Introduction
Schizophrenia and other psychoses are severe neuropsychiatric disorders, heterogeneous in aetiology, clinical trajectory and treatment response (Fanous and Kendler, Reference Fanous and Kendler2005; van Os, Reference van Os2016). In approximately 30% of schizophrenia patients, psychotic symptoms respond poorly, if at all, to most antipsychotics, with the notable exception of clozapine (Elkis and Buckley, Reference Elkis and Buckley2016; Gillespie et al., Reference Gillespie, Samanaite, Mill, Egerton and MacCabe2017; Howes et al., Reference Howes, McCutcheon, Agid, de Bartolomeis, van Beveren, Birnbaum, Bloomfield, Bressan, Buchanan, Carpenter, Castle, Citrome, Daskalakis, Davidson, Drake, Dursun, Ebdrup, Elkis, Falkai, Fleischacker, Gadelha, Gaughran, Glenthoj, Graff-Guerrero, Hallak, Honer, Kennedy, Kinon, Lawrie, Lee, Leweke, MacCabe, McNabb, Meltzer, Moller, Nakajima, Pantelis, Reis Marques, Remington, Rossell, Russell, Siu, Suzuki, Sommer, Taylor, Thomas, Ucok, Umbricht, Walters, Kane and Correll2017). This emerged as the gold-standard pharmacological intervention for ‘treatment-resistant schizophrenia’ (TRS) in 1988 (Kane et al., Reference Kane, Honigfeld, Singer and Meltzer1988). Since then, studies have varied considerably in their definitions of TRS (Howes et al., Reference Howes, McCutcheon, Agid, de Bartolomeis, van Beveren, Birnbaum, Bloomfield, Bressan, Buchanan, Carpenter, Castle, Citrome, Daskalakis, Davidson, Drake, Dursun, Ebdrup, Elkis, Falkai, Fleischacker, Gadelha, Gaughran, Glenthoj, Graff-Guerrero, Hallak, Honer, Kennedy, Kinon, Lawrie, Lee, Leweke, MacCabe, McNabb, Meltzer, Moller, Nakajima, Pantelis, Reis Marques, Remington, Rossell, Russell, Siu, Suzuki, Sommer, Taylor, Thomas, Ucok, Umbricht, Walters, Kane and Correll2017), although there is a consistent minimum requirement of two periods of adherence to two different antipsychotics, each administered at adequate doses (variously defined) for at least 4 weeks, resulting in symptom reductions of <20% (Gillespie et al., Reference Gillespie, Samanaite, Mill, Egerton and MacCabe2017).
Recent evidence suggests that TRS is neurobiologically and categorically distinct from treatment-responsive schizophrenia (Gillespie et al., Reference Gillespie, Samanaite, Mill, Egerton and MacCabe2017). Unlike treatment-responsive patients, treatment-resistant (TR) ones do not exhibit an elevation in dopamine synthesis capacity (Demjaha et al., Reference Demjaha, Murray, McGuire, Kapur and Howes2012), and instead, show elevated glutamate levels in the anterior cingulate cortex (Demjaha et al., Reference Demjaha, Egerton, Murray, Kapur, Howes, Stone and McGuire2014). In addition, previous findings from the ÆSOP-10 study (Aetiology and Ethnicity in Schizophrenia and Other Psychoses) by our group showed that 84% of TR patients were resistant from illness onset (primary TRS), while the remaining 16% made an initial response to antipsychotics, but became TR later (secondary TRS) (Demjaha et al., Reference Demjaha, Lappin, Stahl, Patel, MacCabe, Howes, Heslin, Reininghaus, Donoghue, Lomas, Charalambides, Onyejiaka, Fearon, Jones, Doody, Morgan, Dazzan and Murray2017). Lally et al. (Reference Lally, Ajnakina, Di Forti, Trotta, Demjaha, Kolliakou, Mondelli, Reis Marques, Pariante, Dazzan, Shergil, Howes, David, MacCabe, Gaughran and Murray2016) showed similar results in the GAP (Genetics and Psychosis) first-episode study, with 70% of TR patients having primary TRS.
