Shorter telomere length has been found to be associated with cognitive deficits (Devore et al., Reference Devore, Prescott, De Vivo and Grodstein2011; Kingma et al., Reference Kingma, de Jonge, van der Harst, Ormel and Rosmalen2012; Pearce et al., Reference Pearce, Mann, Martin-Ruiz, Parker, White, von Zglinicki and Adams2012; Valdes et al., Reference Valdes, Deary, Gardner, Kimura, Lu, Spector and Cherkas2010; Yaffe et al., Reference Yaffe, Lindquist, Kluse, Cawthon, Harris, Hsueh and Cummings2011) and with stress-related conditions, including low birth weight (Davy et al., Reference Davy, Nagata, Bullard, Fogelson and Allsopp2009), affective psychiatric disorders (Elvsashagen et al., Reference Elvsashagen, Vera, Boen, Bratlie, Andreassen, Josefsen and Boye2011; Hartmann et al., Reference Hartmann, Boehner, Groenen and Kalb2010; Hoen et al., Reference Hoen, de Jonge, Na, Farzaneh-Far, Epel, Lin and Whooley2011; Karabatsiakis et al., Reference Karabatsiakis, Kolassa, Kolassa, Rudolph and Dietrich2014; Lung et al., Reference Lung, Chen and Shu2007; Simon et al., Reference Simon, Smoller, McNamara, Maser, Zalta, Pollack and Wong2006; Szebeni et al., Reference Szebeni, Szebeni, DiPeri, Chandley, Crawford, Stockmeier and Ordway2014; Verhoeven et al., Reference Verhoeven, Revesz, Epel, Lin, Wolkowitz and Penninx2014; Wikgren et al., Reference Wikgren, Maripuu, Karlsson, Nordfjall, Bergdahl, Hultdin and Norrback2012; Wolkowitz et al., Reference Wolkowitz, Mellon, Epel, Lin, Dhabhar, Su and Blackburn2011) and anxiety disorders (Hoen et al., Reference Hoen, Rosmalen, Schoevers, Huzen, van der Harst and de Jonge2013; Jergovic et al., Reference Jergovic, Tomicevic, Vidovic, Bendelja, Savic, Vojvoda and Sabioncello2014; Kananen et al., Reference Kananen, Surakka, Pirkola, Suvisaari, Lonnqvist, Peltonen and Hovatta2010; Malan et al., Reference Malan, Hemmings, Kidd, Martin and Seedat2011; O’Donovan et al., Reference O’Donovan, Epel, Lin, Wolkowitz, Cohen, Maguen and Neylan2011; Zhang et al., Reference Zhang, Hu, Benedek, Fullerton, Forsten, Naifeh and Ursano2014). However, the direction of causation underlying these associations is unclear.
Telomeres are protein-bound DNA repeat structures that form the ends of linear chromosomes (de Lange, Reference de Lange2002) and maintain genomic stability by protecting the chromosomal termini from end-to-end recombination and chromosomes loss (Blackburn et al., Reference Blackburn, Greider and Szostak2006). They are critical in regulating cellular replicative capacity (Allsopp et al., Reference Allsopp, Vaziri, Patterson, Goldstein, Younglai, Futcher and Harley1992). During somatic cell replication, telomere length progressively shortens because of the inability of DNA polymerase to fully replicate the 3′ end of the DNA strand. Once a critically-short TL is reached, the cell is triggered to enter replicative senescence, which subsequently leads to cell death (Allsopp et al., Reference Allsopp, Vaziri, Patterson, Goldstein, Younglai, Futcher and Harley1992; Blackburn et al., Reference Blackburn, Greider and Szostak2006). The average TL of most proliferating cells (including blood leukocytes) declines substantially with age (Blackburn, Reference Blackburn2001; Iwama et al., Reference Iwama, Ohyashiki, Ohyashiki, Hayashi, Yahata, Ando and Shay1998) and shortened TL has been found to be associated with risk for age-related diseases (Benetos et al., Reference Benetos, Gardner, Zureik, Labat, Xiaobin, Adamopoulos and Aviv2004; Brouilette et al., Reference Brouilette, Moore, McMahon, Thompson, Ford, Shepherd and Samani2007; Brouilette, et al., Reference Brouilette, Singh, Thompson, Goodall and Samani2003; Fitzpatrick et al., Reference Fitzpatrick, Kronmal, Gardner, Psaty, Jenny, Tracy and Aviv2007). In addition, shorter TL has been reported to be associated with a host of other conditions, including psychiatric disorders, cognitive deficits, psychological stress and low birth weight. However, whether telomere shortening actually contributes to these conditions or whether it is merely a consequence of these processes, remains unknown. A recent study showed the genomic variants that affect TL to be associated with various age-related diseases, suggesting a causal role for TL (Codd et al., Reference Codd, Nelson, Albrecht, Mangino, Deelen, Buxton and Samani2013). TL itself is, to a large extent, heritable (Broer et al., Reference Broer, Codd, Nyholt, Deelen, Mangino, Willemsen and Boomsma2013; Codd et al., Reference Codd, Nelson, Albrecht, Mangino, Deelen, Buxton and Samani2013; Nordfjall et al., Reference Nordfjall, Svenson, Norrback, Adolfsson and Roos2010; Slagboom et al., Reference Slagboom, Droog and Boomsma1994).
