Cortisol levels may be altered in childhood in association with maltreatment (neglect, abuse and witnessing abuse) and other adversities, Reference Carlson and Earls1–Reference Lupien, McEwen, Gunnar and Heim4 yet, with few longitudinal studies, Reference Trickett, Noll, Susman, Shenk and Putnam5 little is known about whether effects on cortisol persist into later life. An extensive literature demonstrates the increased risk of adult psychological disorder among those maltreated in childhood. Reference Gilbert, Widom, Browne, Fergusson, Webb and Janson6,Reference Horwitz, Widom, McLaughlin and White7 Childhood experiences may have a lifelong influence on the function of the hypothalamic–pituitary–adrenal (HPA) axis and thus on the regulation of cortisol. Reference Lupien, McEwen, Gunnar and Heim4 It has also been suggested that childhood maltreatment may sensitise the HPA axis resulting in dysfunction and vulnerability to psychological disorders such as depression. Reference Heim and Nemeroff8 Such hypotheses beg the question of whether childhood adversity has a persisting association with cortisol in mid-life. Using data from the 1958 British birth cohort, we aimed to establish whether co-occurring childhood adversities such as maltreatment and household dysfunction were related to cortisol patterns in mid-adulthood (age 45 years) in the general population. A second objective was to establish whether adult anxiety and depression mediate or moderate any association between childhood adversity and cortisol secretion.
Method
Study population
The 1958 cohort comprised a population of about 17 000 live births in England, Scotland and Wales, all born in one week in March 1958 and followed up at ages 7, 11, 16, 23, 33 and 42 years. Reference Power and Elliott9 At age 44–45 years, i.e. during 2002–2004, a biomedical survey was undertaken comprising a home interview by a trained research nurse, two short self-completed questionnaires, physical measurements, blood samples and a saliva sample obtained after the interview: 9377 (78%) participated from the target of 11 971 invited (i.e. participants still in contact with the study and who at age 42 years had not required a proxy interview). Ethical approval for the age 45 year survey was given by the South East Multicentre Research Ethics Committee.
Measures
Salivary cortisol
Two saliva samples were collected in the morning, timed to capture the post-waking peak cortisol concentration and decline following this peak, as key characteristics of the normative cortisol diurnal rhythm. Accordingly, participants were asked to collect two saliva samples on the next convenient day after interview, the first 45 min after awaking (time 1) and the second 3 h later on the same day (time 2). Most participants (n = 9165) consented to saliva collection for cortisol measurement; a reminder was sent to 53% of those who consented but had not responded within 2 weeks of the nurse visit. Samples were received from 6568 participants, of whom 6524 had information on at least one cortisol measure (6467 for the time 1 measure, 6506 for time 2 and 6449 for both). The number responding is likely to reflect our reliance on participants to collect and return their samples.
Participants were instructed to avoid brushing or flossing their teeth and eating or drinking for 15 min before taking each sample. They were asked to chew on a cortisol collection swab (Salivette, Sarstedt, Numbrecht, Germany) until it was soaked, record the date and time of collection and store the sample at room temperature until posting it to the laboratory. Salivary cortisol is stable at room temperature for up to 30 days but samples were frozen after reaching the laboratory to reduce microbial growth. Cortisol levels were measured at the University of Dresden with a commercial chemiluminescence immunoassay kit (IBL International, Hamburg, Germany). The lower sensitivity of this assay is 0.44 nmol/l, with intra-assay and interassay precision below 10% for a wide range of cortisol concentrations. Samples with cortisol levels above 50 nmol/l were rerun in a second assay for confirmation. Participants also reported whether they regularly worked at night (shift work); wakefulness during the previous night; dental work within the previous 3 days; cuts inside their mouth that might bleed; and current medications, categorised as a dichotomous variable.
Childhood psychosocial adversity
Scales for maltreatment and household dysfunction were derived from information collected in childhood and adulthood. Maltreatment scales were constructed from several items selected to represent components of conventional definitions (neglect, abuse, witnessing abuse). Reference Gilbert, Widom, Browne, Fergusson, Webb and Janson6 Information on abuse (physical, sexual or psychological), neglect and witnessing physical or sexual abuse in others in the family was collected solely at age 45 years (see Appendix). A maltreatment scale was created by summing the scores on the three abuse items, one item on witnessing physical or sexual abuse in others in the family and three items on neglect (range 0–7). Further, from information collected in childhood we created an additional scale of neglect, by summing the eight items on the child's physical appearance and the parent's interaction with the child at ages 7 years, 11 years and 16 years (see Appendix). Information collected during childhood (at ages 7, 11 and 16 years) was obtained from structured questionnaires completed by the child's teacher and from a health visitor interview with parents (usually the mother). Information collected in adulthood (age 45 years) was obtained from participants who completed a confidential questionnaire about their childhood to age 16 years, using direct computer data entry. The questionnaire at age 45 years was derived from the Personality and Total Health (PATH) Through Life Project, Reference Rosenman and Rodgers10 originating from the Parental Bonding Instrument, the British National Survey of Health and Development and the US National Comorbidity Survey. Information on household dysfunction, collected in child and adulthood, was used to create a scale of 11 items. For items measured at more than one age, any positive response was classified as adversity. Data on separation or divorce collected from parent interviews at 11 years and 16 years of age were supplemented with data from cohort members at age 33 years.
