelial function assessed by brachial artery ultrasound, secondary outcome measures comprised maximum and pain-free treadmill walking distance, endothelial activation and pro-inflammatory markers. All markers were assessed again after a 3-month washout phase after active treatment.
Materials and methods
Study population
In this prospective single-centre, double-blind, placebo-controlled, randomised trial, seventy patients with stable PAD receiving optimised standard therapy were recruited at the Vascular Medicine outpatient clinic of the Department of Internal Medicine II, Medical University of Vienna, Austria. Key inclusion criteria comprised (1) a history of intermittent claudication (Rutherford categories 2–3), (2) exercise tolerance limited by intermittent claudication during a screening treadmill test and (3) an ankle–brachial index at rest <0·9. Key exclusion criteria comprised (1) rest pain or ischaemic ulcers, (2) exercise tolerance limited by factors other than claudication (e.g. coronary artery disease, dyspnoea, poorly controlled blood pressure any kind of restriction of the musculoskeletal system) and (3) planned revascularisation procedure within the next 3 months (including angioplasty and bypass surgery). Although prior data have shown that n-3 PUFA supplementation in addition to dual antiplatelet therapy does not increase the risk of bleeding(Reference Watson, Joy and Nkonde28), patients on dual antiplatelet therapy (e.g. after stent implantation) were not eligible for enrolment. Guideline-conform medical therapy was ensured in each patient(Reference Tendera, Aboyans and Bartelink29). If changes in standard medication were required at baseline, patient recruitment was postponed for 4 weeks with the exception of lipid-lowering medication, angiotensin (AT)-converting enzyme inhibitors/AT receptor antagonists and nebivolol. As these drugs were shown to improve FMD before, stable therapy was warranted for at least 3 months prior to inclusion.
The Academic Studies Support Office of the Medical University of Vienna oversaw randomisation and allocation concealment. After providing informed consent, the study physician contacted the Academic Studies Support Office for randomisation. Participants were electronically randomised (block-wise randomisation with random block size) to 4 g of n-3 PUFA or an equivalent of placebo with stratification for antiplatelet therapy (clopidogrel or aspirin).
Patients were advised to take two capsules of the study medication (n-3 PUFA or placebo) twice daily with food over the study period of 12 weeks, to abstain from other fish oil supplements and to maintain their habitual diet.
The study drug was a prescription formulation of high-purity n-3 PUFA (850–882 mg EPA and DHA as ethyl esters in the average ratio of EPA/DHA 1·2:1 per 1 g soft gelatine capsule marketed as Omacor®, Manufacturer: Pronova BioPharma, Distributor: SolvayPharma). Placebo was produced with identical appearance to Omacor® consisting of a gelatine-encapsulated nutrition supplement containing fatty acids, which typically occur in the Austrian diet (Manufacturer: Ayanda). Physicians and patients were supplied with medication packages of equal appearance and weight containing active drug or placebo produced by the local hospital pharmacy. Packages were provided with unique numbers, which were also used as participant identification number. Only persons from the Academic Studies Support Office, which were not otherwise involved in the study, had access to the coding.
Primary and secondary endpoints were measured at baseline, 3 months after treatment as well as 3 months after treatment cessation (i.e. 6 months after study inclusion). A safety visit consisting of recording of potential adverse events and collection of blood samples (blood count, liver enzymes) was performed after 4 weeks of treatment.
The study was registered with https://www.clinicaltrials.gov/ (unique identifier: NCT NCT01367145).
