Introduction
Globally, many shorebird populations are in decline, particularly those on the East Asian Australasian Flyway (Stroud et al. Reference Stroud, Baker, Blanco, Davidson, Delany, Ganter, Gill, González, Haanstra, Morrison, Piersma, Scott, Thorup, West, Wilson, Zöckler, Boere, Galbraith and Stroud2006). With resident species it is easier to determine the factors behind population changes (e.g. McCulloch 2008) but with migratory species that cover vast distances it can be very difficult to determine the causes of decline and their relative importance, since birds can be impacted by changes on breeding and wintering grounds and on staging sites in between, in all or any combination.
The Spoon-billed Sandpiper Eurynorhynchus pygmeus (IUCN category: Critically Endangered) is a migratory shorebird whose breeding range is restricted to the Russian far north-east in Chukotka and Koryakya, where it nests along a discontinuous narrow strip of 4,500 km of coastal tundra (Zöckler and Lappo Reference Zöckler and Lappo2008). It migrates to the south-west and passes through key staging sites such as Kamchatka, Korea and Japan to winter across a wide area in South China, Vietnam, Thailand, Myanmar and Bangladesh. Indications of a major decline in the breeding population since the first population estimate in 1977 were found in 2000 (Tomkovich et al. Reference Tomkovich, Syroechkovskiy, Lappo and Zöckler2002), and this led to a series of expeditions to the breeding areas between 2000 and 2009 to monitor numbers and collect information on breeding ecology.
The species has only once been recorded breeding further than 5 km from the coast (7 km) and uses only limited types of habitats, mainly lagoon spits with crowberry-lichen vegetation or dwarf birch, willows and sedges (Tomkovich Reference Tomkovich1995, Tomkovich et al. Reference Tomkovich, Syroechkovskiy, Lappo and Zöckler2002, Zöckler Reference Zöckler2003, Syroechkovskiy Reference Syroechkovskiy and Straw2005). In this paper we use count data to assess the rates of decline and determine the current population size. Using resightings of individually marked birds to estimate adult survival and recruitment and studies of individual nests and broods to estimate productivity, we diagnose the demographic causes behind the observed declines.
Methods
Historic and current population decline
We estimated changes in the numbers of birds on the breeding grounds over the past 30–40 years based on our survey activities (2000–2009) and from the literature (Flint and Kondratiev Reference Flint and Kondratiev1977, Krechmar et al. Reference Krechmar, Andreev and Kondratyev1978, Tomkovich Reference Tomkovich1995, Tomkovich and Soloviev Reference Tomkovich and Soloviev2000, Tomkovich et al. Reference Tomkovich, Syroechkovskiy, Lappo and Zöckler2002, Syroechkovskiy Reference Syroechkovskiy and Straw2005, Zöckler et al. Reference Zöckler, Syroechkovskiy, Lappo, Bunting, Boere, Galbraith and Stroud2006, Syroechkovskiy et al. Reference Syroechkovskiy, Tomkovich, Kashiwagy, Lappo, Zöckler, Buzun and Taldenkov2010). Spoon-billed Sandpipers show a high degree of site-fidelity to their breeding grounds between years (Tomkovich Reference Tomkovich and Kurochkin1994, Reference Tomkovich2003) and trend data were available for more than 70% of known breeding sites, i.e. sites that have been visited at least twice over the last 40 years. Surveying in the remote region of Chukotka (720,000 km2, with a human population of only 50,000) is logistically challenging. With hardly any road network, limited airport access and the sea still covered by ice well into the breeding season, most places can be accessed only via helicopter and all-terrain vehicles (so-called vezdekhods). Around 30% of the known breeding habitat has not been revisited since the 1970s. During the current study period (2000–2009), five sites were revisited once or twice and one site, the key breeding area in Meinypilgyno, South Chukotka, was visited in six seasons in 2003–2005 and 2007–2009. Belyaka Spit in North Chukotka was the site of extensive studies in the late 1980s and was revisited in 2002, 2005, briefly in 2007 and in 2009.
In total, more than 70% of the known and potential breeding sites have been visited recently (see Figure 1 and Table S1 in online Supplementary Materials). To estimate rates of decline at the four sites which had more than two counts between 2002 and 2009 (sites 1, 15, 21, 22 in Table S1, we modelled count as a function of site and year in a generalised linear model with a log link function and a Poisson error distribution, and the annual rate of population change was calculated as the exponential of the year parameter estimate (i.e. the mean annual rate of decline across the sites). If, as is thought, Spoon-billed Sandpipers are very specialised in their use of habitat, we are confident that 70% of the sites with the most suitable habitats—as defined by the presence of suitable vegetation types, gently-sloping coastlines, spits and sandbars near shallow waters and lagoons, were surveyed.