The comparison of the associations of intra-pair differences in birth weight with intra-pair differences in TL in MZ and DZ twin pairs is a strong design to explore whether the association between traits is mediated by genetic factors or whether causality plays a role (Boomsma et al., Reference Boomsma, Willemsen, de Geus, Kupper, Posthuma, Ijzerman, Dolan, Kordon, Gaillard and Christen2005). Differences in TL and birth weight within MZ pairs are caused by non-shared environmental factors, while differences within DZ pairs are influenced by both, environmental and non-shared genetic factors. A larger intra-pair difference in birth weight within TL-discordant DZ pairs than within TL-discordant MZ pairs would thus argue for correlated genetic factors between TL and birth weight.
The first aim of the present study was therefore to assess the association between intra-pair differences for TL and birth weight in MZ and DZ pairs in a large sample from the Brisbane Longitudinal Twin Study (BLTS). Our second aim was to reassess the association between intra-pair differences in TL and IQ (intelligence quotient) and TL and anxiety/depression in the MZ and DZ pairs. We obtained replication data for TL, birth weight, and anxiety/depression (but not IQ) from a large MZ and DZ twin pair sample from the Netherlands Twin Register (NTR). As further replication, we also obtained TL measurements from a small sample of newborn MZ twins from Melbourne who were extremely discordant or concordant for birth weight.
Materials and Methods
Discovery Sample: Brisbane Longitudinal Twins Study (BLTS)
Subjects: The BLTS, which began in 1992, is an ongoing, longitudinal population-based study of melanoma risk factors and cognition and comprises adolescent Australian MZ and DZ twins and their singleton close-age siblings (Wright & Martin, Reference Wright and Martin2004). Twins were enlisted by contacting the principals of primary schools in the greater Brisbane area, by media appeals, and by word of mouth. We estimate that approximately 50% of the eligible birth cohort was recruited into the study. Written, informed consent was obtained from all participants and a parent or guardian. The study was approved by the Human Research Ethics Committee at the QIMR Berghofer Medical Research Institute. Subjects were assessed at ages 12, 14, and 16 for psychiatric symptoms and blood was drawn at these ages. At age 16, twins completed an extensive cognitive test battery, including an IQ assessment. For TL measurement, mean age at accession of the DNA used was 14 years (SD 2.5 years) and only accessions collected on the same day for co-twins were used, which was the case for > 72.2% of twin pairs.
We used data only for pairs with TL measures available for both twins, and the present analyses include three overlapping subsamples of 1,772 subjects: (1) TL and birth weight were available for 242 MZ and 245 same sex DZ complete twin pairs; (2) TL and IQ for 203 MZ and 209 same sex DZ twin pairs; and (3) TL and mental well-being for 137 MZ and 171 DZ same sex twin pairs.
Measures: Psychiatric symptoms were assessed with the Somatic and Psychological Health Report (SPHERE), a 34-item self-report questionnaire that has been developed to screen for mental disorders such as anxiety and depression in general practice (Hickie et al., Reference Hickie, Davenport, Hadzi-Pavlovic, Koschera, Naismith, Scott and Wilhelm2001a; Hickie, et al., Reference Hickie, Davenport, Scott, Hadzi-Pavlovic, Naismith and Koschera2001b). Participants indicated if they had been troubled by symptoms over the past few weeks, making one of three response choices: sometimes/never (coded as zero); often; most of the time (each coded as 1). The total score of all 34 items was used as a measure of anxiety/depression.
At age 16, twins completed three verbal subtests (Information, Arithmetic, Vocabulary) and two performance subtests (Spatial and Object Assembly) from a shortened version of the Multidimensional Aptitude Battery (Jackson, Reference Jackson1984), an extensive cognitive test battery that measures, among others, the IQ.
Mothers of twins and siblings were asked for the birth weight of their children at both the age 12 and 14 visits, or at the age 16 visit, for those twins who were not assessed at age 12 or 14. The birth weight reported at age 12 was used for analyses, being the more proximal measure. If reported birth weight at age 12 was not available, reported birth weight at age 14 or 16 was used. The correlation between the reports at age 12 and 14 respectively, was 0.95.
Replication sample: Netherlands Twin Register (NTR)
Subjects: The NTR was established in the late 1980s (Boomsma et al., Reference Boomsma, Vink, van Beijsterveldt, de Geus, Beem, Mulder and van Baal2002; Reference Boomsma, de Geus, Vink, Stubbe, Distel, Hottenga and Willemsen2006). Most twins were recruited through city councils between 1990 and 1993, when they were adolescents or young adults. Detailed information on the longitudinal survey study and the NTR biobank project has been provided previously (Boomsma et al., Reference Boomsma, de Geus, Vink, Stubbe, Distel, Hottenga and Willemsen2006; Willemsen et al., Reference Willemsen, Vink, Abdellaoui, den Braber, van Beek, Draisma and Boomsma2013). The current analysis included twins who participated in the NTR biobank project, conducted between 2004 and 2008 (Willemsen et al., Reference Willemsen, de Geus, Bartels, van Beijsterveldt, Brooks, Estourgie-van Burk and Boomsma2010). Data on birth weight and TL were available for 514 complete MZ pairs and for 233 DZSS pairs, of whom 437 MZ pairs and 176 DZSS pairs also had data on depression. Blood was drawn when the twins were aged 17–80 (mean age at TL measurement 36 years, SD 11 years). Informed consent was obtained from participants and study protocols were approved by the Medical Ethics Committee of the VU University Medical Centre.