Primary endpoint: flow-mediated vasodilation
FMD measurements were performed in the morning in a fasting state between 07.00 and 10.00 hours at room temperature according to the guidelines for the ultrasound assessment of endothelial-dependent FMD and nitrate-mediated vasodilation of the brachial artery(Reference Corretti, Anderson and Benjamin30). Using high-resolution ultrasound (Hewlett Packard Sonos 2500) with a 7·5 MHz linear array transducer, diameter measurements of the right brachial artery were taken after supine rest for at least 10 min, after cuff deflation completing suprasystolic compression (50 mmHg above systolic pressure) of the right upper arm for 5 min and after sublingual application of 0·8 mg nitroglycerin. After optimal transducer positioning on the brachial artery proximal to the bifurcation of the radial and the ulnar artery, the arm was kept in the same position and the skin was marked. The longitudinal image of the artery was recorded at baseline, continuously from 30 s before to 2 min after cuff deflation and for 5 min after nitroglycerin administration. ECG gating was used during image acquisition to determine the diameter at the same time in the cardiac cycle. The baseline diameter and the maximum FMD and nitrate-mediated vasodilation diameters were measured from one media–adventitia interface to the other. Vasodilatation was then calculated as the percentage change in diameter over the baseline value. To verify that suprasystolic compression of the brachial artery produced adequate increases in blood flow, flow velocity was measured at rest and within 15 s after cuff deflation. Blood flow was calculated by multiplying the velocity time integral by the heart rate and the vessel cross-sectional area. FMD was then calculated as the percentage change in flow during hyperaemia over the baseline value. All measurements were performed by one experienced ultrasound specialist blinded to the treatment strategy.
Secondary endpoints
Maximum and pain-free treadmill walking distance
Treadmill tests were performed with a constant speed of 3·2 km/h, or – in patients with severe walking impairment – 1·6 km/h, respectively, at 12 % slope according to the division’s standard protocol under supervision of a trained nurse with established test–retest reliability (intraclass coefficient of correlation 0·83 for maximum walking distance and 0·75 for pain-free walking distance). In each patient, maximum walking distance and pain-free walking distance were recorded.
Lipid parameters, pro-inflammatory, endothelial and platelet activation markers
After the first 5 ml were discarded, venous blood samples were collected with little or no stasis after a 12-h fast for all measurements.
Routine laboratory assessments including chemistry studies, complete blood count and lipoprotein profile were obtained at baseline and all follow-up time points.
The erythrocyte composition of the two main long-chain n-3 PUFA, EPA and DHA was analysed according to the HS-Omega-3 Index methodology by Omegametrix GmbH.
According to the manufacturer’s protocol, commercially available immunoassays were used to determine plasma levels of ultrasensitive C-reactive protein (American Diagnostica), high-sensitive IL-6 (R&D Systems), high-sensitive TNFα (R&D Systems), asymmetric dimethylarginine (ADMA, DLD Diagnostika GmbH) and plasminogen-activator inhibitor-1 (ABclonal). Soluble P-selectin, soluble E-selectin, soluble intercellular adhesion molecule-1 and soluble vascular cell adhesion molecule-1 were measured using the Human Adhesion Luminex High-Performance Assay 4-Plex Kit (R&D Systems).
Whole-blood flow cytometry for the measurement of monocyte–platelet aggregates was performed as described previously(Reference Moertl, Berger and Hammer20). A quantity of 100 μl of citrate-anticoagulated whole blood was stained with saturating concentrations of the following fluorochrome-conjugated monoclonal antibodies (mAb): allophycocyanin-labelled mAb for the constitutive platelet marker CD42b (glycoprotein Ib of von Willebrand factor receptor complex) and PE-Cy5-labelled mAb for monocyte CD14 (endotoxin receptor) or corresponding isotype controls (all antibodies were purchased from Becton Dickinson). After 10 min of preincubation with antibodies in the dark at room temperature, samples were fixed and erythrolysed with Optilyse B (Instrumentation Laboratories). Flow cytometry was performed on a FACSCalibur (Becton Dickinson). Acquisition was stopped when 5·000 CD14+ events were acquired. The negative and positive delineator were determined by gating 2 % background staining on the isotype control fluorescence. The percentage of monocyte–platelet aggregates was determined as the relative number of monocytes coexpressing the constitutive platelet marker CD42b (CD14+/CD42b+%).