Despite emerging evidence that specific combinations of cognitive deficits define disease heterogeneity as related to treatment response (Gilbert et al., Reference Gilbert, Merette, Jomphe, Emond, Rouleau, Bouchard, Roy, Paccalet and Maziade2014), neuropsychological investigations largely remain an untapped resource for characterising the origin and mechanism of TRS. To date, only six studies have compared neuropsychological function between TR and treatment-responsive patients with psychosis, showing the former to have consistent relative deficits in verbal domains, such as language, verbal intelligence, verbal memory, verbal fluency and verbal interference (Joober et al., Reference Joober, Rouleau, Lal, Dixon, O'Driscoll, Palmour, Annable, Bloom, Lalonde, Labelle and Benkelfat2002; de Bartolomeis et al., Reference de Bartolomeis, Balletta, Giordano, Buonaguro, Latte and Iasevoli2013; Iasevoli et al., Reference Iasevoli, Giordano, Balletta, Latte, Formato, Prinzivalli, De Berardis, Tomasetti and de Bartolomeis2016), less consistently reported deficits in nonverbal domains, such as performance intelligence, processing speed, visuospatial function and visual memory (Joober et al., Reference Joober, Rouleau, Lal, Dixon, O'Driscoll, Palmour, Annable, Bloom, Lalonde, Labelle and Benkelfat2002; Bourque et al., Reference Bourque, Lakis, Champagne, Stip, Lalonde, Lipp and Mendrek2013; Frydecka et al., Reference Frydecka, Beszlej, Goscimski, Kiejna and Misiak2016), and no cognitive differences from treatment-responsive patients in one study (Anderson et al., Reference Anderson, McIlwain, Kydd and Russell2015).
All the above studies have involved cross-sectional investigations of chronically ill samples with schizophrenia or schizoaffective disorder, and with established group differences in treatment response and medication profiles. The respective research designs and methodologies have allowed limited conclusions with regard to two important questions: (1) Do neuropsychological differences between TR and treatment-responsive individuals reflect premorbid differences or the impact of non-remitting psychosis? (2) Do findings from TRS generalise to other psychoses?
To address these questions, we examined baseline neuropsychological data from ÆSOP-10, a population-based study of first-episode psychosis (FEP) with a 10-year follow-up (Fearon et al., Reference Fearon, Kirkbride, Morgan, Dazzan, Morgan, Lloyd, Hutchinson, Tarrant, Fung, Holloway, Mallett, Harrison, Leff, Jones and Murray2006; Morgan et al., Reference Morgan, Dazzan, Morgan, Jones, Harrison, Leff, Murray and Fearon2006, Reference Morgan, Lappin, Heslin, Donoghue, Lomas, Reininghaus, Onyejiaka, Croudace, Jones, Murray, Fearon, Doody and Dazzan2014). All neuropsychological assessments were carried out during the patients’ first episode of psychosis, approximately 10 years before participants were characterised as TR or treatment responsive following detailed re-constructions of their case histories by the ÆSOP team (Demjaha et al., Reference Demjaha, Lappin, Stahl, Patel, MacCabe, Howes, Heslin, Reininghaus, Donoghue, Lomas, Charalambides, Onyejiaka, Fearon, Jones, Doody, Morgan, Dazzan and Murray2017). In line with the TRS literature and with additional neuropsychological findings in support of dimensional models of psychosis (Kravariti et al., Reference Kravariti, Russo, Vassos, Morgan, Fearon, Zanelli, Demjaha, Lappin, Tsakanikos, Dazzan, Morgan, Doody, Harrison, Jones, Murray and Reichenberg2012), we predicted that TR patients would show deficits in verbal tasks of intelligence, fluency and memory compared with treatment-responsive patients and community controls, both among participants with schizophrenia and in the extended sample with various psychoses.