Confounding and mediating factors
Socioeconomic position in childhood and adulthood and adult smoking were included because of their association with both cortisol levels Reference Badrick, Kirschbaum and Kumari11,Reference Li, Power, Kelly, Kirschbaum and Hertzman12 and childhood adversity. Reference Gilbert, Widom, Browne, Fergusson, Webb and Janson6,Reference Anda, Croft, Felitti, Nordenberg, Giles and Williamson13 Socioeconomic position at birth was based on the father's occupation using the UK Registrar General's social class categories, and grouped as professional/managerial, skilled non-manual, skilled manual and semi-skilled or unskilled manual, including single-mother households. Socioeconomic position at age 42 years was based on the participant's current or most recent occupation and categorised as above. Smoking habits, reported at 42 years, ranged from ‘never’ to ‘current smoker, 20 cigarettes a day or more’ (seven categories). Current psychological state at age 45 years was indicated by two or more symptoms of anxiety or depression on the nurse-administered revised Clinical Interview Schedule (CIS-R). Reference Lewis, Pelosi, Araya and Dunn14
Statistical analysis
Extreme cortisol outliers for time 1 (T 1) and time 2 (T 2) were truncated at 2 nmol/l for values below this level (n =24 at T 1, n = 123 at T 2) and at 100 nmol/l for values above 100 nmol/l (n =22 at T 1, n =20 at T 2) in order that extreme values did not exert a disproportionate influence on analyses. Cortisol values were skewed, hence we transformed data using log 10 (C 1 and C 2) to achieve a distribution approximating normality. Not all samples were collected at the specified periods after waking, leading to variation around the target time for T 1 (mean 49 min, s.d. = 15 min) and T 2 (mean 3 h 5 min, s.d. = 23 min). Given that cortisol level was influenced by both time of awaking and time since awaking, we centred the log-transformed cortisol values for each individual at 08.08 h (45 min after the mean awaking time of 07.23 h) and T 2 at 11.08 h (3 h 45 min after mean awaking time) using coefficients for time of waking (T 0), T 1 and T 2 from linear regression models. Specifically, we fitted a linear regression model for C 1 on T 0 and T 1: C 1 = a + bT 0 + cT 1. For each individual the centred value for C 1 (Cort1) was derived as Cort1 = C 1 + b(07.23 h–T 0)+ c(08.08 h–T 1). Similarly, we derived centred cortisol values (at 11.08 h) for T 2. Thus, T 1 and T 2 cortisol levels in all analyses were adjusted for both time of awaking and time since awaking.
To investigate associations with childhood adversity we analysed several cortisol measures derived from transformed and centred values: first, T 1 and T 2 cortisol levels; second, area under the curve (AUC), derived as the sum of T 1 and T 2 cortisol (Cort1 and Cort2 back-transformed to nmol/l, i.e. 10Cort1 and 10Cort2) multiplied by 3 h and divided by 2 (thus, AUC represents the 3 h average of T 1 and T 2 values, allowing for variation in collection times, used here to indicate total 3 h exposure); and third, cortisol T 1 to T 2 slope.
Continuous cortisol variables were analysed using linear regression. Given that T 1 and T 2 cortisol and AUC were log10 transformed, relative change (in percentages) in these cortisol measures was calculated from the regression coefficient (β)as 100×(10β–1). For example, T 1 cortisol changes from a value x to 10β x when the adversity increases by one level, e.g. from 0 to 1 on the maltreatment scale. Scales for adversity were modelled as continuous to identify trend across scores and also as categorical predictors to identify threshold associations. In initial analyses we examined potential influences on cortisol measurement: night work (8%), awake during the previous night (37%), cuts inside mouth (3%), recent dental treatment (2%), current medication and day of the week. Current medication was associated with higher T 1 and lower T 2 cortisol levels, whereas regular night work was associated with lower T 2 levels; hence, we adjusted for these factors in analyses. Because of gender differences in cortisol secretion, Reference Herbert, Goodyer, Grossman, Hastings, De Kloet and Lightman15 and in some childhood adversities, Reference Rosenman and Rodgers10 analyses were conducted separately by gender. However, gender differences were also tested by including an interaction term between gender and each adversity in combined models (P<0.05). Four stages of regression models were undertaken:
-
(a) adjusted for factors affecting cortisol measurement (model 1);
-
(b) additionally adjusted for socioeconomic position in childhood and adulthood, and adult smoking (model 2);
-
(c) further adjusted for anxiety and depression symptoms at age 45 years (model 3);
-
(d) with an interaction term between child maltreatment (or household dysfunction) score and current anxiety or depression to test effect modification.
Because of potential biases associated with sample attrition, Reference Atherton, Fuller, Shepherd, Strachan and Power16 non-response was handled by inverse probability weighting. Several factors associated with non-response at 45 years were used for the weighting, including gender, social class at birth, mathematics score at age 11 years and socioemotional behaviour at ages 7 years and 11 years. We repeated analyses using the sample with complete data and results were similar to those from weighted analyses; the latter are presented here. In addition, we checked the validity of our findings using sensitivity analyses in which assumptions varied about missing adversity measures obtained in adulthood. Specifically, we assumed that one, two or all missing data items were ‘unexposed’. Results were similar across all analyses and conclusions were unaltered: here we present results assuming that individuals with up to two missing items were unexposed.
Results
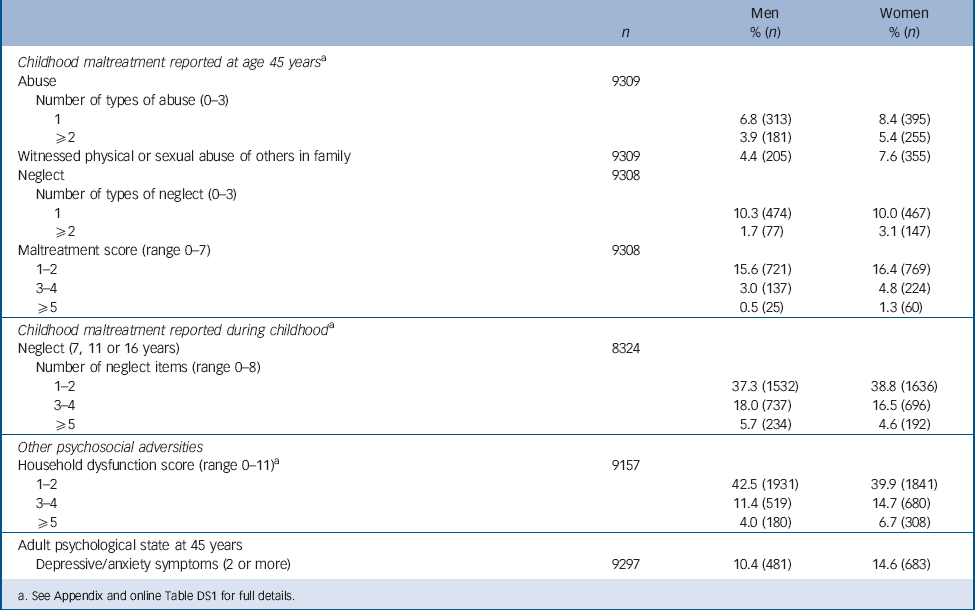
Childhood maltreatment and household dysfunction items and scores are shown for all participants at age 45 years briefly in Table 1 and in full in online Table DS1. The most common maltreatment items reported at this age were psychological abuse and absence of affection from the father. In terms of summary scores, 3.9% men and 5.4% women had two or more reports of abuse, whereas 1.7% and 3.1% respectively had two or more reports of neglect. On the overall maltreatment scale, 3.5% men and 6.1% women had three or more maltreatments reported at this age. For the eight individual neglect items collected during childhood, many were common but only a minority (5.7% boys, 4.6% girls) had five or more items. For household dysfunction the most prevalent items were maternal mental health problems and authoritarian upbringing. Even though some items were common, most individuals (>80%) had two or fewer household dysfunction items; approximately 4% men and 7% women had five or more (Table 1). At age 45 years, 10.4% men and 14.6% women reported two or more depression or anxiety symptoms.