Statistical analysis
For sample size calculation, we assumed that a 2·5 % difference in FMD (with a common sd of the difference of 3) upon fish oil intake would be reasonable for a potential clinical benefit based on prior studies in the field(Reference Brevetti, Silvestro and Schiano6, Reference Rizza, Tesauro and Cardillo9, Reference Schiano, Laurenzano and Brevetti12). These assumptions, an α<0·05 and a power of 0·9 for a two-sided independent-group comparison, yielded a sample size of sixty-two (thirty-one in each group). Assuming a dropout rate of 10 %, the study aimed to recruit seventy patients.
Categorical data are presented as numbers and percentages and compared by χ2 statistics between the groups. As several parameters were non-normally distributed, all continuous data are presented as medians and interquartile ranges (IQR). Between-group comparisons were made by the Mann–Whitney U test and within-group comparisons by the Wilcoxon signed-rank test. For the primary endpoint, an additional analysis was performed using a linear mixed-effects model examining the main effects (treatment group; visit) and interaction effects (treatment group × visit). All analyses were made with SAS 9.4. A P value <0·05 was considered as statistically significant.
Results
Overall, seventy patients were randomised to either n-3 PUFA or placebo in a 1:1 fashion between February 2011 and March 2013. The last follow-up visit was performed in October 2013. Patient characteristics are given in Table 1. No differences were seen between the groups except for intake of Ca channel blockers. The Consolidated Standards of Reporting Trials flow chart is depicted in Fig. 1. Regarding severe adverse events, one patient suffered a myocardial infarction 4 weeks after randomisation in the n-3 PUFA group, which was treated by primary percutaneous coronary intervention with good outcome. In the placebo group, one patient underwent coronary angiography for new-onset angina and was subsequently excluded from the study.
Table 1. Baseline characteristics
(Medians and interquartile ranges (IQR); numbers and percentages)
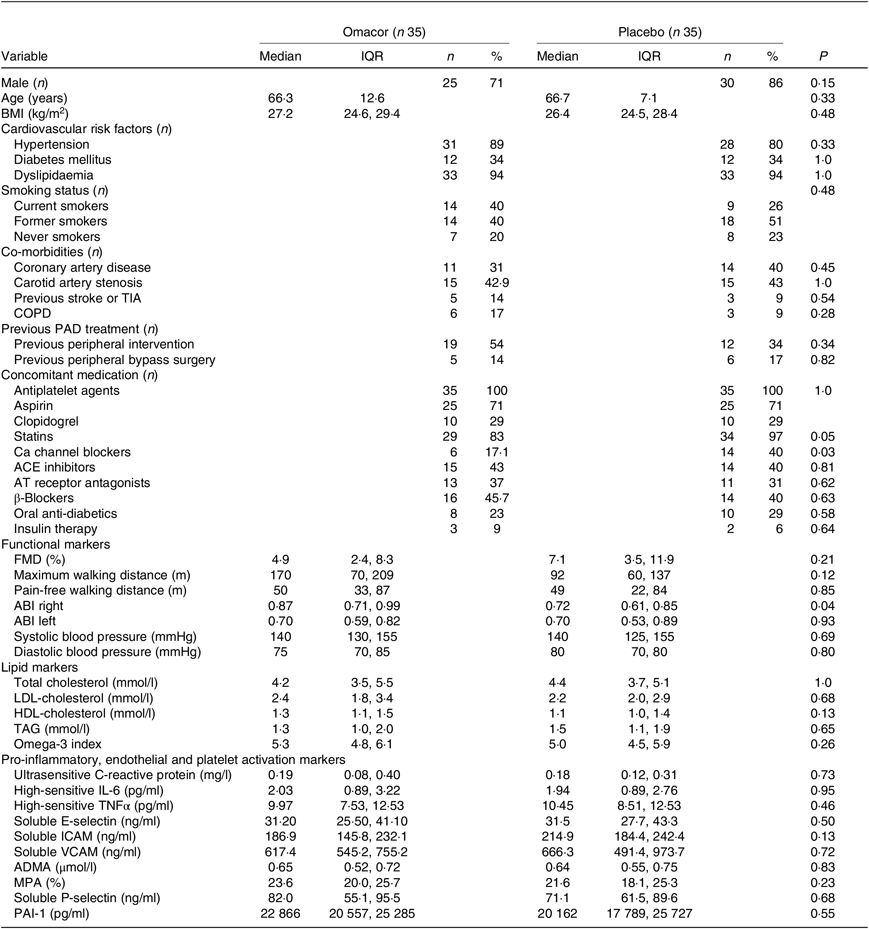
TIA, transient ischaemic attack; COPD, chronic obstructive pulmonary disease; PAD, peripheral arterial disease; ACE, angiotensin-converting enzyme; AT, angiotensin; FMD, flow-mediated dilation; ABI, ankle–brachial index; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule; ADMA, asymmetric dimethylarginine; MPA, monocyte–platelet aggregates; PAI-1, plasminogen activator inhibitor-1.