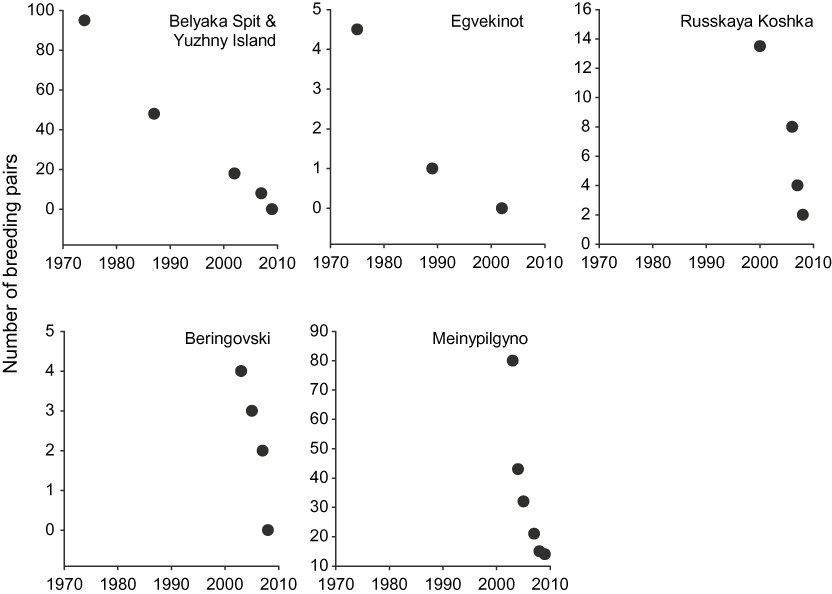
Figure 1. Population trends of Spoon-billed Sandpipers at breeding locations where two or more counts were available for the period 1970–2009. Site details may be found in Table S1 in online Supplementary Materials.
Estimating rates of decline on the wintering grounds
In order to compile all existing knowledge of Spoon-billed Sandpiper distribution and population, a geo-referenced database of records from the non-breeding areas was created in 2004 (Bunting and Zöckler Reference Bunting, Zöckler, Boere, Galbraith and Stroud2006) and includes information from BirdLife International (2001), which summarises all published sightings before 2000. Since then, more recent published records have been added, as well as many posted on the internet and provided by a growing network of national contacts.
Surveys on the breeding grounds have been supplemented by an increase in monitoring in non-breeding areas over the last 8–10 years, especially in Korea, Japan, China, Bangladesh and Myanmar. However, non-breeding survey coverage is fragmented and varies in frequency, with few systematic monitoring schemes. Every year only a small proportion (10–20%) of the population, as estimated from the breeding grounds, has been observed in non-breeding areas until 2008, when large wintering concentrations were found in Myanmar. However, trends can tentatively be identified from the few regular key monitoring sites in non-breeding areas (Yellow Sea, Korea: Moores Reference Moores2001, Moores et al. Reference Moores, Rogers, Kim, Hassell, Gosbell, Kim and Park2008; Japan: M. Kashiwagi in litt. 2006; Bangladesh: E. Ul Haque in litt. 2006 and more recently from Myanmar, Zöckler et al. Reference Zöckler, Htin Hla, Clark, Syroechkovskiy, Yakushev, Daengphayon and Robinson2010). The status and trends from flyway range countries in the non-breeding season presented here were based on expert assessment at a workshop in preparation of a species action plan in Thailand in December 2006 with recent updates.
Estimates of nesting success at Meinypilgyno (2003–2007)
Meinypilgyno is a coastal spit about 60 km long and up to 3 km wide with sparse vegetation of mostly Crowberries Empetrum nigrum on pebbled sand, interspersed by broad channels running parallel to, and partly connected to, the sea (Figure 2). The site was discovered as a Spoon-billed Sandpiper breeding location in 2001 since when it has held more pairs than any other known site. During each survey, the spit and adjacent moraine hills were searched for displaying Spoon-billed Sandpipers during June. Taking the vast potential breeding area into account, the coverage varied from year to year and was limited to the central and western spit only in 2004, 2007 and 2008 (approximately 60-70% of the area surveyed in 2003) and to approximately 80-85% of the 2003 study area in 2005 and 2009. From around mid-June, adult birds started to lay and the first brooding birds were found from 13 June onwards. Nests were found in previously identified territories by watching adults return to their nests. Both parents shared the brooding equally and, once a nest was found, the location was recorded using a GPS and, where possible, the adults were trapped and fitted with an individual combination of coloured leg rings. During 2003 and 2007, 83 adults were captured and colour-ringed at the breeding site so they could be identified as individuals (Figure 3). Chicks were also ringed, but not as individuals. A summary of the ringing and resightings is presented in Table S2 in Supplementary Materials. From 2008 onwards, adult birds were not trapped to prevent any further risk to breeding birds.

Figure 2. Habitat at Meinypilgyno showing typical breeding habitats of Spoon-billed Sandpipers.

Figure 3. Colour-ringed adult male and brood of four Spoon-billed Sandpipers.
The nests were checked for hatching and hatched chicks were followed for as long as possible to assess their survival. Most broods could be followed after hatching for up to six days, but rarely for the entire period of 18–20 days to fledging and thus the brood sizes reported are for birds up to a week old. Given the nature of the terrain, fledgling success was even more difficult to estimate but from behaviour of the adults and observation of juvenile birds, fledgling success of broods was estimated at 50% of the mean observed brood size in 2003, 2004 and 2007 and 40% in 2005. For further details on breeding survey techniques see Tomkovich and Soloviev (Reference Tomkovich and Soloviev2000), Tomkovich et al. (Reference Tomkovich, Syroechkovskiy, Lappo and Zöckler2002) and Syroechkovskiy (Reference Syroechkovskiy and Straw2005).