TABLE 1 Prevalence of childhood psychosocial adversity and adult psychological state
| n | Men %(n) |
Women %(n) |
|
|---|---|---|---|
| Childhood maltreatment reported at age 45 years Footnote a | |||
| Abuse | 9309 | ||
| Number of types of abuse (0–3) | |||
| 1 | 6.8 (313) | 8.4 (395) | |
| ⩾2 | 3.9 (181) | 5.4 (255) | |
| Witnessed physical or sexual abuse of others in family | 9309 | 4.4 (205) | 7.6 (355) |
| Neglect | 9308 | ||
| Number of types of neglect (0–3) | |||
| 1 | 10.3 (474) | 10.0 (467) | |
| ⩾2 | 1.7 (77) | 3.1 (147) | |
| Maltreatment score (range 0–7) | 9308 | ||
| 1–2 | 15.6 (721) | 16.4 (769) | |
| 3–4 | 3.0 (137) | 4.8 (224) | |
| ⩾5 | 0.5 (25) | 1.3 (60) | |
| Childhood maltreatment reported during childhood Footnote a | |||
| Neglect (7, 11 or 16 years) | 8324 | ||
| Number of neglect items (range 0–8) | |||
| 1–2 | 37.3 (1532) | 38.8 (1636) | |
| 3–4 | 18.0 (737) | 16.5 (696) | |
| ⩾5 | 5.7 (234) | 4.6 (192) | |
| Other psychosocial adversities | |||
| Household dysfunction score (range 0–11)Footnote a | 9157 | ||
| 1–2 | 42.5 (1931) | 39.9 (1841) | |
| 3–4 | 11.4 (519) | 14.7 (680) | |
| ⩾5 | 4.0 (180) | 6.7 (308) | |
| Adult psychological state at 45 years | |||
| Depressive/anxiety symptoms (2 or more) | 9297 | 10.4 (481) | 14.6 (683) |
a See Appendix and online Table DS1 for full details.
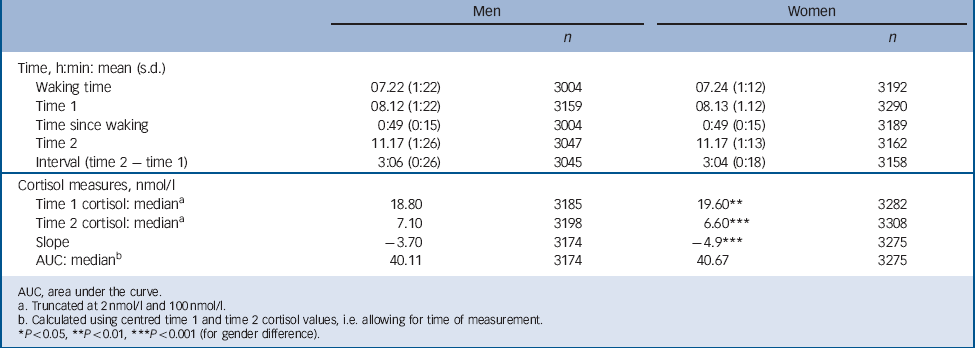
TABLE 2 Cortisol measures and times of sampling (n = 3209 men, n = 3315 women)
| Men | Women | |||
|---|---|---|---|---|
| n | n | |||
| Time, h:min: mean (s.d.) | ||||
| Waking time | 07.22 (1:22) | 3004 | 07.24 (1:12) | 3192 |
| Time 1 | 08.12 (1:22) | 3159 | 08.13 (1.12) | 3290 |
| Time since waking | 0:49 (0:15) | 3004 | 0:49 (0:15) | 3189 |
| Time 2 | 11.17 (1:26) | 3047 | 11.17 (1:13) | 3162 |
| Interval (time 2 – time 1) | 3:06 (0:26) | 3045 | 3:04 (0:18) | 3158 |
| Cortisol measures, nmol/l | ||||
| Time 1 cortisol: medianFootnote a | 18.80 | 3185 | 19.60** | 3282 |
| Time 2 cortisol: medianFootnote a | 7.10 | 3198 | 6.60*** | 3308 |
| Slope | –3.70 | 3174 | –4.9*** | 3275 |
| AUC: medianFootnote b | 40.11 | 3174 | 40.67 | 3275 |
AUC, area under the curve.
* P<0.05, ** P<0.01, *** P<0.001 (for gender difference).
a Truncated at 2 nmol/l and 100 nmol/l.
b Calculated using centred time 1 and time 2 cortisol values, i.e. allowing for time of measurement.