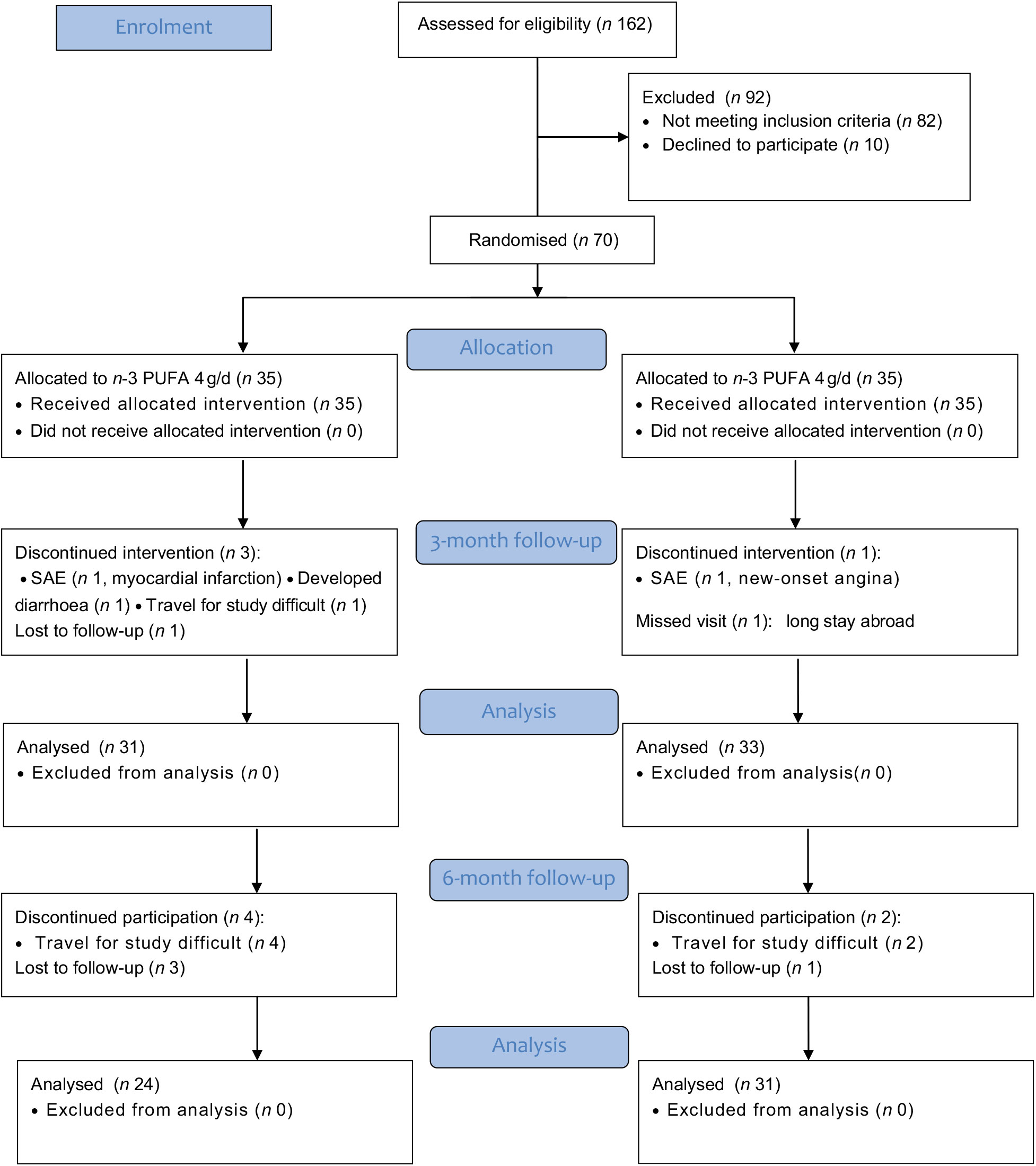
Fig. 1. Consolidated Standards of Reporting Trials (CONSORT) flow chart. SAE, severe adverse event.