Methods
The ÆSOP-10 study
The present analysis included baseline neuropsychological, sociodemographic and clinical data from ÆSOP-10 (Aetiology and Ethnicity in Schizophrenia and Other Psychoses), a 10-year longitudinal follow-up, population-based study of FEP (Fearon et al., Reference Fearon, Kirkbride, Morgan, Dazzan, Morgan, Lloyd, Hutchinson, Tarrant, Fung, Holloway, Mallett, Harrison, Leff, Jones and Murray2006; Morgan et al., Reference Morgan, Dazzan, Morgan, Jones, Harrison, Leff, Murray and Fearon2006). The study identified all individuals aged 16–65 years with FEP [codes F20–F29 and F30–F33 in the International Classification of Diseases, 10th Revision (ICD-10) manual (World Health Organisation: WHO, 1992a)], who presented to specialist mental health services in tightly defined catchment areas in Southeast London, Nottingham and Bristol between September 1997 and August 2000. Exclusion criteria were the previous contact with health services for psychosis, organic causes of psychotic symptoms, transient psychosis due to acute intoxication (as defined by ICD–10) and IQ<50. [Due to the primary focus of this analysis on neuropsychological functions, we used a higher threshold of inclusion herein: IQ>69, as assessed by the Wechsler Intelligence Scale-Revised (WAIS-R; Wechsler, Reference Wechsler1981)]. The study further included a random sample of community controls with no past or present psychotic disorder, recruited using mainly a sampling method that matched cases and controls by area of residence. Across the three centres, 568 cases with consensus diagnoses of psychotic illness who met the study inclusion criteria, and 412 community controls, were identified. Patients provided detailed contact information for themselves, their General Practitioners (GPs) and relatives, and consent to be re-contacted for follow-up. Ethical approvals for the baseline and follow-up studies were obtained from local research ethics committees. Detailed overviews of the ÆSOP study design and procedures have been published elsewhere (Fearon et al., Reference Fearon, Kirkbride, Morgan, Dazzan, Morgan, Lloyd, Hutchinson, Tarrant, Fung, Holloway, Mallett, Harrison, Leff, Jones and Murray2006; Morgan et al., Reference Morgan, Dazzan, Morgan, Jones, Harrison, Leff, Murray and Fearon2006, Reference Morgan, Lappin, Heslin, Donoghue, Lomas, Reininghaus, Onyejiaka, Croudace, Jones, Murray, Fearon, Doody and Dazzan2014; Demjaha et al., Reference Demjaha, Lappin, Stahl, Patel, MacCabe, Howes, Heslin, Reininghaus, Donoghue, Lomas, Charalambides, Onyejiaka, Fearon, Jones, Doody, Morgan, Dazzan and Murray2017).
Baseline assessment of sociodemographic and clinical characteristics
Sociodemographic data were collected by interviews with the participants using the Medical Research Council Sociodemographic Schedule (Mallett, Reference Mallett1997). Information gaps were filled using additional data sources, including case notes and other informants. Clinical data were collected as soon as possible after the first contact with psychiatric services using the Schedules for Clinical Assessment in Neuropsychiatry (SCAN; WHO, 1992b). The SCAN incorporates the Present State Examination (PSE) – Version 10, which was used to elicit symptom-related data at presentation. Where a patient interview was not possible, case notes and, when available, information from informants, were used to complete the SCAN Item Group Checklist. Baseline symptom scores were further subjected to factor analysis, giving rise to five psychopathological dimensions: manic, reality distortion, negative, depressive and disorganization symptom dimensions (for full details, see Demjaha et al., Reference Demjaha, Morgan, Morgan, Landau, Dean, Reichenberg, Sham, Fearon, Hutchinson, Jones, Murray and Dazzan2009). Patients’ ICD–10 diagnoses were determined using the SCAN data on the basis of consensus meetings involving a principal investigator (PI) and other members of the research team with satisfactory interrater reliability (κ values ranged from 0.63 to 0.75, p < 0.001). Duration of untreated psychosis (DUP) was defined as the period from the onset of psychosis to the first contact with statutory mental health services (for full details, see Morgan et al., Reference Morgan, Dazzan, Morgan, Jones, Harrison, Leff, Murray and Fearon2006). Data on illicit substance use before the first presentation to mental health services were collected retrospectively using an ad hoc secondary data collection schedule, which collated data on prevalence and type of illicit substance use from relatives or carers, from the SCAN (WHO, 1992b) and from clinical case notes. Controls were screened for psychosis using the Psychosis Screening Questionnaire (Bebbington and Nayani, Reference Bebbington and Nayani1995). Those with a positive rating were further assessed using the SCAN (WHO, 1992b) and, where appropriate, excluded.