Measures: Data on birth weight were collected as part of multiple NTR surveys and projects. Data reported by the twins themselves and/or by their parents were combined and consistency across family members and time was checked. When multiple data points differed, the average was taken, but only if the difference was less than 200 grams. When possible, data were also checked against reports from National Youth Health Services.
Data on depression were collected in multiple NTR surveys. For the current analyses, depression scores from NTR surveys 5 (conducted in 2000), 6 (2002), 8 (2009), and 9 (2011) were used. In NTR surveys 5 and 6, the Young Adult Self-Report Score (YASR; Achenbach, Reference Achenbach1997) was used to score depressive symptoms and in survey 8 and 9, the Adult Self-Report (Achenbach & Rescorla, Reference Achenbach and Rescorla2003) was collected as part of the survey. Within surveys, depression scores were converted to z scores. To obtain one score for each subject, the depression z score was selected from the time point closest to the moment of blood draw, for which both twins had data (i.e., data for co-twins were selected from the same survey).
Replication sample: Melbourne peri/postnatal epigenetics twin study (PETS)
The longitudinal Peri/postnatal Epigenetics Twin Study (PETS) is a cohort of 251 twin pairs and their mothers, recruited midway through pregnancy (Loke et al., Reference Loke, Novakovic, Ollikainen, Wallace, Umstad, Permezel and Craig2013; Saffery et al., Reference Saffery, Morley, Carlin, Joo, Ollikainen, Novakovic and Craig2012). To date, lifestyle/environment data and tissue samples have been collected at birth and at 18 months of age, with six-year follow-up currently underway. Mothers are predominantly of white European descent. For the current study, we used purified cord blood mononuclear cells (CBMCs) from MZ twin pairs that were either highly discordant (7 pairs, birth weight difference 456 g to 991 g, mean difference 613 g) or concordant in birth weight (12 pairs, birth weight difference 40 g to 155 g, mean difference 91 g).
TL measurement and quality control analysis
TL was measured in the Brisbane and Amsterdam samples in the same laboratory at the University of Leicester, and the Melbourne samples in the laboratory at the Murdoch Childrens Research Institute. Both laboratories used the same established quantitative PCR–based technique (Cawthon, Reference Cawthon2002, Reference Cawthon2009). In brief, this method expresses TL as a ratio (T/S) of telomere repeat length (T) to copy number of a single copy gene (S), in each sample. Samples were measured in duplicate for both the telomere (T) and 36B4 (S) assays. The single-copy gene, 36B4, serves as a reference gene in the conventional qPCR for telomere measurement (Cawthon, Reference Cawthon2002). To minimize inter-assay variation, the T/S ratio was calculated relative to a calibrator sample (DNA from the K562 cell line) run on each PCR plate. Any sample that was outside the linear range of the assay was diluted and rerun until results were observed within the linear range. For quality control, all samples were checked for concordance between duplicate values. Samples showing a difference between the duplicate measurements of greater than 0.2 cycles were excluded and rerun. To ensure reproducibility of the assay, samples were regularly rerun at random on a different day and/or machine. All samples reproduced well (r 2 = 0.97, p < .0001).
Statistical analyses
In the Brisbane and Amsterdam twins, T/S ratio was z-standardized and birth weight was transformed using the square root (Blair et al., Reference Blair, Liu, de Klerk and Lawrence2005). In the Brisbane twins, Blom rank normalization was applied for the IQ score, and the SPHERE-34 item responses of the repeated measurements at ages 12, 14 and 16 were transformed into theta scores according to the principles of Item Response Theory (Wray et al., Reference Wray, Coventry, James, Montgomery, Eaves and Martin2008). In the Amsterdam twins, the depression score was z-standardized. Analyses were calculated in IBM SPSS statistics 20.0 (http://www.spss.com). TL was corrected for sex, age at blood withdrawal, and age squared. To minimize the impact of age and laboratory batch variation, we used an intra-twin pair design, using only DZSS pairs. Where intra-pair differences were being correlated for different variables, the same order of twins was preserved for each phenotype.
Results
Table 1a shows the number of pairs and intra-pair differences for the raw TL, birth weight, IQ, and anxiety/depression scores in the three overlapping subsamples from Brisbane, and Table 1b likewise for the Amsterdam and Melbourne samples. Mean age of samples at TL analysis for Brisbane was 14 (SD 2.5), Amsterdam 36 (SD 11), and Melbourne twins were all newborns.
TABLE 1a Absolute Intra-Pair Differences (Diff.) for Monozygotic and Dizygotic Twin Pairs of Raw Telomere Length (TL), Birth Weight, IQ, and Anxiety/Depression Scores in the Three Subsamples of the Brisbane (Discovery) Sample

Differences = diff., TL = telomere length, Monozygotic = MZ, Dizygotic = DZ.