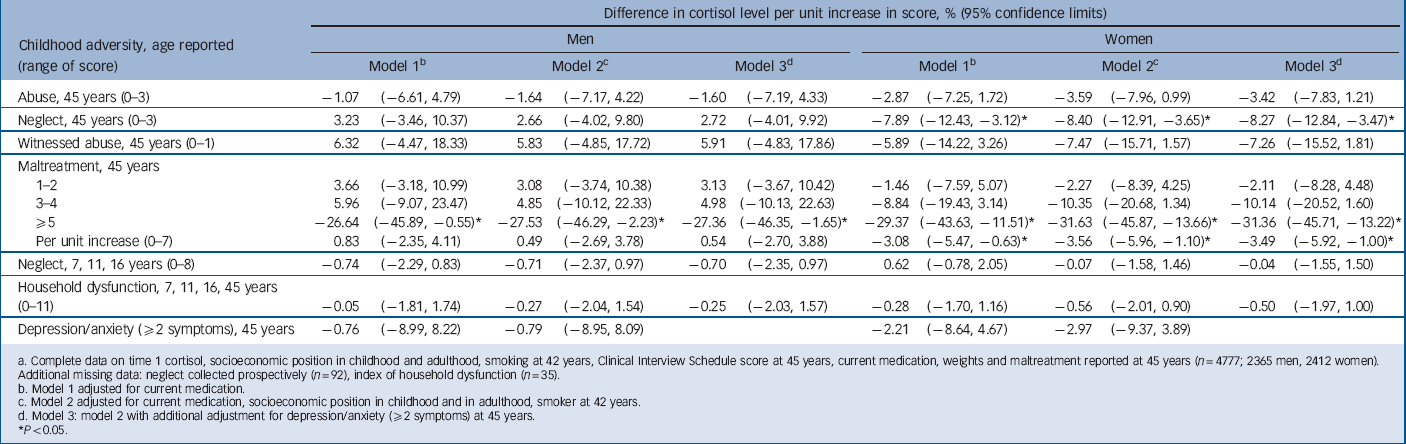
Table 2 presents information for those with at least one cortisol measure: men had a lower median cortisol level at T 1 but a higher level at T 2 (18.8 nmol/l and 7.1 nmol/l respectively) than women (19.6 nmol/l and 6.6 nmol/l respectively). For T 1 cortisol there was no association with either abuse or household dysfunction in childhood or with depression/anxiety symptoms at 45 years (Table 3). In women but not in men, T 1 cortisol was lowered by 7.9% per unit increase in childhood neglect score (range 0–3 for 45 year score; gender interaction P = 0.01). Also for women, there was a trend of lowered T 1 cortisol by 3.1% per unit increase in maltreatment score over the range 0–7. No trend was seen for men, but for both men and women T 1 cortisol was lowered by more than 25% for those with five or more maltreatments v. no maltreatment, i.e. a dose–response relationship for women and threshold effect for men. All associations remained after adjustment for current medication, socioeconomic position in child and adulthood, adult smoking and depression/anxiety. Among women the association between maltreatment score and T 1 cortisol level did not vary according to current depression or anxiety symptoms. In men the lower T 1 cortisol level for those with five or more maltreatments was seen only in those without (i.e. with fewer than two) depression or anxiety symptoms (for interaction P = 0.003).
TABLE 3 Change in time 1 cortisol level per unit increase in score for childhood maltreatment, household dysfunction and adult depression/anxietyFootnote a
| Difference in cortisol level per unit increase in score, % (95% confidence limits) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Childhood adversity, age reported
(range of score) |
Men | Women | ||||||||||
| Model 1Footnote b | Model 2Footnote c | Model 3Footnote d | Model 1Footnote b | Model 2Footnote c | Model 3Footnote d | |||||||
| Abuse, 45 years (0-3) | –1.07 | (–6.61, 4.79) | –1.64 | (–7.17, 4.22) | –1.60 | (–7.19, 4.33) | –2.87 | (–7.25, 1.72) | –3.59 | (–7.96, 0.99) | –3.42 | (–7.83, 1.21) |
| Neglect, 45 years (0-3) | 3.23 | (–3.46, 10.37) | 2.66 | (–4.02, 9.80) | 2.72 | (–4.01, 9.92) | –7.89 | (–12.43, 73.12)* | –8.40 | (–12.91, 73.65)* | –8.27 | (–12.84, 73.47)* |
| Witnessed abuse, 45 years (0-1) | 6.32 | (–4.47, 18.33) | 5.83 | (–4.85, 17.72) | 5.91 | (–4.83, 17.86) | –5.89 | (–14.22, 3.26) | –7.47 | (–15.71, 1.57) | –7.26 | (–15.52, 1.81) |
| Maltreatment, 45 years | ||||||||||||
| 1-2 | 3.66 | (–3.18, 10.99) | 3.08 | (–3.74, 10.38) | 3.13 | (–3.67, 10.42) | –1.46 | (–7.59, 5.07) | –2.27 | (–8.39, 4.25) | –2.11 | (–8.28, 4.48) |
| 3-4 | 5.96 | (–9.07, 23.47) | 4.85 | (–10.12, 22.33) | 4.98 | (–10.13, 22.63) | –8.84 | (–19.43, 3.14) | –10.35 | (–20.68, 1.34) | –10.14 | (–20.52, 1.60) |
| ⩾5 | –26.64 | (–45.89, –0.55)Footnote * | –27.53 | (–46.29, –2.23)Footnote * | –27.36 | (–46.35, –1.65)Footnote * | –29.37 | (–43.63, –11.51)Footnote * | –31.63 | (–45.87, –13.66)Footnote * | –31.36 | (–45.71, –13.22)Footnote * |
| Per unit increase (0-7) | 0.83 | (–2.35, 4.11) | 0.49 | (–2.69, 3.78) | 0.54 | (–2.70, 3.88) | –3.08 | (–5.47, 70.63)Footnote * | –3.56 | (–5.96, 71.10)Footnote * | –3.49 | (–5.92, 71.00)Footnote * |
| Neglect, 7, 11, 16 years (0-8) | –0.74 | (–2.29, 0.83) | –0.71 | (–2.37, 0.97) | –0.70 | (–2.35, 0.97) | 0.62 | (–0.78, 2.05) | –0.07 | (–1.58, 1.46) | –0.04 | (–1.55, 1.50) |
| Household dysfunction, 7, 11, 16, 45
years (0-11) |
–0.05 | (–1.81, 1.74) | –0.27 | (–2.04, 1.54) | –0.25 | (–2.03, 1.57) | –0.28 | (–1.70, 1.16) | –0.56 | (–2.01, 0.90) | –0.50 | (–1.97, 1.00) |
| Depression/anxiety (⩾2 symptoms), 45 years | –0.76 | (–8.99, 8.22) | –0.79 | (–8.95, 8.09) | –2.21 | (–8.64, 4.67) | –2.97 | (–9.37, 3.89) | ||||
* P<0.05.
a Complete data on time 1 cortisol, socioeconomic position in childhood and adulthood, smoking at 42 years, Clinical Interview Schedule score at 45 years, current medication, weights and maltreatment reported at 45 years (n = 4777; 2365 men, 2412 women). Additional missing data: neglect collected prospectively (n = 92), index of household dysfunction (n = 35).
b Model 1 adjusted for current medication.
c Model 2 adjusted for current medication, socioeconomic position in childhood and in adulthood, smoker at 42 years.
d Model 3: model 2 with additional adjustment for depression/anxiety (⩾2 symptoms) at 45 years.
