Acute gastroenteritis is one of the most common diseases in humans, and continues to be a significant cause of morbidity and mortality worldwide [Reference Parashar1]. Despite much progress in the understanding of its pathogenesis, and its management with widespread use of oral rehydration therapies, diarrhoeal illness remains one of the most important causes of global childhood mortality and morbidity [Reference Freedman2]. Human sapoviruses, formerly known as Sapporo-like viruses, are single-stranded positive-sense RNA viruses in the family Caliciviridae [Reference Dey3]. Sapovirus infects both children and adults, and has been found to cause outbreaks of gastroenteritis in kindergartens, hospitals and mental healthcare facilities. Sapovirus-associated diarrhoea is usually mild, compared to that caused by norovirus [Reference Nguyen4]. Sapovirus was first named after its discovery in an outbreak of acute gastroenteritis in a children's home in Sapporo, Japan in October 1977. Sapoviruses have a typical ‘Star of David’ configuration by electron microscopy and can be divided into five genogroups (GI–GV), of which GI, GII, GIV and GV have been identified in humans [Reference Dey3]. Recently, the diversity of sapovirus was described in genogroups I and II, and could be classified into eight and five genotypes, respectively [Reference Akihara5]. Moreover, porcine enteric calicivirus has been reported to be a member of genogroup III [Reference Akihara5]. The objectives of this study were: to determine the seasonal trend and genotype distribution of sapovirus infection in Japan.
A total of 3232 faecal specimens were collected from infants and children with acute gastroenteritis in five different regions from north to south of Japan (Sapporo, Tokyo, Maizuru, Osaka, Saga) during July 2003 to June 2009. In this period, sapovirus analysis was performed systematically on all stool samples by reverse transcription–polymerase chain reaction. Sapovirus was further characterized for genotypes by sequencing methods. We determined the seasonality of sapovirus-associated disease by plotting the percentage of the total number of annual cases that occurred each month. Monthly distribution of sapovirus was defined after adjusting the epidemic curves according to a 3-month unweighted moving average (mean) [Reference Dey6]. The ‘peak’ month during the sapovirus season was then defined as that during which the greatest numbers of sapovirus-positive specimens were collected. We analysed the laboratory-confirmed sapovirus cases between 2003 and 2009. We used July as the start month of the sapovirus season for convenience, given that the annual sapovirus season began between October and March during the study period.
To ascertain any shift in the peak, the Mann–Whitney U test was used for analysis of relationship between duration from August to the peak month (beginning peak duration) during the six seasons. Inter-period differences in the mean were tested by one-way ANOVA followed by Mann–Whitney U test. All calculations were performed with SPSS for Windows version 13.0 (SPSS Inc., USA), and significance was concluded at P<0·05 [Reference Dey6].
Between 2003 and 2009, 3232 stool samples were received for microbiological study from patients aged <15 years with acute gastroenteritis. Sapovirus was detected in 123 (3·8%) patients. The annual number of patients detected with sapovirus in 2003–2009 was 14 (3·5%), 31 (4·8), 17 (2·8%), 24 (3·8%), 18 (3·8%), and 19 (4·0%), respectively (Table 1). The highest detection rates of sapovirus were in November (22·9%, 30/131) followed by December (13·7%, 18/131), March (12·8%, 17/131) and January (11·4%, 15/131) and the lowest detection rates were in July (1·5%, 2/131) followed by May (3%, 4/131), August (4·5%, 6/131) and September (4·5%, 6/131).
Table 1. Distribution of genogroups and genotypes of sapoviruses in Japan, 2003–2009
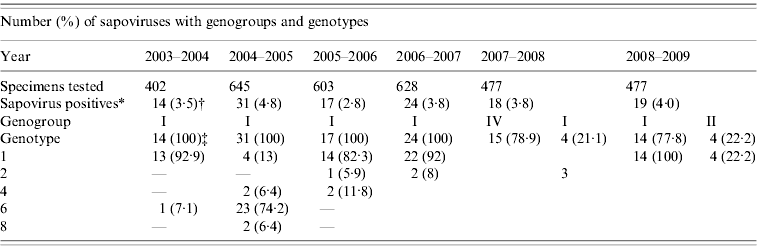
* Refers to monoinfection and mixed infection of sapoviruses.
† Refers to number of sapovirus positives over number of specimens tested.
‡ Refers to number of sapovirus types over number of sapovirus positives.
Seasonal decrease in sapovirus diarrhoea occurred annually in Japan (Fig. 1). Peak sapovirus activity occurred between November and March during the study period (2003–2009). The comparisons of sapovirus-positive cases (mean) for each month showed that there was no significant difference in each month. Sapovirus was detected all year round, during which two peaks occurred: one peak was apparently confined within a 4-month period (October–January) and the other was confined within a 3-month period (February–April). From this study, it is clear that sapovirus infection is common in winter and late winter season in Japan. The sapovirus peak in Japan was different from that of norovirus (November–January) and rotavirus (January–April) infection. A marked finding of this study was the low number of sapovirus-positive cases that occurred in the warm months of summer in Japan with the sapovirus peak starting a little earlier during 2004–2005. During the last 6 years, sapovirus GI/1 was the predominant strain in Japan followed by GI/6, GIV, GI/4, GI/8 and GII/1, respectively.