TABLE 1b Absolute Intra-Pair Differences for MZ Twin Pairs of Raw TL, Birth Weight, and Depression Scores in the Two Replication Samples

Pearson correlations between intra-pair differences in TL, birth weight, IQ, and anxiety/depression in MZ and DZ twin pairs are shown in Table 2. We found a significant correlation between intra-pair differences in TL and birth weight in MZ twin pairs (r = 0.149, p = .021, 95% CI: 0.023–0.270) with the heavier twin having higher TL, but not in DZ pairs (r = 0.048, p = 0.457, 95% CI: –0.078–0.172). We also found a significant correlation between intra-pair differences in TL and IQ in MZ (r = 0.285, p < 0.001, 95% CI: 0.153–0.407), and in the DZ pairs (r = 0.149, p = 0.031, 95% CI: 0.014–0.279). Correlations between intra-pair differences in birth weight and IQ are positive and appreciable in both MZ (p = 0.269, p < 0.001, 95% CI: 0.138–0.391) and DZ twins (r = 0.204, p = 0.003, 95% CI: 0.070–0.331), indicating that the heavier twin at birth has higher IQ, and these correlations remained significant when controlled for birth weight. Correlations with anxiety/depression are all small and non-significant, indicating that low birth weight, shorter TL or lower IQ are not associated with teenage mental health.
TABLE 2a Pearson Correlations of Intra-Pair Differences in Telomere Length (TL), Birth Weight, IQ, and Anxiety/Depression in Monozygotic (MZ, Above the Diagonal) and Same Sex Dizygotic (DZ) Twin Pairs (Below Diagonal) of the Brisbane Sample. p Values are Two-Tailed.
M Z twins (113– 242 pairs)

D Z twins (171–245 pairs)
* p ≤ 0.05 ** p ≤ 0.01 *** p ≤ 0.001.
In the replication sample from Amsterdam, there was a significant correlation between the intra-pair difference in TL and birth weight in the MZ (r = 0.092, p = 0.038, 95% CI: 0.006–0.177), but not in the DZ twin pairs (r = –0.037, p = 0.572, 95% CI: –0.165–0.092). We found no evidence for a relationship between intra-pair difference in depression and intra-pair difference in TL, either in MZ twins (r = –0.056, p = 0.244, 95% CI: –0.149–0.038) or DZ twins (r = –0.108, p = 0.155, 95% CI: –0.252–0.041). Neither did the intra-pair difference in depression correlate with the intra-pair difference in birth weight in MZ (r = –0.043, p = 0.365, 95% CI: –0.136–0.051) or DZ twin pairs (r = 0.027, p = 0.720, 95% CI: –0.121–0.174). These findings support the results in the Brisbane sample.
To further explore whether the correlation of the intra-twin pair difference in birth weight and TL was carried by MZ twin pairs with an extreme difference in birth weight, we calculated the difference in birth weight between the heavier and the lighter twin (BWH-L) and the corresponding difference TLH-L. We then split the Brisbane MZ twin pairs into two groups, that is, in the most birth weight discordant twin pairs (top 10%: N = 24 twin pairs with difference in birth weight ranging between 652 and 1,200 g and a mean difference of 882 g) and least birth weight discordant in twin pairs (bottom 90%: N = 218 twin pairs with difference in birth weight ranging between 0 g and 624 g with a mean difference of 222 g). The Amsterdam MZ twin pairs were also split into the top 10% (51 pairs, birth weight difference 684–1,500 g, mean difference 971 g) and bottom 90% birth weight difference groups (463 pairs, birth weight difference 0–650 g, mean difference 206 g). For the Melbourne sample, we compared the mean of the seven most birth weight discordant pairs compared with the 12 least discordant (Figure 1). For all three samples, the results went in the same direction of the most birth weight discordant twin pairs having larger TL differences, with the lighter twin having shorter TL, but t-tests revealed that this difference was only significant for Brisbane (p = 0.001, one-tail), but not for Amsterdam (p = 0.288) or Melbourne (p = 0.469). The combined one-tail p value for all three samples is p = 0.0145.