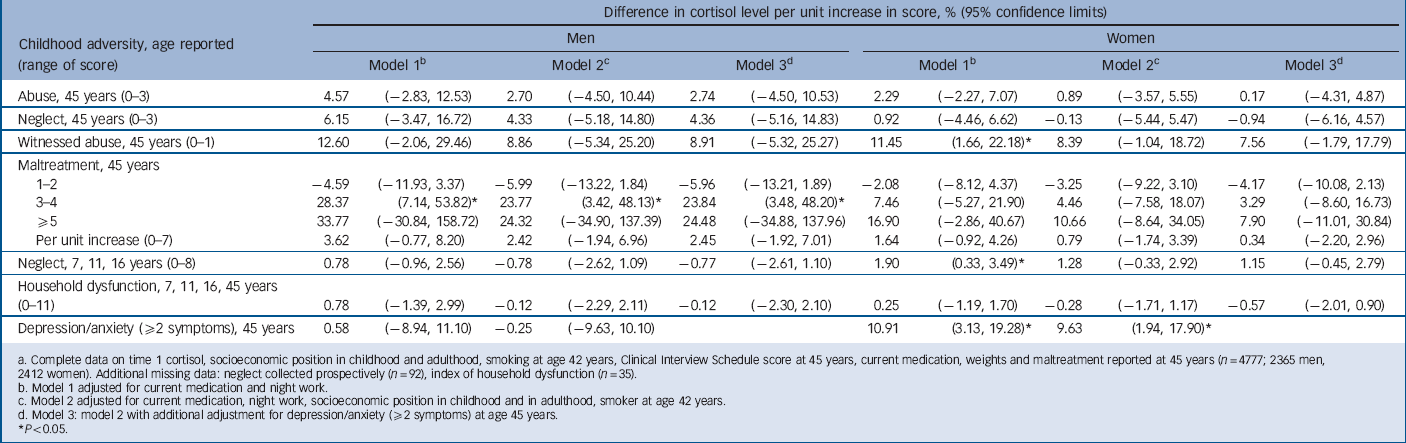
For T 2 cortisol there was no association with either childhood abuse or household dysfunction (Table 4). Men with a maltreatment score of 3 or more had an elevated T 2 cortisol (by approximately 30%) compared with those with no maltreatment. This association remained although it was slightly attenuated after adjustment. A weaker (non-significant) trend with maltreatment score was observed for women. However, cortisol level at T 2 was elevated among women who witnessed abuse and there was a trend for neglect score (using childhood measures). These associations were weakened after adjustment partly owing to the elevated T 2 cortisol among women with current depression/anxiety symptoms (Table 4).
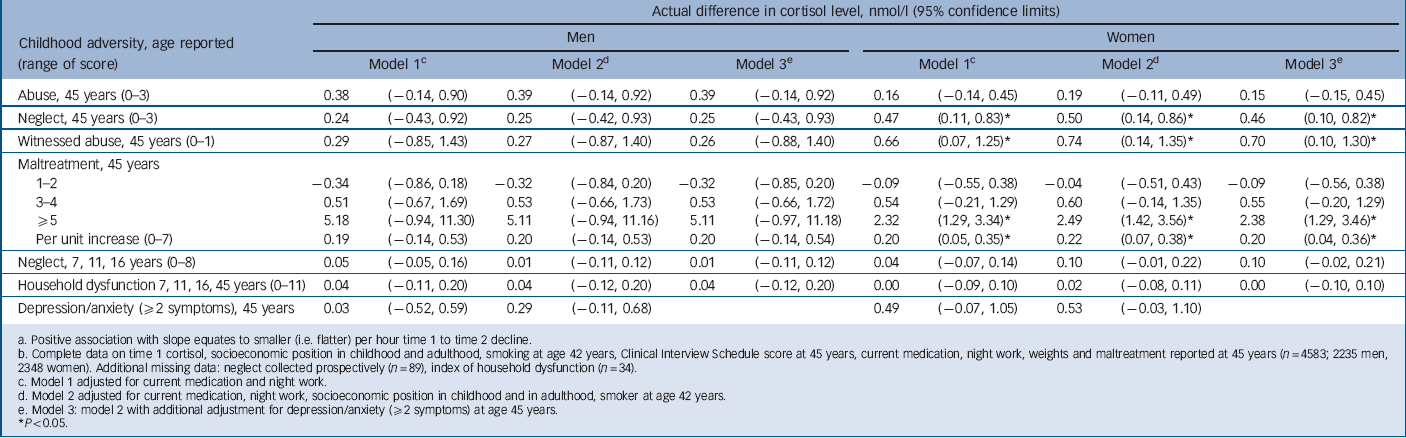
Most participants had a higher cortisol levels at T 1 than at T 2, i.e. there was a negative T 1 to T 2 slope (Table 2). If T 1 is low and/or T 2 is elevated the negative slope will be less steep, indicating a slower decline in cortisol levels over the morning. In women, neglect (age 45 years), witnessing abuse and maltreatment scores were associated with less steep negative slopes before and after adjustment (Table 5). Total 3 h cortisol exposure, indicated by AUC, was lower by 4.3% (95% CI –8.1 to –0.3) for each increment in neglect score (45 years) for women but not for men (gender interaction P = 0.01). This association did not diminish after adjustment (data not presented).
Discussion
Cumulative maltreatments in childhood were associated with flattened morning cortisol secretion (T 1 cortisol or T 1 to T 2 decline) in mid-adult life. For women, the total burden of childhood maltreatment was associated with reduced T 1 cortisol level at age 45 years, with a notable contribution of neglect (reported retrospectively) to this association. Specifically, T 1 cortisol declined by more than 3% for each increment across a 0–7 maltreatment scale, regardless of concurrent adult depressive and anxiety symptoms, and correspondingly there was a less steep morning (T 1 to T 2) decline in cortisol levels. Consistently for both men and women, those with the most maltreatments (five or more) had lower T 1 cortisol levels by more than 25%. In men, T 2 cortisol was strongly elevated for those with three or more maltreatments, even after adjustment; this pattern was similar, albeit weaker, for women. No independent association was found between childhood abuse or household dysfunction and T 1 or T 2 cortisol.
Methodological considerations
Ascertainment of childhood maltreatment and other psychosocial adversities is not straightforward because all ascertainment methods have biases and inconsistencies. Reference Gilbert, Widom, Browne, Fergusson, Webb and Janson6 Our neglect and household dysfunction measures were based on information collected in childhood (from parents and teachers) and in adulthood from study participants. Measures of abuse were constructed from adult reports alone. There are different limitations associated with each method; information from parents may be influenced by socially desirable responding and concealment, whereas reports from study participants in adulthood may be affected by recollection. Nonetheless, we used conventional definitions for childhood abuse and neglect. For example, child neglect is defined as failure to meet a child's basic physical, emotional, medicinal/dental or educational need; to provide adequate nutrition, hygiene and shelter; or to ensure a child's safety. Reference Gilbert, Widom, Browne, Fergusson, Webb and Janson6 Our neglect measures collected in childhood largely – although not completely – capture this definition. Any single study, including ours, cannot entirely overcome inherent problems of measurement. However, we used multiple informants, time points and indicators to best identify a range of childhood adversities. Such a range of measures provides insights into whether associations with adult cortisol levels are robust to data ascertainment method. Wherever possible we created scores to reflect burden of adversity, rather than relying on any single item. It is also noteworthy that all reports of childhood adversity were collected masked to knowledge of cortisol levels.