Estimating survival and recruitment
There were sufficient adult birds marked and resighted to estimate survival and recruitment into the adult breeding population at Meinypilgyno using Pradel survival and recruitment models (Pradel Reference Pradel1996) in Program Mark (White and Burnham Reference White and Burnham1999). These models allow a diagnosis of the demographic causes of population change. They measure apparent survival and recruitment into the adult breeding population. We used the program U-Care (Choquet et al. Reference Choquet, Lebreton, Gimenez, Reboulet and Pradel2009) to check whether there was heterogeneity in the encounter histories. None of the tests was significant, indicating that there were no issues with, for example, transients in the population, trap happiness or other significant heterogeneity in resighting rates of individuals. The survival and recruitment models were run with time-dependent or constant survival, reporting and recruitment rates and models ranked by AIC to determine the most parsimonious model.
Results
Breeding population trends
Of the 32 breeding sites visited before 2000, only five were not surveyed again between 2000–2009 (Table S1 in online Supplementary Materials) and none of those sites not resurveyed previously held large populations (Flint and Kondratiev 1977, Krechmar et al. Reference Krechmar, Andreev and Kondratyev1978). Among the re-surveyed sites, 10 lost their Spoon-billed Sandpiper populations altogether and six sites just held one pair. The global population is estimated to have declined by two-thirds between the late 1990s and 2006 (Table 1; Table S1). The recent rate of decline has been extremely rapid at the four sites where there were more than two population estimates between 2002 and 2009 (see Figure 1). These declining trends are not localised (i.e. they are widespread across the breeding range), the species is extinct in many sites where it was previously known to have occurred (Figure 4), and nowhere is a population known to be stable or increasing. The mean rate of decline at the four best monitored breeding sites was calculated as 26.4% per annum (range 19–50% pa) or an 88.2% decline from 2002 to 2009. This included two years of data from Meinypilgyno in which survey effort was reduced. However, when these points were removed, the rate of population decline was similar at 27.1% per annum.
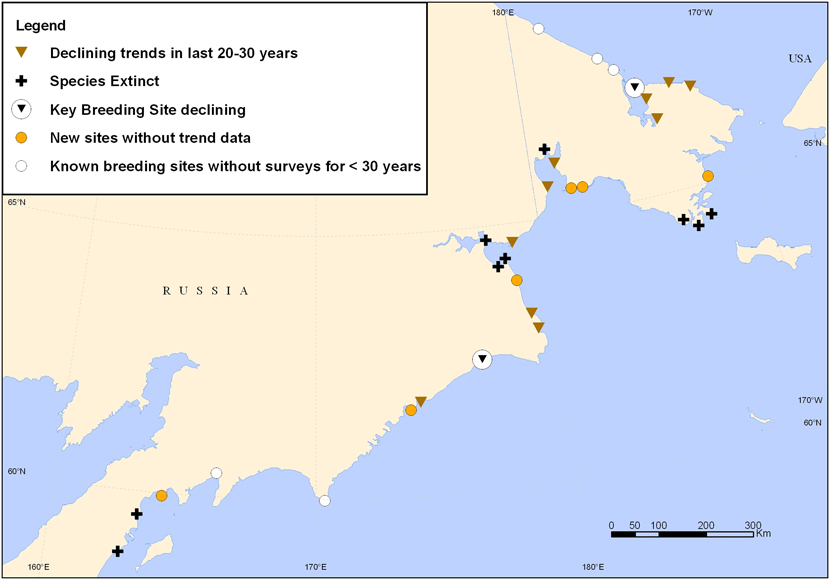
Figure 4. Map of all known Spoon-billed Sandpiper breeding sites, including population trend information at breeding sites, based on field surveys carried out between 2000 and 2009. The key breeding sites are Belyaka Spit (northern point) and Meinypilgyno (southern point).
Table 1. Population trends in Spoon-billed Sandpiper breeding pairs in Chukotka, Russia

A maximum of 57 pairs were recorded from sites visited between 2000 and 2009 (see Table S1). These sites covered approximately 70% of the known breeding sites and are an optimistic estimate as the numbers of pairs at several sites (accounting for 19 pairs) will have declined further since visits in 2000–2004. If declines at these sites have been similar to those in Figure 1, this would give a total of 45 pairs in 2009. To account for potential additional pairs at the remaining 30% of sites not revisited and unknown sites we have increased the estimates by 30–50% using the recommendation of Syroechkovskiy (Reference Syroechkovskiy and Straw2005), who based this extrapolation on historical data from 1960–1990 and aerial surveys of suitable habitats in 2002 and 2003. This accounts for a maximum of an extra 15–30 pairs.
Furthermore we accounted for additional oversights within the identified range and outside in yet-to-be-discovered locations and roughly estimate these at an additional maximum of 60–100 pairs, based on potential habitats at coastal stretches in the southern range that have never been surveyed. We therefore estimate the total breeding population at: 45–57 + 15–30 + (60–100) = 120–200 pairs or approximately 500–800 individuals, when juveniles and non-breeders are included (assuming an optimistic ratio of two non-breeding first- or second-years per pair).
Breeding ecology at Meinypilgyno, South Chukotka
Breeding success
Between 2003 and 2007, 10–25 nests were monitored at Meinypilgyno each year, with the exception of 2006 (Table 2). The breeding performance parameters measured were fairly consistent and with only four years’ worth of data it was not possible to discern any trend (Table 3). In none of these study years were there any complete breeding failures (i.e. no chicks fledged) and the main factor impacting the number of fledged chicks being produced was the total number of breeding pairs.