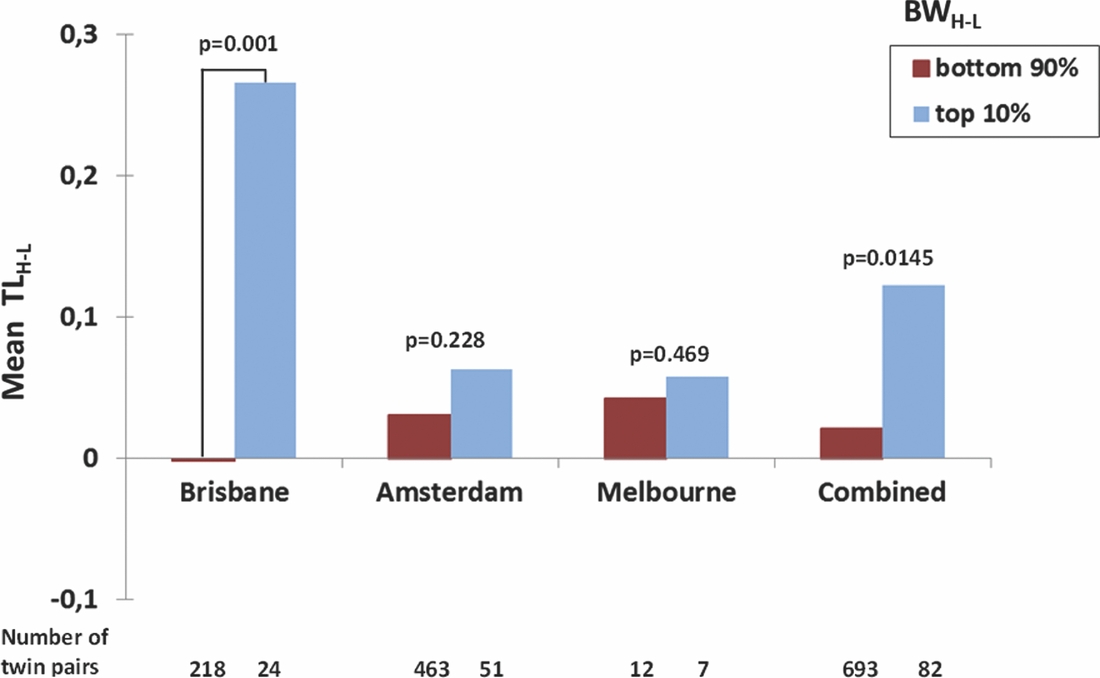
FIGURE 1 Mean TLH-L (Difference in TL between the Heavier and the Lighter Twin) in the Top 10% and Bottom 90% of BWH-L Values (Difference in BW between the Heavier and the Lighter Twin) in the Brisbane, Amsterdam, and Melbourne and Combined Monozygotic Twin Pairs.
Our top 10% cut-off was somewhat arbitrary and, being a relative measure, differs between samples, so we further investigated in an explorative way the influence of the absolute threshold in birth weight difference (BWH-L) in grams on the association between birth weight and TL difference. For this purpose, we dichotomized both birth weight difference and TL difference as follows: birth weight difference was coded as ‘+’ if twin 1 was the heavier twin and ‘–’ if twin 2 was the heavier twin. Similarly, TL difference was coded as + if twin 1 had longer telomeres and – if twin 2 had longer telomeres. A chi-square contingency test was then applied to test whether the heavier twin also showed larger TL and vice versa. We did this at different BWH-L cut-offs (> 0 g, > 100 g, > 200 g, > 300 g, > 400 g, > 500 g, > 600 g) in the Brisbane, Amsterdam and Melbourne MZ twin pairs and the combined MZ twin pair sample; that is, first we included all pairs with a BWH-L > 0 g, then all pairs with a BWH-L > 100 g and so on. If there is a very strong relationship between birth weight discordance and TL difference, then we should expect to see a stronger association in twin pairs with a higher minimum birth weight difference. In fact, we observed an increase of the effect size (Phi, OR) until a cut-off at BWH-L 500 g, and then the effect size decreased, probably because of limited power due to decreasing number of twin pairs per cell (Table 3).
TABLE 2b Pearson Correlations of Intra-Pair Differences in TL, Birth Weight, and Depression in MZ (Above the Diagonal) and Same Sex DZ Twin Pairs (Below Diagonal) of the Amsterdam Sample. p Values are Two-tailed MZ twins (437–514 pairs)

D Z twins (176–233 pairs)
* p ≤ 0.05 ** p ≤ 0.01 *** p ≤ 0.001
TABLE 3 Significance of Association in Monozygotic (MZ) Pairs of the Concordance of Direction of the Twin1–Twin2 Differences in Telomere Length with the Twin1– Twin2 Differences in Birth Weight, Depending on Cut-Off for Absolute Birth Weight Difference (BWH-L), by Study and for the Combined Sample. One-Tailed p-Values are Given

Discussion
We assessed the association of TL with birth weight, IQ and anxiety/depression in a large Brisbane sample of MZ and same sex DZ twin pairs, making use of intra-pair differences. In MZ twin pairs, the intra-pair difference in TL was significantly correlated with the intra-pair difference in birth weight (r = 0.149, p = 0.021), but not in DZ twin pairs (r = 0.048). We replicated this pattern in the twin pairs from Amsterdam (MZ: r = 0.092, DZ: r = –0.037), suggesting that the association between birth weight and TL is not mediated by genetic factors but by non-shared environmental factors. In the Brisbane MZ twin pairs, the intra-pair difference in TL was correlated with the intra-pair difference in IQ (0.285, p < 0.001); also, the intra-pair difference in IQ was correlated with the intra-pair difference in birth weight (0.269, p < 0.001). Both correlations were lower but substantial in the DZ twin pairs (r = 0.149, p = 0.031; r = 0.204, p = 0.003, respectively) suggesting that these associations are mediated by environmental and/or non-shared genetic factors.
We further hypothesized that if the same factors that cause an extreme discordant birth weight difference in the MZ twin pairs also influence TL length in both co-twins, then the correlation between TL and birth weight should be stronger in the MZ twin pairs most highly discordant in birth weight. Indeed, in the Brisbane MZ twin pairs the correlation between TL and birth weight was only significant in 10% of the most birth weight discordant MZ twin pairs but not in the twin pairs with a low-to-medium birth weight difference. An effect in the same direction –– although non-significant –– was also present in the MZ twin pairs from Amsterdam and a second replication sample from Melbourne. In all three samples, the most birth weight discordant twin pairs had larger TL differences, with the lighter twin having shorter TL. Further analysis using increasing thresholds of MZ birth weight discordance showed steadily increasing effects on TL up to a 500 g discordance, beyond which our number of twin pairs per cell became too small to obtain reliable results.