TABLE 4 Change in time 2 cortisol level per unit increase in score for childhood maltreatment, household dysfunction and adult depression/anxietyFootnote a
| Difference in cortisol level per unit increase in score, % (95% confidence limits) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Childhood adversity, age reported
(range of score) |
Men | Women | ||||||||||
| Model 1Footnote b | Model 2Footnote c | Model 3Footnote d | Model 1Footnote b | Model 2Footnote c | Model 3Footnote d | |||||||
| Abuse, 45 years (0-3) | 4.57 | (–2.83, 12.53) | 2.70 | (–4.50, 10.44) | 2.74 | (–4.50, 10.53) | 2.29 | (–2.27, 7.07) | 0.89 | (–3.57, 5.55) | 0.17 | (–4.31, 4.87) |
| Neglect, 45 years (0-3) | 6.15 | (–3.47, 16.72) | 4.33 | (–5.18, 14.80) | 4.36 | (–5.16, 14.83) | –0.92 | (–4.46, 6.62) | –0.13 | (–5.44, 5.47) | –0.94 | (–6.16, 4.57) |
| Witnessed abuse, 45 years (0-1) | 12.60 | (–2.06, 29.46) | 8.86 | (–5.34, 25.20) | 8.91 | (–5.32, 25.27) | –11.45 | (–1.66, 22.18)Footnote * | –8.39 | (–1.04, 18.72) | –7.56 | (–1.79, 17.79) |
| Maltreatment, 45 years | ||||||||||||
| 1-2 | –4.59 | (–11.93, 3.37) | –5.99 | (–13.22, 1.84) | –5.96 | (–13.21, 1.89) | –2.08 | (–8.12, 4.37) | –3.25 | (–9.22, 3.10) | –4.17 | (–10.08, 2.13) |
| 3-4 | 28.37 | (–7.14, 53.82)Footnote * | 23.77 | (–3.42, 48.13)Footnote * | 23.84 | (–3.48, 48.20)Footnote * | –7.46 | (–5.27, 21.90) | –4.46 | (–7.58, 18.07) | –3.29 | (–8.60, 16.73) |
| ⩾5 | 33.77 | (–30.84, 158.72) | 24.32 | (–34.90, 137.39) | 24.48 | (–34.88, 137.96) | 16.90 | (–2.86, 40.67) | 10.66 | (–8.64, 34.05) | 7.90 | (–11.01, 30.84) |
| Per unit increase (0-7) | 3.62 | (–0.77, 8.20) | 2.42 | (–1.94, 6.96) | 2.45 | (–1.92, 7.01) | –1.64 | (–0.92, 4.26) | 0.79 | (–1.74, 3.39) | 0.34 | (–2.20, 2.96) |
| Neglect, 7, 11, 16 years (0-8) | 0.78 | (–0.96, 2.56) | –0.78 | (–2.62, 1.09) | –0.77 | (–2.61, 1.10) | 1.90 | (–0.33, 3.49)Footnote * | 1.28 | (–0.33, 2.92) | 1.15 | (–0.45, 2.79) |
| Household dysfunction, 7, 11, 16, 45
years (0-11) |
0.78 | (–1.39, 2.99) | –0.12 | (–2.29, 2.11) | –0.12 | (–2.30, 2.10) | 0.25 | (–1.19, 1.70) | –0.28 | (–1.71, 1.17) | –0.57 | (–2.01, 0.90) |
| Depression/anxiety (⩾2 symptoms), 45 years | 0.58 | (–8.94, 11.10) | –0.25 | (–9.63, 10.10) | –10.91 | (–3.13, 19.28)Footnote * | –9.63 | (–1.94, 17.90)Footnote * | ||||
* P<0.05.
a Complete data on time 1 cortisol, socioeconomic position in childhood and adulthood, smoking at 42 years, Clinical Interview Schedule score at 45 years, current medication, weights and maltreatment reported at 45 years (n = 4777; 2365 men, 2412 women). Additional missing data: neglect collected prospectively (n = 92), index of household dysfunction (n = 35).
b Model 1 adjusted for current medication and night work.
c Model 2 adjusted for current medication, night work, socioeconomic position in childhood and in adulthood, smoker at age 42 years.
d Model 3: model 2 with additional adjustment for depression/anxiety (52 symptoms) at age 45 years. (⩾2 symptoms) at 45 years.