Table 2. Summary of the number of pairs of breeding Spoon-billed Sandpipers and the number of nests and broods found in the Meinypilgyno breeding area (South Chukotka, Beringovski Region) between 2003 and 2009. Survey effort in 2004, 2007 and 2008 was lower due to less time available and with only 2 rather than 4 people undertaking fieldwork. Additional territories refer to displaying or alarming pairs which were not confirmed breeding.
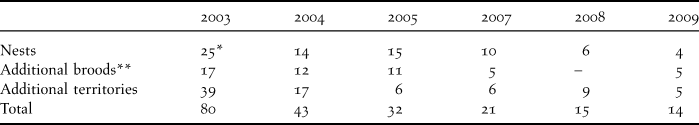
* Two nests were found outside the survey area of Meinypilgyno.
** Broods found in addition to pairs with nests.
Table 3. Nesting success of Spoon-billed Sandpiper in the Meinypilgyno area based on the authors’ observations for 2003 and 2005 and Taldenkov and Golub (Reference Taldenkov and Golub2005) for 2004 and N. Yakushev (in litt.) for 2007. Mean brood sizes were calculated from pairs where the nest was found and also other broods where the nest was not found.
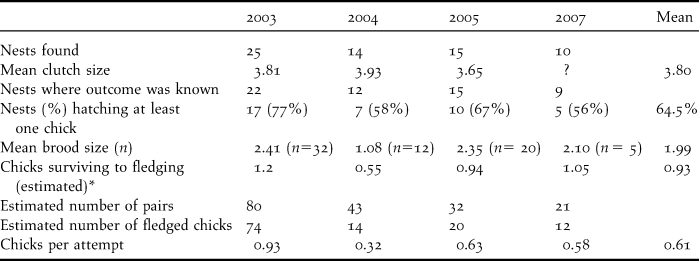
* = Fledgling survival in number of fledged chicks/pair, estimated at 50% of brood size in 2003, 2004 and 2007, based on observations of juvenile losses and fledged birds, but only 40% in 2005.
Hatching success averaged 64.5% over the four years studied. Arctic-breeding waders tend to have one breeding attempt only per annum due to the short breeding season but, in 2005, one pair laid a second clutch after their first nest failed at 3-5 days. The mean size of broods of up to one week old number was 1.99 chicks per nest. Fledging success was difficult to measure as broods were not easy to find after they were one week old. In 2003, it was estimated at 50%. However, in 2005, observations of 22 broods of up to one week old contained two (five cases) or just one chick (eight cases) indicating a lower average fledging success in 2005 which was estimated at 40%. Between 2003 and 2007, the mean fledgling success of observed broods was estimated at 0.61 ± 0.14 SE individuals per brood, ranging from 0.32 in 2004 to 0.93 in 2003. There was no discernible temporal trend in the estimated number of birds fledged per nesting attempt.
Breeding site fidelity
During our surveys in 2003-2009 no individually-marked adults were subsequently observed further than 3 km from the original site of ringing. I. Taldenkov (in litt.) noted year-to-year distances between five nests of the same pairs in Meinypilgyno in 2003 and 2004 ranging from 59 to 180 m (average 128.5 m, n = 5). In 2004, most of the adult birds individually marked in 2003 were recorded nesting within 200 m of the territory they held in the previous year (87.5%, n = 21). Also in 2005, none of the 19 resighted birds was observed further than 3 km from its ringing site. Only three birds were found further from their previous-year territories (distances of 1,900–3,000 m) but in two cases the birds probably were just feeding far away from their home territory. In 2009 two colour-marked adult birds were breeding 2.1 and 1.8 km away from where they nested six and two years previously (Tomkovich in litt.), confirming the high site fidelity observed by Tomkovich (Reference Tomkovich and Kurochkin1994) in North Chukotka.
Survival and recruitment of adult birds
In total, resighting histories were available for 82 birds ringed and resighted between 2003 and 2009. Of the general models the most parsimonious was one with constant survival with time-dependent reporting and recruitment (Model 1, Table 4). Despite being a small sample the encounter probability was high in the first two years (0.63 and 0.67), then fell to 0.46 in 2005 and 0.33 in 2007 (no visit was made in 2006). In 2008 and 2009, the reporting probability was low at 0.07 and 0.09. The most parsimonious model was one with a constant survival rate of 0.76 ± 0.077 SE. Given the very low reporting rate and number of resightings for the last two years, survival estimates for this period must be treated with caution.
Table 4. Results from the survival modelling of adult birds ringed 2003 to 2007 at Meinypilgyno ranked by Akaike Information Criterion corrected for small sample sizes (AICc). φ - survival, p – reporting rate, f – recruitment to the adult population. t – time dependent parameter; . – constant parameter.