Primary endpoint
FMD showed a significant median absolute increase of Δ 3·7 (IQR –1·8, 7·1) % in response to 4 g/d n-3 PUFA treatment (P = 0·03), while no such increase was seen in the placebo group (median Δ –0·5 (IQR –6·5, 3·0) %, P = 0·18) yielding a significant difference between the groups at 3 months (P = 0·01, Table 2, Fig. 2). After an additional 3 months washout phase, the benefit of n-3 PUFA treatment was not sustained. Compared with baseline, the median change was Δ 0·6 (IQR –2·2, 5·6) % in the n-3 PUFA group (P = 0·10) and Δ –0·9 (IQR –6·6, 6·7) % in the placebo group (P = 0·96) yielding no difference between the groups (P = 0·20, Table 3, Fig. 2). In an additional analysis using a linear mixed-effects model, a significant group by time interaction was observed (P<0·05). In line with the primary analysis, FMD improved between baseline and 3 months in the 4 g/d n-3 PUFA group (P = 0·04) but not at 6 months or in the placebo group. A signal for a between-groups difference was seen at 3 months (P = 0·08), which diminished at 6 months (P = 0·8).
Table 2. Absolute change of functional and laboratory markers after 3 months of 4 g/d n-3 PUFA or placebo
(Medians and interquartile ranges (IQR))
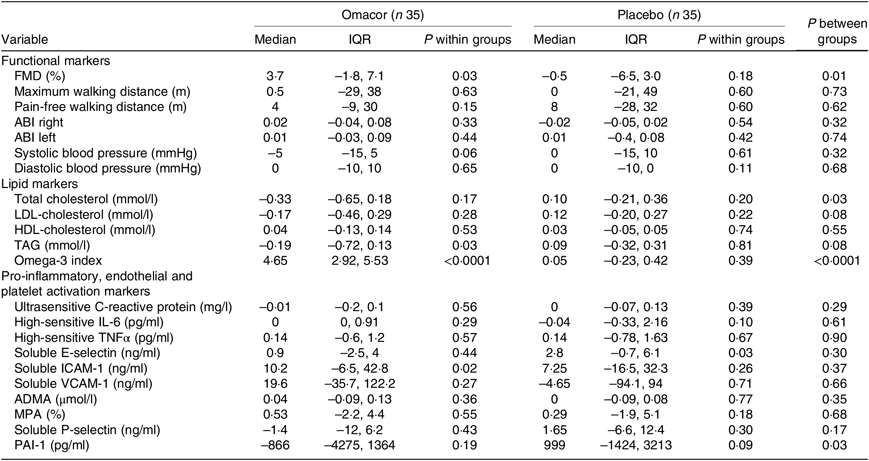
FMD, flow-mediated dilation; ABI, ankle–brachial index; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule; ADMA, asymmetric dimethylarginine; MPA, monocyte–platelet aggregates; PAI-1, plasminogen activator inhibitor-1.