These findings indicate that prenatal factors that decrease birth weight in one twin more than the other have a negative influence on TL in the lighter twin. This relationship between low birth weight and shorter TL has been found previously in placenta samples (Davy et al., Reference Davy, Nagata, Bullard, Fogelson and Allsopp2009) but was not found in a small study measuring TL in cord blood (Akkad et al., Reference Akkad, Hastings, Konje, Bell, Thurston and Williams2006) nor in three cohorts (including 124 MZ and DZ twin pairs) from Finland (Kajantie et al., Reference Kajantie, Pietilainen, Wehkalampi, Kananen, Raikkonen, Rissanen and Hovatta2012). These negative reports do not necessarily contradict our findings. In our study, we only observed an association between birth weight and TL within MZ twin pairs, most strongly in those highly discordant for birth weight. We did not observe an association when analyzing the twins in a conventional between-subject analysis, even allowing for relatedness, and when analyzing within twin pair differences in DZ pairs. Thus one could hypothesize that in DZ twins and the general population, the genetic effects influencing TL may override the perinatal factors. Also, we found the strongest associations between the intra-pair difference in TL and birth weight in the Brisbane sample, in which TL was measured at a mean age of 14 years, and a smaller association in the Amsterdam sample where TL was measured at a mean age of 36 years. The Finnish twins were young adults when TL was assessed. In older age, the effects of prenatal stress on TL may weaken and be overlaid by other postnatal factors influencing TL such as, for example, environmental stress factors (Ahola et al., Reference Ahola, Siren, Kivimaki, Ripatti, Aromaa, Lonnqvist and Hovatta2012; Drury et al., Reference Drury, Theall, Gleason, Smyke, Vivo, Wong and Nelson2012; Reference Drury, Mabile, Brett, Esteves, Jones, Shirtcliff and Theall2014; Humphreys et al., Reference Humphreys, Epel, Cooper, Lin, Blackburn and Lee2012; Kananen et al., Reference Kananen, Surakka, Pirkola, Suvisaari, Lonnqvist, Peltonen and Hovatta2010; O’Donovan et al., Reference O’Donovan, Epel, Lin, Wolkowitz, Cohen, Maguen and Neylan2011; Shalev et al., 2013b; Surtees et al., Reference Surtees, Wainwright, Pooley, Luben, Khaw, Easton and Dunning2011; Tyrka et al., Reference Tyrka, Price, Kao, Porton, Marsella and Carpenter2010; Uchino et al., Reference Uchino, Cawthon, Smith, Light, McKenzie, Carlisle and Bowen2012).
Our findings suggest that low birth weight per se is not associated with shorter TL, but that specific non-shared prenatal factors in MZ twins lead to TL shortening. Prenatal factors that could potentially account for our findings are placental differences between MZ and DZ twins and between monochorionic and dichorionic MZ twins (for review, see Loos et al., Reference Loos, Ridgway, Ong, Lawlor, Lawlor and Mishra2009). Peripheral umbilical cord insertion on the placenta and fused dichorionic placentas occur more often or have less favorable outcomes (including lower birth weight) in MZ than in DZ twins. Also, monochorionic MZ twins who share one placenta have a higher prevalence of vascular anastomoses, velamentous cord insertion and single umbilical arteries, which may result in an unequal supply of blood to one of the co-twins. These placental differences between MZ co-twins and MZ and DZ twins may account for our finding that the intra-twin pair correlation between TL and birth weight was only significant in the highly birth weight discordant MZ twin pairs, and not in the DZ pairs. A very severe form of unfavorable placentation of one twin is the twin-to-twin transfusion syndrome (TTTS). TTTS complicates 8–10% of twin pregnancies with monochorionic diamniotic (MCMZ) pregnancy (Simpson, Reference Simpson2013). In such cases, connections (anastomoses) are formed between placental blood vessels from each twin. This results in unequal blood flow between the twins, which in turn causes one sac to increase in size at the expense of the other, resulting in high birth weight discordance (Simpson, Reference Simpson2013). As a result, both twins have a higher risk of mortality and morbidity including compromised neurodevelopment. We hypothesized that a common cause of the correlation of short TL with lower IQ was the extremely low birth weight of twins who had suffered TTTS. However, direct reporting of TTTS was only available for the Melbourne sample, and the one confirmed MZ pair with TTTS had the second highest birth weight difference (746 g) and the fifth highest difference in TL.
We also observed a higher correlation of the intra-pair differences in TL and IQ and IQ and birth weight in MZ pairs, suggesting that the same non-shared prenatal factors that account for the association of TL and birth weight may also mediate the association between TL and IQ, that is, less favorable placentation in MZ twins may increase the risk for placental undersupply in one co-twin and affect cognitive ability in later life.