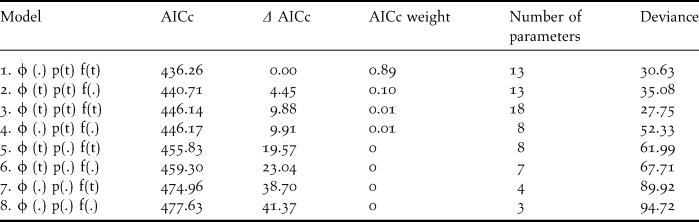
Recruitment into the adult population was extremely low and was effectively zero in most years. There was evidence of good recruitment in 2005 (0.32 +/- 0.16) which was larger than the adult mortality and some recruitment in 2007 (0.06 +/- 0.14). The decline in the population at Meinypilgyno is consistent with an ageing population of adult birds returning to breed with little or no recruitment in most years.
If natal philopatry were high, as we assume from the results from North Chukotka (Tomkovich Reference Tomkovich and Kurochkin1994) this result could be supported by resightings in subsequent years of birds marked as chicks (see Table S2 for numbers ringed). We predicted that first-year birds mostly remain in the non-breeding areas (Tomkovich Reference Tomkovich1995) and would only have expected to find returning juveniles two years after ringing. However, in 2003 and 2004 none of the 30 chicks ringed in 2001 was resighted. In 2005 only four birds marked as chicks were resighted (most likely from 93 chicks marked in 2003), of which none was observed nesting and only one was briefly seen displaying. In 2007 only two birds originally ringed as chicks were re sighted. The juvenile ringing dataset is independent of the adult survival and recruitment modelling and the years in which juveniles were observed back on the breeding grounds correspond to the years in which recruitment was greater than zero. Low return rates of birds marked as chicks should not necessarily be taken as indicative of low recruitment to the breeding population as natal philopatry in Arctic-breeding waders varies widely and can be low (Nol et al. Reference Nol, Williams and Sandercock2010). However, that colour ringed chicks were only seen in years when recruitment in to the adult breeding population was shown to occur does lend support to (i) some degree of natal philopatry and (ii) low, or zero recruitment in other years. Tomkovich (Reference Tomkovich2003) also found that birds ringed as chicks returned to the population in which they were originally marked.
Similarly, in 2002 at Belyaka spit, North Chukotka, 27 juvenile birds were colour-ringed (Syroechkovskiy et al. Reference Syroechkovskiy, Tomkovich, Kashiwagy, Lappo, Zöckler, Buzun and Taldenkov2010). After spending the first summer in the non-breeding areas the birds were expected to have returned in 2004. The area was surveyed again in 2005 and, according to I. Taldenkov (in litt. 2005), none of the 27 marked juveniles was observed, again suggesting extremely low juvenile return rates and perhaps recruitment.
Non-breeding population trends
By early 2010 over 1,000 observations were listed in the Spoon-billed Sandpiper database. Although this is enough to give some initial insight into distribution patterns and even trends (Table 5), there were huge variations in survey effort between different areas. Apparent increases in some areas largely reflect increased observer activities in certain places. In the majority of countries there was a strong decline, especially in those areas most important for the species such as South Korea and Vietnam. Myanmar has recently been identified as a very important wintering area (Zöckler et al. Reference Zöckler, Htin Hla, Clark, Syroechkovskiy, Yakushev, Daengphayon and Robinson2010) but there is at present little trend information from this country. Although the monitoring effort in the non-breeding areas (both structured and casual recording) has greatly increased over the last decade, the size of flocks recorded has declined dramatically in most locations where birds have been regularly recorded. This is contrary to expectations if the population was stable.
Table 5. Status and trends from flyway range countries in the non-breeding season, based on expert assessment at a workshop in preparation of a species action plan in Thailand in December 2006 (Zöckler et al. Reference Zöckler, Syroechkovskiy and Bunting2008) and updated to February 2010 (Zöckler et al. Reference Zöckler, Htin Hla, Clark, Syroechkovskiy, Yakushev, Daengphayon and Robinson2010).

Discussion
From the evidence presented, the Spoon-billed Sandpiper is in widespread and rapid population decline across its breeding range in both core and peripheral breeding areas. The species is thought to have bred in 32 locations in 2009. There are a further 8–12 potential sites within the potential breeding range that have not been visited but have suitable coastal habitat, based on remote sensing analyses, but only 2-3 are likely to hold significant numbers (Syroechkovskiy Reference Syroechkovskiy and Straw2005). The global population in 2009 is therefore most likely to be less than 200 breeding pairs. Unless the current rate of decline (approximately a quarter of the adult population per year) is stemmed, the Spoon-billed Sandpiper will become extinct in little over a decade.
Population change
Taking the breeding season evidence alone, the recent rapid declines observed with zero recruitment into the adult population in most years is the pattern of a species in severe trouble. During the study period (2003–2009) there was only one year in which recruitment into the adult population was greater than the average annual mortality, and then only just. The evidence for rapid population change is strong. Coupled with the systematic surveys in the breeding grounds, the declining numbers of observations in the wintering grounds and at major staging areas confirm the overall declining trend of the population (Moores Reference Moores2001, Zöckler et al. Reference Zöckler, Balachandran, Bunting, Fanck, Kashiwagi, Lappo, Maheswaran, Sharma, Syroechkovskiy and Webb2005, Moores et al. Reference Moores, Rogers, Kim, Hassell, Gosbell, Kim and Park2008). Numbers in Bangladesh in 2006–2009 ranged around 15–20 and have not exceeded 100 since 1990 (E. Ul Haque in litt.) and the numbers on migration in Korea dropped significantly from almost 200 birds in 2000 to only 20 in 2008 (Moores et al. Reference Moores, Rogers, Kim, Hassell, Gosbell, Kim and Park2008) suggesting also a 90% drop in numbers for two key non-breeding sites.