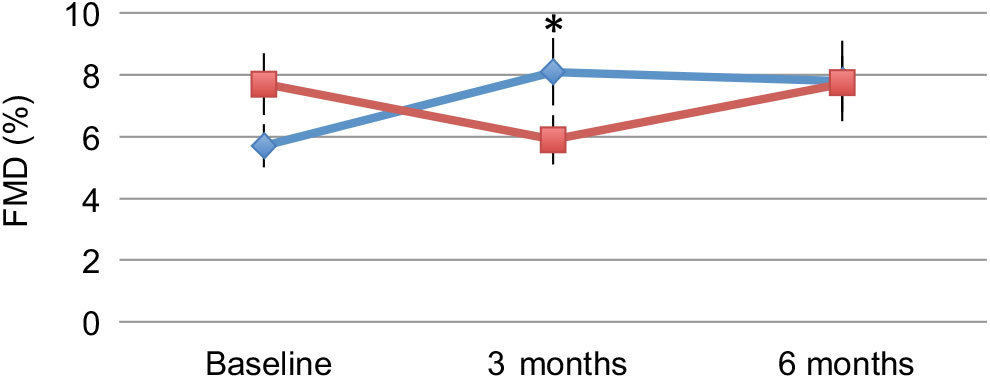
Fig. 2. Flow-mediated dilation (FMD) at baseline, after 3 months of treatment with 4 g/d n-3 PUFA (-♦-) or placebo (-▪-) and after 6 months (3 months treatment plus 3 months washout). Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from that at baseline (P < 0·05).
Table 3. Absolute change of functional and laboratory markers after 6 months (3 months treatment plus 3 months washout) with 4 g/d n-3 PUFA or placebo (Medians and interquartile ranges (IQR))
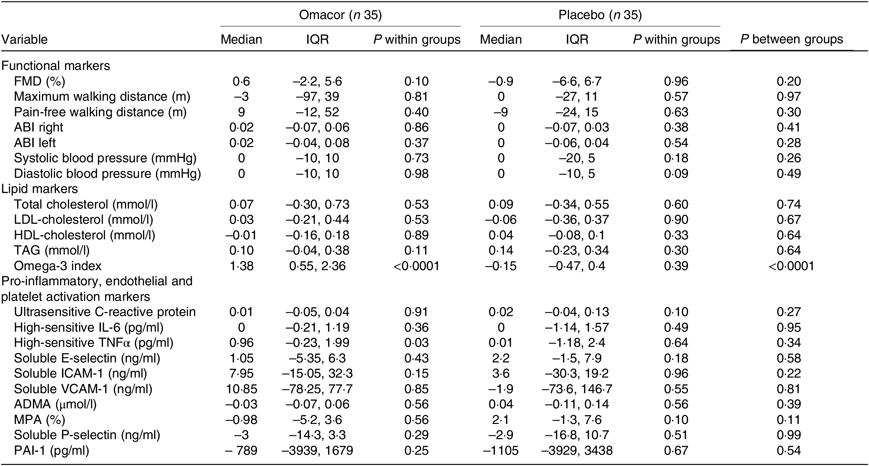
FMD, flow-mediated dilation; ABI, ankle–brachial index; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule; ADMA, asymmetric dimethylarginine; MPA, monocyte–platelet aggregates; PAI-1, plasminogen activator inhibitor-1.