TABLE 5 Change in time 1 to time 2 cortisol slope per unit increase in score for childhood maltreatment, household dysfunction and adult (45y) depression/anxietyFootnote a , Footnote b
| Actual difference in cortisol level, nmol/l (95% confidence limits) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Childhood adversity, age reported
(range of score) |
Men | Women | ||||||||||
| Model 1Footnote c | Model 2Footnote d | Model 3Footnote e | Model 1Footnote c | Model 2Footnote d | Model 3Footnote e | |||||||
| Abuse, 45 years (0-3) | 0.38 | (–0.14, 0.90) | 0.39 | (–0.14, 0.92) | 0.39 | (–0.14, 0.92) | 0.16 | (–0.14, 0.45) | 0.19 | (–0.11, 0.49) | 0.15 | (–0.15, 0.45) |
| Neglect, 45 years (0-3) | 0.24 | (–0.43, 0.92) | 0.25 | (–0.42, 0.93) | 0.25 | (–0.43, 0.93) | 0.47 | (– (0.11, 0.83)Footnote * | 0.50 | (– (0.14, 0.86)Footnote * | 0.46 | (– (0.10, 0.82)Footnote * |
| Witnessed abuse, 45 years (0-1) | 0.29 | (–0.85, 1.43) | 0.27 | (–0.87, 1.40) | 0.26 | (–0.88, 1.40) | 0.66 | (0.07, 1.25)Footnote * | 0.74 | (0.14, 1.35)Footnote * | 0.70 | (0.10, 1.30)Footnote * |
| Maltreatment, 45 years | ||||||||||||
| 1-2 | –0.34 | (–0.86, 0.18) | –0.32 | (–0.84, 0.20) | –0.32 | (–0.85, 0.20) | –0.09 | (–0.55, 0.38) | –0.04 | (–0.51, 0.43) | –0.09 | (–0.56, 0.38) |
| 3-4 | 0.51 | (–0.67, 1.69) | 0.53 | (–0.66, 1.73) | 0.53 | (–0.66, 1.72) | 0.54 | (–0.21, 1.29) | 0.60 | (–0.14, 1.35) | 0.55 | (–0.20, 1.29) |
| ⩾5 | 5.18 | (–0.94, 11.30) | 5.11 | (–0.94, 11.16) | 5.11 | (–0.97, 11.18) | 2.32 | (– (1.29, 3.34)Footnote * | 2.49 | (– (1.42, 3.56)Footnote * | 2.38 | (– (1.29, 3.46)Footnote * |
| Per unit increase (0-7) | 0.19 | (–0.14, 0.53) | 0.20 | (–0.14, 0.53) | 0.20 | (–0.14, 0.54) | 0.20 | (0.05, 0.35)Footnote * | 0.22 | (0.07, 0.38)Footnote * | 0.20 | (0.04, 0.36)Footnote * |
| Neglect, 7, 11, 16 years (0-8) | 0.05 | (–0.05, 0.16) | 0.01 | (–0.11, 0.12) | 0.01 | (–0.11, 0.12) | 0.04 | (–0.07, 0.14) | 0.10 | (–0.01, 0.22) | 0.10 | (–0.02, 0.21) |
| Household dysfunction, 7, 11, 16, 45
years (0-11) |
0.04 | (–0.11, 0.20) | 0.04 | (–0.12, 0.20) | 0.04 | (–0.12, 0.20) | 0.00 | (–0.09, 0.10) | 0.02 | (–0.08, 0.11) | 0.00 | (–0.10, 0.10) |
| Depression/anxiety (⩾2 symptoms), 45 years | 0.03 | (–0.52, 0.59) | 0.29 | (–0.11, 0.68) | 0.49 | (–0.07, 1.05) | 0.53 | (–0.03, 1.10) | ||||
* P<0.05.
a Positive association with slope equates to smaller (i.e. flatter) per hour time 1 to time 2 decline.
b Complete data on time 1 cortisol, socioeconomic position in childhood and adulthood, smoking at age 42 years, Clinical Interview Schedule score at 45 years, current medication, night work, weights and maltreatment reported at 45 years (n = 4583; 2235 men, 2348 women). Additional missing data: neglect collected prospectively (n = 89), index of household dysfunction (n = 34).
c Model 1 adjusted for current medication and night work.
d Model 2 adjusted for current medication, night work, socioeconomic position in childhood and in adulthood, smoker at age 42 years.
e Model 3: model 2 with additional adjustment for depression/anxiety (52 symptoms) at age 45 years.
A further consideration is our measurement of cortisol twice in the morning on one day. There is a lack of consensus on the measurement of HPA axis function; several measures are used, for example, based solely on cortisol or its ratio to dehydroepiandrosterone (DHEA). Reference Herbert, Goodyer, Grossman, Hastings, De Kloet and Lightman15 Cortisol measurement is usually timed to take account of the well-established normative diurnal rhythm of a post-waking peak followed by a decline over the next few hours. Reference Chida and Steptoe17 The cortisol awakening response (CAR) is frequently used, based on a measure immediately on waking and then at intervals (e.g. 30 min) over the subsequent hour. Reference Chida and Steptoe17 Ideally, multiple saliva collections over several days best characterise an individual's diurnal cortisol rhythm, including measures such as CAR. We lacked a measure on waking so were unable to assess CAR, but our post-waking measure was timed to capture the period of peak concentration (a systematic review of CAR gives a range of 20–45 min after waking as the period of peak concentration, and several other studies have also used 45 min). Reference Chida and Steptoe17 In our large population study a maximum of two samples on one day was feasible. Our second cortisol measure was timed at 3 h after T 1 (i.e. before lunch) to assess whether levels declined as expected in accord with the normative diurnal rhythm. To summarise limitations, from our two morning measures we could not assess decline throughout the rest of the day or CAR, and no childhood cortisol measure was available. Nevertheless, the two samples provide an approximation of both post-waking peak concentration and the average rate of subsequent decline in adult cortisol level. Because of the study size, precision in estimation of effects is gained at the group level, although estimates for individuals may be less reliable. Accordingly, differences in adult cortisol patterns within this population have been shown for social classes and other groups. Reference Li, Power, Kelly, Kirschbaum and Hertzman12 Furthermore, we found associations between current medication use and T 1 and T 2 cortisol. Given the numerous drugs and medical conditions that could affect cortisol, these associations warrant separate investigation. Owing to sample attrition complete data were available for less than half of the original cohort and underrepresented participants with the most adverse childhood backgrounds. We therefore undertook a weighted analysis using factors associated with non-participation to allow for differential loss to follow-up. Reference Atherton, Fuller, Shepherd, Strachan and Power16
Our study overcomes several limitations of research to date, such as clinical or special populations with specific types of adversity, Reference Roy18–Reference Weissbecker, Floyd, Dedert, Salmon and Sephton20 small sample size, focus solely on women, Reference King, Mandansky, King, Fletcher and Brewer2,Reference Roy18,Reference Stein, Yehuda, Koverola and Hanna21 or short-term follow-up. Reference Carlson and Earls1,Reference King, Mandansky, King, Fletcher and Brewer2 As it was a general population study we were able to capture experiences that vary widely in severity and may accumulate across types of adversity. If multiple adversities represent the most severely affected individuals, one could argue that population-based studies are less efficient than clinical studies. We argue that they are complementary because those with the greatest burden of adversity in a general population will not necessarily be identified through clinical and administrative referral.