As with many other species of Arctic-breeding waders, adult Spoon-billed Sandpipers are strongly site-faithful in the breeding grounds (Tomkovich Reference Tomkovich and Kurochkin1994) and there is no evidence that they are nomadic or occupy rapidly changing ephemeral habitats such that birds have to continually colonise new areas. The conclusions from Tomkovich (Reference Tomkovich and Kurochkin1994) are supported in this study by two independent data sources. First, birds individually-marked as adults and chicks were recorded returning to the same breeding territories in subsequent years after 14 and 16 years (Tomkovich Reference Tomkovich2003) and, second, the survival and recruitment analysis, which measures apparent survival rate (i.e. both true mortality and emigration) indicated an apparent survival rate of 0.76. This rate is similar or higher than other species of similar size (e.g. Western Sandpiper C. mauri and Semipalmated Sandpiper C. pusilla, Johnson et al. Reference Johnson, Ruthrauff, McCaffery, Haig and Walters2010) and supports the assertion that rates of permanent emigration were low.
Low rates of natal philopatry, i.e. chicks returning to breed where they were born, have been recorded amongst Arctic-breeding waders (e.g. Nol et al. Reference Nol, Williams and Sandercock2010) and thus the low return rate of birds marked as chicks to the study area is not necessarily indicative of low recruitment rates. However, the survival and recruitment analysis of the adult breeding birds, which is independent of the chick marking data, measures recruitment into the adult population. This was effectively zero in all but two years and in only one year was it high. Given these were the two years in which any birds colour-ringed as chicks were recorded, there is some degree of natal philopatry but could have included some birds hatched elsewhere.
The evidence is therefore that the population at Meinypilgyno is ageing as recruitment into the adult population is very low. It is possible that chicks recruited into the adult population are moving into new breeding areas but no marked birds have been recorded outside their nesting sites, and the decline at Meinypilgyno is consistent with the decline in other sites and in none of the other known sites are populations stable or increasing.
Why is recruitment into the adult population so low?
In all the years studied, apart from 2009 due to bad weather, there was never a complete breeding failure at Meinypilgyno and the decline in the number of fledged chicks was largely a function of the number of breeding pairs. There are a number of uncertainties regarding the estimates of breeding success. Arctic-breeding waders will only raise a maximum of one brood per year due to the short breeding season available to them. One Spoon-billed Sandpiper pair laid a second clutch in 2005 after their first nest was lost early on during incubation. The estimates of nest failure presented here include this and the proportion of nests failing and second clutches being relaid remains low and may vary between years depending on a number of factors such as weather and predation pressure.
The brood sizes in the first week after leaving the nest (1.99 chicks per brood) is in agreement with the observations by Tomkovich (Reference Tomkovich1995) in Belyaka, who observed median brood sizes of two chicks. The major uncertainty in terms of breeding success parameters was the number of chicks fledged per pair and given the nature of the terrain this was extremely difficult to obtain and is a best estimate from the observers.
The number of chicks fledged per pair based on the parameters measured, assuming one attempt per pair and the crude estimates of fledging success, indicates an estimate of 0.601 chicks fledging per pair. If juvenile survival was the same as for adults, then this would not be sufficient to maintain the population and would result in a slow decline in the population but not the rapid decline as currently observed.
Lack of recruitment into the adult population is therefore unlikely to be a result of breeding failure and the problem is largely related to losses once birds have left the nest. Little is known about what might happen to young birds in the breeding area once they have fledged and should be a subject for future research. It is however most likely that the losses of juvenile and second-year birds occur outside the breeding areas on the flyway or in wintering areas. Recent work in Myanmar and Bangladesh during 2006–2009 has shown that hunting and trapping of shorebirds occurs at all sites known to support > 10 wintering Spoon-billed Sandpipers (Zöckler et al. Reference Zöckler, Htin Hla, Clark, Syroechkovskiy, Yakushev, Daengphayon and Robinson2010). The Bay of Martaban in Myanmar held an estimated 220 Spoon-billed Sandpipers in 2010 and shorebirds are regularly hunted there. A survey of the hunters there indicated that they regularly catch small numbers of Spoon-billed Sandpipers throughout the year. Immature birds may well be more susceptible to capture than adults, as (i) they do not return to the breeding areas until they are two years old and hence are exposed for longer to hunting activities and (ii) young birds have been shown to be more vulnerable to capture in mist-nets than adults (e.g. Pienkowski and Dick Reference Pienkowski and Dick1976, Goss-Custard et al. Reference Goss-Custard, Durrell, Sitters and Swinfen1981). Although habitat loss at staging areas and other threats to the breeding areas may well be important, it is inevitable that high hunting pressure at a site where more than half of the world population winters will have a major impact on the species as a whole.