Secondary endpoints
Changes of secondary endpoints in response to treatment after 3 and 6 months are shown in Tables 2 and 3. With respect to lipid markers, a significant difference was observed for total cholesterol between n-3 PUFA and placebo treatment after 3 months (n-3 PUFA, median cholesterol Δ –0·33 (IQR –0·65, 0·18) mmol/l v. placebo, cholesterol Δ 0·10 (IQR –0·21, 0·36) mmol/l, P = 0·03 between the groups). In addition, a trendwise difference was seen for LDL-cholesterol and TAG between the groups after 3 months, and the decrease in TAG compared with baseline reached statistical significance in the n-3 PUFA group (Δ –0·19 (IQR –0·72, 0·13) mmol/l, P = 0·03 within-group comparison). The omega-3 index increased significantly upon n-3 PUFA intake (Δ 4·65 (IQR 2·92, 5·53), P<0·0001) yielding a significant difference between the groups at 3 months (P<0·0001). This difference was less pronounced but still significant after 6 months, that is, 3 months after n-3 PUFA treatment cessation (n-3 PUFA, omega-3 index Δ 1·38 (IQR 0·55, 2·36) v. placebo, omega-3 index Δ –0·15 (IQR –0·47, 0·4), P<0·0001 between the groups).
No statistically significant effect of n-3 PUFA treatment was observed for maximum and pain-free walking distance as well as measurements of systolic and diastolic blood pressure and ankle–brachial index. Similarly, n-3 PUFA treatment did not affect several markers of inflammation as well as endothelial and platelet activation (ultrasensitive C-reactive protein, high-sensitive IL-6, high-sensitive TNFα, soluble E-selectin, soluble intercellular adhesion molecule-1, soluble vascular cell adhesion molecule-1, ADMA, soluble P-selectin, monocyte–platelet aggregates). Plasminogen-activator inhibitor-1 levels did not increase significantly in response to n-3 PUFA treatment either.
Discussion
In the present study in patients with symptomatic PAD, we found that 3 months of treatment with 4 g/d n-3 PUFA improved endothelial function without an effect on walking distance. This improvement was accompanied by significant changes in erythrocyte EPA and DHA content and TAG levels but not in pro-inflammatory as well as other endothelial and platelet activation markers. The effect seen on FMD was not sustained 3 months after treatment cessation. n-3 PUFA supplementation was well tolerated, only one patient stopped intake due to diarrhoea.
Some but not all prior studies showed an improvement of FMD by n-3 PUFA as summarised before in a meta-analysis(Reference Wang, Liang and Wang31). Also in PAD patients, discrepant results have been described previously. One prior small study comprising thirty-two patients randomised to 1 g/d n-3 PUFA or no intervention did find a positive effect of n-3 PUFA on endothelial function after 3 months(Reference Schiano, Laurenzano and Brevetti12), while short-term (1 month) 4·4 g/d n-3 PUFA supplementation in eighty patients randomised to active treatment or placebo did not report a benefit(Reference Grenon, Owens and Nosova16). Several factors including characteristics of the patients enrolled, length of treatment and study quality were postulated to potentially modify the effects of n-3 PUFA on endothelial dysfunction(Reference Wang, Liang and Wang31). Thus, the treatment of patients with established CVD compared with healthy volunteers for longer time intervals seemed to be associated with a more pronounced benefit of n-3 PUFA. This is also in line with the significant improvement we saw in our patient population after 3 months of treatment in patients with symptomatic PAD, which are known to suffer from high CV morbidity and mortality(Reference Criqui and Aboyans1). The benefit of n-3 PUFA supplementation on FMD was not sustained after a 3-month washout period, although the omega-3 index was still significantly higher in the treatment compared with the control group. Thus, the long-term intake might be necessary for a persistent improvement of endothelial function that could translate into potential clinical benefits. Importantly, we did not observe a difference in maximum and pain-free walking distance as functional PAD-specific markers. As exercise training was shown to improve walking capacity(Reference Lane, Harwood and Watson32) and markers of endothelial function(Reference Schlager, Giurgea and Schuhfried33) in PAD, a combination of both treatments could be beneficial.