Nutrition is an important factor in the development of the brain (Kretchmer et al., Reference Kretchmer, Beard and Carlson1996). Micronutrient deficiencies (iodine, iron, zinc, and vitamin B-12) during pregnancy and malnutrition from early childhood on (Engle & Fernandez, Reference Engle and Fernandez2010; Grantham-McGregor, Reference Grantham-McGregor1995) have found to negatively influence neurocognitive development and cognitive function (Black, Reference Black2003; Blusztajn & Mellott, Reference Blusztajn and Mellott2012; Larque et al., Reference Larque, Gil-Sanchez, Prieto-Sanchez and Koletzko2012; Mitka, Reference Mitka2013; Nyaradi et al., Reference Nyaradi, Li, Hickling, Foster and Oddy2013; Zimmermann, Reference Zimmermann2007). Also, associations between low birth weight and cognition have been described repeatedly (Shenkin et al., Reference Shenkin, Starr and Deary2004; Singh et al., Reference Singh, Kenney, Ghandour, Kogan and Lu2013). Finally, there are findings that suggest an influence of micronutrient deficiencies on TL (Moores et al., Reference Moores, Fenech and O’Callaghan2011; Paul, Reference Paul2011). All these studies in combination with our findings suggest that placental undersupply may have a negative influence on TL and IQ in adolescence and young adulthood. The correlations between TL and IQ (0.149, p = 0.031) and IQ and birth weight (r = 0.204, p = 0.003) in DZ twins however suggests that in these associations non-shared genetic or further non-shared environmental effects that also affect DZ twins may also play a role.
We did not observe an association between TL and anxiety/depression, either in the Brisbane sample or in the replication sample from Amsterdam. Previous studies on the association between TL and longitudinally assessed psychiatric symptoms and psychiatric disorders are largely inconsistent. Hoen et al. (Reference Hoen, Rosmalen, Schoevers, Huzen, van der Harst and de Jonge2013), for example, reported in a prospective study that persistence of anxiety disorders was associated with shorter TL measured after 2 years follow-up, but depressive disorders did not. Their results indicate that at least anxiety symptoms may have a negative impact on TL. The authors argue that the physiological hyperarousal and physiological stress associated with anxiety disorders may cause telomere damage, consistent with other studies linking psychological stress with shorter TL (Ahola et al., Reference Ahola, Siren, Kivimaki, Ripatti, Aromaa, Lonnqvist and Hovatta2012; Drury et al., Reference Drury, Theall, Gleason, Smyke, Vivo, Wong and Nelson2012; Reference Drury, Mabile, Brett, Esteves, Jones, Shirtcliff and Theall2014; Entringer et al., Reference Entringer, Epel, Kumsta, Lin, Hellhammer, Blackburn and Wadhwa2011; Reference Entringer, Epel, Lin, Buss, Shahbaba, Blackburn and Wadhwa2013; Epel et al., Reference Epel, Blackburn, Lin, Dhabhar, Adler, Morrow and Cawthon2004; Humphreys et al., Reference Humphreys, Epel, Cooper, Lin, Blackburn and Lee2012; Kananen et al., Reference Kananen, Surakka, Pirkola, Suvisaari, Lonnqvist, Peltonen and Hovatta2010; O’Donovan et al., Reference O’Donovan, Epel, Lin, Wolkowitz, Cohen, Maguen and Neylan2011; Price et al., Reference Price, Kao, Burgers, Carpenter and Tyrka2013; Shalev et al., Reference Shalev, Entringer, Wadhwa, Wolkowitz, Puterman, Lin and Epel2013a; Shalev et al., Reference Shalev, Moffitt, Sugden, Williams, Houts, Danese and Caspi2013b; Surtees et al., Reference Surtees, Wainwright, Pooley, Luben, Khaw, Easton and Dunning2011; Tyrka et al., Reference Tyrka, Price, Kao, Porton, Marsella and Carpenter2010; Uchino et al., Reference Uchino, Cawthon, Smith, Light, McKenzie, Carlisle and Bowen2012). On the other hand, there are also studies that do not support such an association, among them a most recent study assessing life stress in a 30-year birth cohort (Jodczyk et al., Reference Jodczyk, Fergusson, Horwood, Pearson and Kennedy2014; Phillips et al., Reference Phillips, Robertson, Carroll, Der, Shiels, McGlynn and Benzeval2013; Rius-Ottenheim et al., Reference Rius-Ottenheim, Houben, Kromhout, Kafatos, van der Mast, Zitman and Giltay2012). Our findings thus add further support to the assumption that shorter TL is not per se a risk factor for psychiatric disorders. Rather, psychiatric disorders and the psychological and physiological stress associated with them may possibly influence TL shortening.
Our study has several strengths. First, to our knowledge, it is the largest study and the largest twin study to date investigating the relation between TL and IQ, and the largest twin study investigating the association between TL and birth weight and TL and anxiety/depression. Second, our study design, that is, the investigation of correlations of intra-pair differences separately in MZ and DZ pairs, allowed us to disentangle whether non-shared genetic factors and/or environmental factors account for the association between TL and cognition. Finally, we replicated our results that TL does not appear to affect anxiety/depression later in life, in two independent twin samples.