Interpretation of findings
Childhood adversity, notably maltreatment, is associated with multiple health hazards many years later in adulthood. Reference Gilbert, Widom, Browne, Fergusson, Webb and Janson6 One potential biological mechanism linking childhood adversity and later outcome involves regulation of the HPA axis, although few studies exist to establish cortisol patterns into middle age. This is an important omission because the impact of childhood insults on cortisol regulation may evolve over the lifespan. Studies restricted to childhood have reported severe tactile deprivation in the first 2 years of life and sexual abuse of young girls to be associated with lower early morning cortisol levels, Reference Carlson and Earls1,Reference King, Mandansky, King, Fletcher and Brewer2 whereas diverse forms of maltreatment were associated with elevated levels. Reference Cicchetti and Rogosch3 Some studies suggest that associations can persist into adulthood, such that severe childhood stress and trauma may be related to adult hypocortisolism. Reference Roy18,Reference Weissbecker, Floyd, Dedert, Salmon and Sephton20–Reference Gunnar and Vasquez22 One notable prospective study highlighted the changing relationships with age: high cortisol levels were seen when childhood maltreatment was first experienced, followed by lower levels as the HPA axis evolved from child to adulthood differently in the maltreated and non-maltreated groups. Reference Trickett, Noll, Susman, Shenk and Putnam5 This pattern may be due to downregulation of the HPA system in the maltreated individuals in response to initially high levels of circulating glucocorticoids. Reference Dozier and Peloso23 Whether cortisol levels are elevated, reduced or show no differences in relation to child maltreatment may therefore depend on life stage, i.e. the time elapsed since maltreatment. Our findings need to be considered in light of such evolving life-course patterns, in that associations with cortisol in mid-adulthood might not be evident across all childhood maltreatments. Consistent with reports of adult hypocortisolism in relation to severe childhood stress and trauma, Reference Trickett, Noll, Susman, Shenk and Putnam5,Reference Roy18,Reference Weissbecker, Floyd, Dedert, Salmon and Sephton20–Reference Gunnar and Vasquez22 we found incremental reductions in adult T 1 cortisol and flattened decline over the morning among women with accumulating burden of childhood maltreatment. Our study adds to the growing literature suggesting that the HPA axis may become downregulated in response to maltreatment in childhood, leading to a flattened cortisol diurnal rhythm. Cortisol pattern may have implications for subsequent health outcomes, with some recent studies suggesting that a flattened diurnal pattern is associated with poorer physical function at older ages, Reference Gardner, Lightman, Gallacher, Hardy, Kuh and Ebrahim24 and increased risk of cardiovascular mortality. Reference Kumari, Shipley, Stafford and Kivimaki25
It has been argued that associations with cortisol patterns may differ, for example by type of maltreatment, Reference Gunnar and Vasquez22 possibly due to variation in age in childhood or developmental stage of the brain when different maltreatments occurred. Reference Lupien, McEwen, Gunnar and Heim4 In our study, variations were observed related to childhood adversity measures and gender. Although no association was observed for childhood abuse or household dysfunction, we found that women (but not men) had lower average T 1 cortisol with childhood neglect (at 45 years). These results parallel the findings from a study of mental health in which the risk of major depressive disorder was elevated in association with childhood neglect but not with sexual abuse. Reference Horwitz, Widom, McLaughlin and White7 Women might be subject to more – or more upsetting – life events such as abuse than men, Reference Bebbington26 as observed in our study. Research also suggests that women are more vulnerable to effects of life events on mental health, Reference van Os, Jones, Lewis, Wadsworth and Murray27 possibly reflecting gender differences in social roles that enable men to distance themselves from life events. Reference Nazroo, Edwards and Brown28 On the other hand, higher T 2 cortisol levels were seen in men but not in women in relation to cumulative maltreatment.
Our finding of lack of association between childhood neglect and adult cortisol level for men was robust to data ascertainment method, whereas for women associations varied for neglect ascertained in child and adulthood. Plausibly, current mental health could affect retrospective report of childhood adversities differently for men and women. Our analyses show higher T 2 cortisol for women with current symptoms of anxiety or depression, as suggested elsewhere. Reference Herbert, Goodyer, Grossman, Hastings, De Kloet and Lightman15 However, adjustment for current symptoms had little effect on the adversity–cortisol associations, for men or women. This does not support a ‘reappraisal bias’ according to current mental health. In the absence of population-based longitudinal data, the natural history of maltreatment and cortisol secretion remains a matter of speculation. Our observation of reduced adult T 1 cortisol with cumulative childhood maltreatment is consistent with reports of low cortisol output and psychiatric ill health in adulthood. Reference Strickland, Deakin, Percival, Dixon, Gater and Goldberg29 However, our analyses suggest that altered adult cortisol secretion patterns associated with cumulative child maltreatments were not operating through current psychological state. Studies of samples such as the 1958 birth cohort have the capacity to put childhood maltreatment in the context of other influences that might mediate the association with adult cortisol secretion.
Appendix
Questionnaire items at age 45 years
Abuse
Psychological abuse by a parent (verbally abused or humiliated, ridiculed, bullied/mental cruelty)
Physical abuse by a parent (punched, kicked or hit or beaten with an object, or needed medical treatment)
Sexual abuse by a parent
Witnessed abuse
Witnessed physical or sexual abuse of others in family
Neglect
Neglected
Father not at all affectionate
Mother not at all affectionate
Childhood maltreatment reported during childhood
Neglect (7, 11 or 16 years of age)
Scruffy, dirty or underfed appearance (7, 11 years)
Mother hardly ever reads to child (7 years)
Father hardly ever reads to child (7 years)
Hardly ever takes outings with mother (7, 11 years)
Hardly ever takes outings with father (7, 11 years)
Mother little interest in education (7, 11 or 16 years)
Father little interest in education (7, 11 or 16 years)
Low parental aspirations: leave school at minimum age (11 or 16 years)
Other psychosocial adversities
Household dysfunction (7, 11, 16 or 45 years)
Domestic tension (7, 45 years)
Parental drink or drug problem (7, 45 years)
Doesn't get on well with mother (16 years)
Doesn't get on well with father (16 years)
Institutional care by age 16 years (16 years)
Father suffered from nervous or emotional trouble or depression (45 years)Mother suffered from nervous or emotional trouble or depression (45 years)
Strict, authoritarian or regimented upbringing (45 years)
Too much physical punishment – hitting, smacking, etc. (45 years)
Separation or divorce of parents by age 16 years
Grew up in poverty (45 years)
Funding
Analysis was funded by the UK Medical Research Council (MRC) (grant G0000934) and the Human Early Learning Partnership, Vancouver, Canada. The work was undertaken at the Great Ormond Street Hospital/University College London Institute of Child Health, London, who received a proportion of funding from the UK Department of Health's National Institute for Health Research Biomedical Research Centres funding scheme. The Centre for Paediatric Epidemiology and Biostatistics also benefits from funding support from the MRC in its capacity as the MRC Centre of Epidemiology for Child Health. L.L. was funded by an MRC career development award in biostatistics.
Acknowledgements
We are grateful to participants in the 2002–2004 clinical follow-up of the 1958 birth cohort and to the Medical Research Council for support of this survey. Cortisol levels were measured under the direction of Professor Kirschbaum, Biological Psychology, Department of Psychology, University of Dresden, Germany.








eLetters
No eLetters have been published for this article.