Baseline neuropsychological assessment
The present analysis included neuropsychological data collected at baseline using the National Adult Reading Test-Revised (NART-R) (Nelson and Willison, Reference Nelson and Willison1991), assessing premorbid intelligence; the Vocabulary, Comprehension, Block Design, and Digit Symbol subtests of the WAIS-R (Wechsler, Reference Wechsler1981), assessing verbal and non-verbal intelligence; trials 1–5 and 7 of the Rey Auditory Verbal Learning Test (RAVLT) (Spreen and Strauss, Reference Spreen and Strauss1991), assessing immediate and delayed verbal recall and learning; the immediate Visual Reproduction trials of the Wechsler Memory Scale – Revised (WMS-R) (Wechsler, Reference Wechsler1987), assessing visual memory; the Trail Making A (Reitan, Reference Reitan1958), Trail Making B (Reitan, Reference Reitan1958) and Letter-Number Span (Gold et al., Reference Gold, Carpenter, Randolph, Goldberg and Weinberger1997) tests of processing speed, working memory and executive function; and Category and Letter Verbal Fluency (category: ‘body parts’, ‘fruits’ and ‘animals’; letter: F, A, S), assessing verbal ability and executive control.
Follow-up clinical assessment
At approximately 10 years after inclusion, we made an attempt to trace, re-contact and re-assess 557 ÆSOP participants with psychosis, who had initially been identified in the Southeast London and Nottingham centres. Using an extended version of the WHO Life Chart Schedule (LCS) (WHO, 1992c) and a Medication History Timeline, comprehensive information on psychopathology, all prescribed antipsychotic medications (start and end date, dosage, adherence, reasons for change or termination), substance use and contact with mental health services was collected and rated for the entire follow-up period using a wide range of information sources: medical case records, follow-up interview with participant or informants (where possible), treating clinicians, ward and community prescriptions, medication charts and clinical documentation (including, where available, reports of drug level testing, and correspondence from the prescribing clinician/to GPs). Using the above sources, adherence to each prescribed antipsychotic throughout the period it was prescribed was rated on a three-point scale (1: 0–33%; 2: 34–67%; 3: 68–100%), using 68% as the cut-off for ‘adherence’. Presence of symptoms at follow-up was further assessed using the Scan – Version 2 (WHO, 1994). Based on all available information, case histories were reconstructed for the entire follow-up period to complete all sections of the Life Chart. A detailed overview of the follow-up clinical assessment procedures has been published elsewhere (Morgan et al., Reference Morgan, Lappin, Heslin, Donoghue, Lomas, Reininghaus, Onyejiaka, Croudace, Jones, Murray, Fearon, Doody and Dazzan2014; Demjaha et al., Reference Demjaha, Lappin, Stahl, Patel, MacCabe, Howes, Heslin, Reininghaus, Donoghue, Lomas, Charalambides, Onyejiaka, Fearon, Jones, Doody, Morgan, Dazzan and Murray2017).
Representativeness of the follow-up sample
Of the 557 cases who were initially recruited, 434 (78%) underwent follow-up assessments. There were no marked differences in gender, ethnicity, duration of untreated psychosis, or diagnosis between the cases who underwent follow-up assessments and those who were lost to follow-up, except that the follow-up sample was younger (t = 2.5; p = 0.02).
Criteria for treatment response and treatment resistance
‘Response to treatment’ was defined as a state of no or mild symptoms (SCAN score<2), not interfering with daily functioning, lasting at least 6 months (Andreasen et al., Reference Andreasen, Carpenter, Kane, Lasser, Marder and Weinberger2005). In line with National Institute for Health and Care Excellence (NICE) criteria (National Institute for Health and Care Excellence, 2014), patients were classified as ‘Treatment Resistant’, if, despite recorded adherence to medication, they continued to show positive symptoms of at least moderate severity (SCAN score⩾2) following two sequential trials of antipsychotic medication at a daily dose of 400–600 mg of chlorpromazine equivalence, each lasting at least 4 weeks. Patients were classified as ‘Treatment Resistant from Illness Onset’ (TRO) if they met resistance criteria after the first two trials of antipsychotic medication, and as ‘Delayed Onset Treatment Resistant’ (DOTR), if such criteria were met after a period of response to treatment. An individual meeting treatment resistance criteria, but later meeting treatment response criteria, would have been classified as treatment resistant. However, the ÆSOP-10 Study did not identify individuals whose response to medication improved during the course of illness. Of the 50 patients who received clozapine (by definition TR), 14 (28%) were clozapine responders, 12 (24%) were clozapine resistant, and the remaining 24 (48%) could not be classified, due to a suboptimal clozapine trial or insufficient clinical and response data (Demjaha et al., Reference Demjaha, Lappin, Stahl, Patel, MacCabe, Howes, Heslin, Reininghaus, Donoghue, Lomas, Charalambides, Onyejiaka, Fearon, Jones, Doody, Morgan, Dazzan and Murray2017).