Our study also has several limitations. First, we did not consider –– because data were not available –– monochorionic and dichorionic twins separately in our analyses, which may have elucidated whether our findings are the result of these placental factors. Second, we were not able to disentangle potential shared environmental effects such as, for example, maternal psychosocial stress during pregnancy, on the association of TL and birth weight. Third, in the Brisbane sample, anxiety/depression was measured in adolescence and we do not know whether our study participants will develop psychological problems at some point later in life (they are currently being followed up in their 20s). However, in the Amsterdam sample depression was measured in middle age and still we saw no association between TL and depression. Fourth, TL was not measured at birth in the Brisbane and Amsterdam twins. However, it was measured at birth in the Melbourne sample in which we observed a correlation between TL and birth weight in the same direction as in the two other samples –– although non-significant. Fifth, although the SPHERE is a screening instrument for mental disorders such as anxiety and depression in general practice (Hickie et al., Reference Hickie, Davenport, Hadzi-Pavlovic, Koschera, Naismith, Scott and Wilhelm2001a; Hickie et al., Reference Hickie, Davenport, Scott, Hadzi-Pavlovic, Naismith and Koschera2001b), it is a self-rating questionnaire and not a diagnostic instrument such as, for example, the SCID-I (Structured Clinical Interview for DSM-IV Axis I Disorders), which gives valid psychiatric diagnoses. Sixth, stochastic effects have also influenced our intra-twin pair correlations; however, the magnitude of these effects is unknown. Finally, one of our most important results –– the correlation between TL and birth weight in the 10% most birth weight discordant MZ twin pairs –– was non-significant in the Amsterdam and Melbourne samples. In the Melbourne sample, the directional effect is there throughout the range of birth weight discordance (Table 3), but the small sample size, which decreases the power to detect an effect, may explain the non-significance. In the Amsterdam sample, the higher age of the twins may be an important factor in diminishing the effect of birth weight on current TL. Nevertheless, as Table 3 shows, in the Amsterdam sample the directional effect is there throughout the range of MZ birth weight discordance and is significant at the 100 g, 200 g, and 300 g thresholds, even with a low-powered nonparametric test.
In summary, our findings suggest that environmental effects that occur prenatally and decrease birth weight have a negative influence on TL. Our findings further indicate that the association between TL and IQ may partly be driven by the same perinatal effects that decrease birth weight, although the positive correlation in DZ twins suggests genetic factors may also play a part. Finally, we have shown that twin pairs who are discordant for TL and birth weight do not differ in anxiety/depression later in life.
Acknowledgments
Brisbane Longitudinal Twin Study: We thank the Brisbane twins and siblings for their participation; Marlene Grace and Ann Eldridge for sample collection; Kerrie McAloney for study coordination; Anthony Conciatore for IT support, and Leanne Wallace and the Molecular Genetics Laboratory for sample preparation. The research was supported by the Australian Research Council (A7960034, A79906588, A79801419, DP0212016, DP0343921) and by the National Health and Medical Research Council (389891, 1049911, 1069141). Measurement of telomere length (by VC in the laboratory of NS) was partially funded by the EU ENGAGE consortium (FP7-HEALTH-F4-2007-201413).
Netherlands Twin Register: We would like to thank all twins and family members and the funding agencies for their support; the Netherlands Organisation for Scientific Research (NWO 900-562-137, 904-61-090, 985-10-002, 904-61-193, 56-464-14192, 400-03-330, 480-04-004, 400-07-080, 911-09-032, 451-06-004, 451-08-026, 451-10-005), the Netherlands Organisation for Health Research and Development (ZonMW 3100.0038, 940-37-024, 31160008), EMGO + Institute for Health and Care Research, Neuroscience Campus Amsterdam, BBMRI -NL (184.021.007: Biobanking and Biomolecular Resources Research Infrastructure), National Institutes of Health (NIH 5R37DA018673-03, R01 MH059160, 1RC2 MH089951-01, 4R37DA018673-06, 1R01 MH087646-01A1), National Institute of Mental Health (RFA MH08120), Brain and Behavior Research Foundation (2011 NARSAD Distinguished Investigator Grant; 18633), FP7 ENGAGE (FP7-HEALTH-F4-2007-201413), European Research Council (230374-GMI, 284167), Rutgers University (3797).
Melbourne Peri/postnatal Epigenetics Twin Study: We wish to thank Ruth Morley, John Carlin, Mark Umstad, Euan Wallace, Michael Permezel, Sarah Healy, Tina Vaiano, Nicole Brooks, Jennifer Foord, Sheila Holland, Anne Krastev, Siva Illancheran and Joanne Mockler, Xin Li, Ji Hoon E. Joo, Anna Czajko, Geraldine McIlroy for their contributions to PETS and all mothers and twins who participated in this study. RS is supported by an NHMRC Senior Research Fellowship and BN by an NHMRC CJ Martin Early Career Fellowship. JMC is supported by the NHMRC, the Australian Twin Registry and the MCRI. PETS is supported by the Victorian Government Operational Infrastructure support program.
DN is supported by the Australian Research Council (FT0991022) and National Health and Medical Research Council (APP0613674). The visit of JS to QIMR was supported by the grant ‘Exzellenzinitiative II / Maßnahme 7 / Mobilitätsmaßnahmen im Rahmen der internationalen Forschungskooperation 2013–2014’ from the University of Heidelberg. LR visited QIMR with support from the Psychosis Centre, Department for Psychiatry and Psychotherapy, University Medical Center Hamburg-Eppendorf, Hamburg, Germany. Support was also received from the NHMRC-EU Project Grant ID-496739.