Threats
There are a number of known threats in their remote breeding areas. Although in the northern breeding grounds at the Chukchi Peninsula, predation by Arctic Foxes Alopex lagopus might have increased after 1990 due to the collapse of the previously subsidised fur market (Syroechkovkiy Reference Syroechkovskiy and Straw2005) it is not likely to be a major threat. According to data collated from the Arctic Birds Breeding Condition Survey, Arctic Foxes have declined over the past 20 years and their southern range limit has retreated further north (P. Tomkovich and M. Soloviev in litt.). In the southern part of the breeding range, Arctic Foxes are absent and are replaced by the less abundant Red Fox Vulpes vulpes. Severe habitat destruction by military activities and the construction of country houses near breeding sites has been observed at five out of 32 sites. The collecting of skins and clutches was responsible for losses at a considerable 17% of sites between 1990 and 2005, when Russian middlemen were still operating on behalf of West European and American collectors (Zöckler et al. Reference Zöckler, Syroechkovskiy and Bunting2008). Problems in the breeding areas also include subtle changes in climate leading to observed changes in the soil moisture and changes in vegetation on the breeding grounds (Tomkovich et al. Reference Tomkovich, Syroechkovskiy, Lappo and Zöckler2002, Zöckler Reference Zöckler2003, Syroechkovskiy Reference Syroechkovskiy and Straw2005, Zöckler et al. Reference Zöckler, Syroechkovskiy and Bunting2008). Surveys in 2008 and 2009 in Northern Kamchatka found that the vegetation on coastal spits is changing, presumably because of a warming climate (Syroechkovskiy and Zöckler Reference Syroechkovskiy and Zöckler2009). In the higher Arctic sites where Spoon-billed Sandpiper nests in Chukotka, the crowberry (Empetrum sp.) vegetation is less than 5 cm high. However, at the survey sites in Kamchatka it was found to be 10-20 cm high and wader chicks are not able to move around or feed in this habitat. This change in vegetation has been observed in previously known breeding locations at Kayum and Tumlat spit, where broods were recorded in 1972 and 1979 (Gerassimov and Vyatkin 1973, Lobkov Reference Lobkov1986), but in 2009 hardly any suitable habitat remained. There has also been an encroachment of Siberian Dwarf Pine bushes Pinus pumila. These were unknown to local people in the 1950s and 1960s at all the spits visited in Kamchatka, but in 2009 up to 20% cover of this species was found. This suggests gradual changes in the breeding habitats in the southern part of the breeding range of the Spoon-billed Sandpiper, which is likely to force the species to retreat further north within its range. These changes might have contributed to the observed decline in the southern part of the range, but do not sufficiently explain the very rapid decline of the population in both northern and southern parts of its range since 2002.
Among the long list of threats identified in the non-breeding areas (see Zöckler et al. Reference Zöckler, Syroechkovskiy and Bunting2008 for details) the large-scale reclamation and urban development of intertidal mudflats, the main habitats in the non-breeding areas, may well have had the most striking impact on the survival of adult and juveniles. For the Yellow Sea area alone, Barter (Reference Barter2003) estimated the loss of 40% of mudflats in the previous 50 years with a further 43% reclaimed since or in planning. Of the latter, the largest single reclamation development, the Saemangeum area in South Korea, destroyed 40,000 ha of habitat when it was completed in 2006 and a 90% decline in spring counts from 34 in 2006 to three in 2008 has been observed (Moores et al. Reference Moores, Battley, Rogers, Park, Sung, van de Kam and Gosbell2006). Japan has lost 70% of its mudflats and continues to lose the remaining area to coastal development (M. Kashiwagi pers. comm.). China is accelerating its coastal development with rapid destruction of coastal mudflats. Bangladesh is also rapidly converting its intertidal mudflats into salt pans and shrimp farms, leaving less and less habitat for Spoon-billed Sandpipers, which specialise on intertidal mudflats with high sandy componments (E. Ul Haque pers. comm., Zöckler et al. Reference Zöckler, Syroechkovskiy and Bunting2008).
The main wintering areas and habitat requirements are still not totally known. Additional stressors in the known areas include hunting or further habitat destruction. Surveys in Bangladesh in 2006 and Myanmar in 2008, 2009 and 2010 revealed that some wintering areas have been gradually encroached by small-scale reclamation of the intertidal mudflats, mostly for prawn-pond development and saltpans. Most importantly though, the surveys of the main wintering areas in the Bay of Bengal are threatened by regular trapping of waders on the mudflats by local people and probably represent a major source of mortality for this species (Zöckler et al. Reference Zöckler, Htin Hla, Clark, Syroechkovskiy, Yakushev, Daengphayon and Robinson2010). At present, annual surveys in the wintering grounds aim to enhance the knowledge on the wintering behaviour of the species, a task increasingly difficult with diminishing total numbers.
Population size
Historical estimates gave a figure of 2,000–2,800 pairs in the 1970s (Flint and Kondratyev Reference Flint and Kondratiev1977). This estimate was based on the present range and the knowledge of the habitat at the time, collected during various surveys undertaken in the 1960s and 1970s in several sites across the breeding range.
Our present estimate of a global population of around 120–200 breeding pairs already constitutes an overall decline of at least 90% over the last 30 years. The estimate is likely to be on the optimistic side, considering that 70% of the most suitable breeding habitats had been surveyed by 2009 and many sites also experienced a stronger decline than previously estimated. For example, four coastal segments monitored in 2004–2006 contained only eight pairs, but had been predicted to host 25–65 pairs (Syroechkovskiy Reference Syroechkovskiy and Straw2005). Even if we add into our extrapolations from the remaining sites an additional one-third of pairs, we would still only add 60–100 pairs.