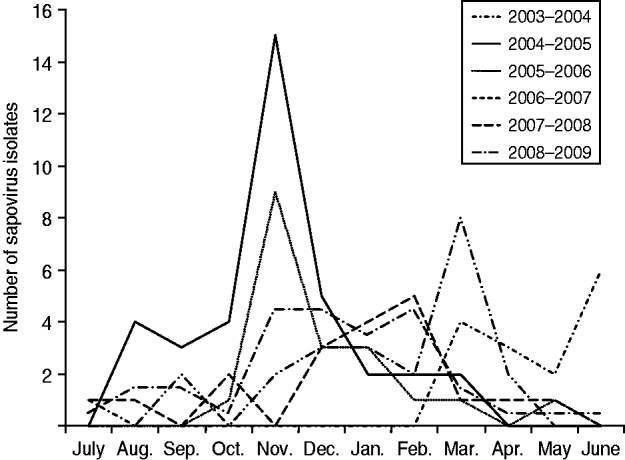
Fig. 1. Seasonal pattern of sapovirus infection in Japan, 2003–2009.
Sapovirus is one of the global causes of viral gastroenteritis and also associated with sporadic cases and outbreaks of gastroenteritis worldwide and its prevalence is shown to range from 0·3% to 9·3%. Seasonality of pathogens can be defined as the appearance of recurrent epidemics at defined periods of the year [Reference Dey7]. Sapovirus epidemic characteristics, and timing, are markedly inconsistent from year to year, with a peak incidence during wintertime. Many research groups in Japan have confirmed a large portion of gastroenteritis cases to be caused by sapovirus [Reference Dey7–Reference Chan-it10]. Our analysis, based on laboratory studies during the past 6 years, shows that the sapovirus peak was observed mainly in the cold season (November–March) in Japan, with statistical significance (Fig. 1, Table 1), in accord with laboratory data from five regions (Sapporo, Tokyo, Maizuru, Osaka, Saga) of Japan for 2003–2009. In most studies, seasonality did not vary much over long periods, although variations in the timing of peak activity have been reported. The typical pattern of regular sapovirus epidemics that appear in cold months in other countries is not similar to that observed in Japan in the 2003–2009 period of this study. Seasonality in disease incidence often reflects associations with weather factors [Reference Dey6].
Previously published data for 2003–2009 show sapovirus to be a universal cause of acute gastroenteritis in Japanese infants and children [Reference Dey7–Reference Chan-it10]. Sapovirus GI/1 was the predominant strain in Japan followed by GI/6, GIV, GI/4, GI/8 and GII/1, respectively, for 2003–2009. Sapovirus GII/4 was predominant during 2003–2004 (92·9%), 2005–2006 (92·9%), 2006–2007 (92%) and 2008–2009 (77·8%) (Table 2). However, a changed prevalence pattern of sapovirus genotypes was found for the years 2004–2005 and 2007–2008 with the emergence of GI/6 (74·2%) and GIV (78·9%) and a decrease of GI/1 for unknown reasons. During 2008–2009, GI/1 (~80%) remained the predominant genotype in the Japanese paediatric population; with GII/2 next (Table 2). We also detected some unusual genotypes of sapovirus such as GI/6 and GI/8 in 2004–2005. Some recombinant strains of sapovirus were also detected during the study periods. It should be noted that sapovirus shows great genomic diversity in the Japanese paediatric population.
Table 2. Comparison of mean of sapovirus cases in Japan, 2003–2009
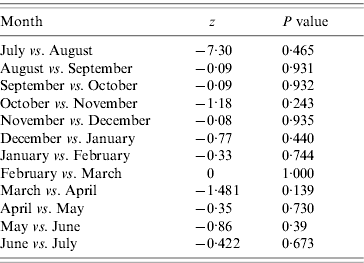
Mann–Whitney U test; P value <0·05 means significant.
In this study we found that sapovirus infection was mostly associated with gastroenteritis in infants and children aged <3 years. Over a 6-year period, the present study collected information on cases of sapovirus infection diagnosed and their epidemiological features in a specific geographic area and a well-defined population, i.e. children aged <15 years.
The data from our study demonstrate the cold weather predominance of sapovirus-associated disease in Japan. This finding was independent of the study setting, the age of the patients, or the detection methods used and may have implications concerning the route of transmission of sapovirus. Winter seasonal transmission is a key epidemiological feature of rotavirus, an important gastrointestinal pathogen for which airborne transmission has been postulated [Reference Dey6]. The speed with which sapovirus can spread through a community has led some investigators to postulate that airborne spread may also be important for these viruses. Although sapoviruses are primarily transmitted by the faecal–oral route, the winter seasonality of sapovirus-associated disease would lend support to airborne spread as a secondary route.
In conclusion, sapovirus is an important enteropathogen responsible for viral gastroenteritis in the paediatric population in Japan. The data about sapovirus seasonality is still limited. The winter seasonality of sapovirus provides important clues to the aetiology of gastroenteritis in infants and children in Japan.
ACKNOWLEDGEMENTS
We are grateful to Shuichi Nishimura, Hideaki Kikuta, Kumiko Sugita, Tsuneyoshi Baba, and Atsuko Yamamoto for faecal specimen collection. This study was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Sciences and Technology, and the Ministry of Health, Labour and Welfare, Japan.
DECLARATION OF INTEREST
None.