While the exact mechanisms are still under debate, several pathways have been proposed how n-3 PUFA improves endothelial function including increased membrane fluidity of endothelial cells as well as anti-inflammatory and antiplatelet effects(Reference Mozaffarian and Wu7). We studied a variety of biomarkers to potentially link plausible biological mechanisms with the observed benefit on FMD but no changes were seen for circulating pro-inflammatory (ultrasensitive C-reactive protein, high-sensitive IL-6, high-sensitive TNFα), endothelial function (soluble intercellular adhesion molecule-1, s-VCAM-1, soluble E-selectin), plasminogen-activator inhibitor-1 and platelet activation (soluble P-selectin, monocyte–platelet aggregates) markers. This lacking benefit is in contrast to prior reports in the literature(Reference Mozaffarian and Wu7, Reference Borow, Nelson and Mason17, Reference Calder18). However, we observed rather low baseline values leaving little room for further improvement due to n-3 PUFA intake. One reason might be the optimised medical background therapy in our patients including high rates of AT-converting enzyme inhibitors, AT receptor blockers and statins for CV risk factor control.
Similarly, we also did not observe an effect of n-3 PUFA on ADMA, which is an endogenous nitric oxide synthase inhibitor associated with decreased nitric oxide expression and endothelial dysfunction. This is in line with prior data in patients after acute myocardial infarction, in whom n-3 PUFA also improved FMD but did not affect ADMA levels(Reference Haberka, Mizia-Stec and Mizia11). The authors hypothesised that ADMA measurements might not be the appropriate marker to evaluate drug efficacy with respect to endothelial function.
So far, the mechanisms of FMD improvement in our study remain unclear. Multiple physiological roles including effects on cell membrane structure, cell function and responses have been attributed to n-3 PUFA and this potential complex interplay is probably difficult to elucidate by measuring downstream markers(Reference Calder34). Refined techniques such as determination of patterns of gene expression could be necessary to clarify mechanistic pathways.
Strengthening our study, we used a prescription formulation containing n-3 PUFA that has been approved in the USA for the treatment of hypertriacylglycerolaemia (marketed as Lovaza) as well as in European countries (marketed as Omacor) for the treatment of hypertriacylglycerolaemia and for secondary prevention after myocardial infarction. In contrast, commonly used dietary supplements are not subject to the same manufacturing and regulatory standards and the accuracy of the stated amount of n-3 PUFA has been found to be inconsistent(Reference Bradberry and Hilleman35).
All study patients were claudicants managed in a dedicated vascular outpatient department that ensured good control of CV risk factors. We cannot exclude that the effect of n-3 PUFA supplementation would be different in a less stable PAD study population or patients with critical limb ischaemia.
Limitations
We did not register dietary habits at baseline or any potential dietary changes over the study period. However, substantial changes in the placebo group with respect to n-3 PUFA are unlikely as the omega-3 index measurements remained unchanged over time. Due to the small number of patients in the trial, no subgroup analyses were performed. Due to organisational issues, reproducibility of FMD measurements was not assessed. However, all FMD measurements in the study were performed at consistent conditions by the same experienced investigator following published guidelines.
Conclusions
In conclusion, our study reports a beneficial effect of high-dose n-3 PUFA supplementation on endothelial function in patients with PAD. This makes n-3 PUFA intake a potential candidate for reduction of the excess CV risk in PAD patients, which needs testing in large-scale trials.
Acknowledgements
This investigator-initiated study was funded through the department’s allocated research budget; no external funding was involved.
The authors' contributions were as follows: A. H.: Conception and design of the study, study execution and data acquisition, data analysis and interpretation, article writing and critical revising. D. M.: Conception and design of the study, study execution and data acquisition, data analysis and interpretation, article writing and critical revising. O. S.: Design of the study, study execution and data acquisition, data analysis and interpretation, article critical revising. M. M.: Data analysis and interpretation, article critical revising. D. S.: Design of the study, study execution and data acquisition, data analysis and interpretation, article critical revising. R. K.: Design of the study, study execution and data acquisition, data analysis and interpretation, article critical revising. S. S.: Conception and design of the study, study execution and data acquisition, data analysis and interpretation, article writing and critical revising.
None of the authors has any conflicts of interest to declare.