Although there is a degree of uncertainty in these estimates of population size, there is very strong support for the rapid population decline. Even if new areas were discovered and the population size was even several-fold higher, if the rate of population decline of approximately a quarter of the population each year continued, the population would rapidly be reduced to unviable levels.
Recommendations
Low recruitment and an ageing population are the most likely cause for the decline of this species, which is on the brink of extinction. The low recruitment points to losses along the flyway in staging or wintering areas. The continuing decline requires immediate action at a global level. The species uses a flyway that is extremely vulnerable and exposed to major environmental changes. Considerable efforts are required to sustain the stopover and wintering sites for the species and address the issue of hunting in the main wintering areas.
The current estimate of 120–200 pairs may be optimistic. To improve estimates of population size, surveys of the remaining 30% of potential breeding areas are needed, particularly of those in the north along the Chukchi Sea and along the southern range boundaries in North Kamchatka. In order to determine where suitable breeding sites occur a GIS analysis is recommended to model potential breeding sites using existing nest locations. Continued monitoring in the core breeding area in Meinypilgyno is essential to assess the rate of decline, the level of recruitment, juvenile dispersal and threats occurring during the breeding season. It is important that research focuses on chick survival (hatching to fledging), survival of first-year birds and adults along the migration route and their winter habitat requirements. Regional differences in population decline across breeding areas must be explored, and reasons for any differences investigated.
This review suggests that it is most important to improve survey and monitoring efforts in non-breeding areas, especially in South China, Vietnam, Cambodia, Myanmar and Bangladesh and use geolocators to explore further the flyway route and identify crucial stopover sites. Clearly, we need improved knowledge of the main non-breeding areas of the species and address those threats identified along the flyway and in the wintering areas, such as hunting, and secure the protection of all major stopover and wintering sites along the flyway. Recent surveys in Kamchatka, Myanmar and Bangladesh revealed that widespread hunting of waders at a massive scale is a major cause of mortality that must be having a significant negative effect on the global population (Zöckler et al. Reference Zöckler, Htin Hla, Clark, Syroechkovskiy, Yakushev, Daengphayon and Robinson2010). Moreover it is a problem that can be directly addressed by conservation action. The survey found that hunters in Myanmar would be more than willing to give up bird hunting in return for livelihood support.
In addition, unless immediate conservation action is taken in the countries along the flyway and in the wintering areas, this species will probably become extinct, most likely in little more than a decade. It is important to raise wider awareness of the species and its precarious situation in the countries along the flyway and urgent conservation action is required in the entire flyway context, potentially engaging as many as 14–16 range countries and territories. Given the rapid and continuing nature of the decline, a captive breeding programme should be considered now. The greatest likelihood of success in founding a captive population is likely to come from the collection of eggs which will have less impact on the wild population if it is done when only a small proportion of the total production would be needed. In addition, as the population continues to decline it will lose genetic diversity which will in turn reduce the chance of successful establishment. Such a captive breeding programme should not deflect efforts from conservation measures throughout the flyway and should be seen as an insurance policy for the survival of the species.
Acknowledgements
The surveys in Meinypilgyno and other monitoring sites along Chukotka’s coasts were extensively carried out and supported by Russian and foreign colleagues. We would like to thank Elena Lappo, Minoru Kashiwagi, Vladimir Morozov, John O’Sullivan and Maxim Suldin, who worked in Meinypilgyno. In particular we would like to thank Nikolay Yakushev, who provided additional unpublished data from 2007 and 2008. We also thank Pavel Tomkovich, Chris Schenk, Manfred Trobitz, Jens Gregersen, Weiting Liu, James McCallum, Chris Kelly, Heikki Karhut, Rob Schuckard, Thomas Heinicke, Tom Noah, Alexander Kuzmich, Pavel Pinchuk Sergey Golubev and many other friends who have participated in Spoon-billed Sandpiper monitoring work during our expeditions. Ivan Taldenkov provided unpublished data for 2004 and 2005. Pavel Tomkovich and Vladimir Arkhipov provided some unpublished data for 2009. Last, but not least, we would like to thank our friends Roman and Svetlana Belogorodtsevy, and Andrey and Lena Golub’ from Meinypilgyno, who supported our work throughout our stay in so many ways. We thank Dimitriy Dobrinin and Gillian Bunting (ArcCona Consulting) for GIS support and cartography, two anonymous reviewers, Stuart Butchart, Suhel Quader, Humphrey Sitters, Pavel Tomkovich and Paul Donald for valuable comments on an earlier draft and for encouragement to undertake this paper.
We are grateful to JAWAN, BirdLife Asia, the German Ornithological Society (DO-G) and the Manfred–Hermsen–Foundation, Bremen as well as WWF-Japan, KNCF, Toyota Foundation, the Packard Foundation and BirdLife International’s Species Champion Programme, UNEP/GEF ECORA project, Environment Australia, Club-300 (Sweden), CIC–Migratory Bird Commission, Russian Academy of Sciences, Administration of Chukotka Autonomous Area, Arctic Ecology and Anthropology Center (Moscow) and private sources of Dr. Werner Trense, volunteers and Russian businessmen, who generously provided financial support to the expeditions over the last nine survey years.