Representativeness of the follow-up sample that was evaluated for treatment response
Of the 434 cases who were assessed at follow-up, 212 (49%) met criteria for treatment response, 74 (17%) met criteria for treatment resistance (of whom 62: 84% were TRO) and 37 (9%) had never received an adequate trial of antipsychotic medication and could not be included in either category. The remaining 111 participants (26%) had incomplete clinical information documentation and could not be classified. Cases with complete information did not differ notably from the remainder of the follow-up sample in terms of age, gender, ethnicity, DUP or diagnosis (Demjaha et al., Reference Demjaha, Lappin, Stahl, Patel, MacCabe, Howes, Heslin, Reininghaus, Donoghue, Lomas, Charalambides, Onyejiaka, Fearon, Jones, Doody, Morgan, Dazzan and Murray2017).
Statistical analysis
Data reduction and generation of composite neuropsychological scores
To avoid the caveats of multiple testing and of experimenter assumptions during the grouping of cognitive tasks into overarching constructs (e.g. executive function), we reduced an original set of 13 neuropsychological variables to a small number of components, using Principal Component Analysis (PCA) with promac oblique rotation in Stata/MP 14.0 (StataCorp, 2015). This was performed on the present analytic cohort, i.e. 402 ÆSOP participants (145 cases, 257 controls) with available neuropsychological data at baseline (all participants), and with entire case history reconstruction at the 10-year follow-up (cases only). Where appropriate, variables were inverse transformed to achieve normality of distribution. The selected cut-off for the variable loadings on the PCA components was 0.30, in line with recommendations that this has practical significance for sample sizes of at least 350 (Hair et al., Reference Hair, Tatham, Anderson and Black1998, p. 112). The oblique method was preferred over the varimax solution for its superior capacity to identify a simple factorial structure, particularly in datasets where the latent traits are correlated (Finch, Reference Finch2006). The Kaiser-Meyer-Olkin (KMO) test of sampling adequacy was used to determine the factorability of the data. To generate composite neuropsychological scores (each reflecting a participant's composite ability across variables with primary loadings on a certain component), we estimated PCA scores using Stata's predict command with the score option immediately after the PCA command. The PCA scores are expressed in standardised units based on linear combinations of the retained components.
Comparison of composite neuropsychological scores across study groups
Statistical analysis was performed in Stata/MP 14.0 (StataCorp, 2015). Each of the main and sensitivity (see below) analyses was a multivariable regression analysis with robust standard errors, comparing selected study groups or subgroups in each composite neuropsychological score. All main and sensitivity analyses co-varied for sociodemographic and (where appropriate) clinical variables that were associated with each composite score at p < 0.1 in preliminary univariable linear regression analyses (see online Supplementary Table S1). Composite neuropsychological scores were compared across the TR, treatment-responder (non-TR) and community control groups, as well as between TR and non-TR cases in the full patient sample and in the diagnostic subgroup with schizophrenia.
Performing separate analyses for cases who were TR from Illness Onset (TRO) and those who had Delayed-Onset Treatment Resistance (DOTR) would enhance the interpretability of our findings. However, this was not possible due to the small size of the DOTR subgroup (n = 6). We instead repeated all data analytic steps in sub-analyses which excluded the DOTR patients.
Sensitivity analysis
All main statistical analyses uniformly controlled for the effects of ethnicity and education. However, as the TR group had a higher proportion of Black ethnic minorities (47%) than the non-TR (24%) and control (14%) groups (see Results and Table 1), it was important to further address potential confounding influences of language in our analysis. We, therefore, assessed the sensitivity of our findings to excluding all participants who had been born outside the UK, regardless of ethnicity or first language (n = 58; 15 non-TR, 6 TR, 37 controls). This left in the analysis an all-UK-born sample who had attended compulsory schooling in the UK.
Table 1. Sociodemographic and clinical characteristics in the treatment-responder, treatment-resistant and community control groups
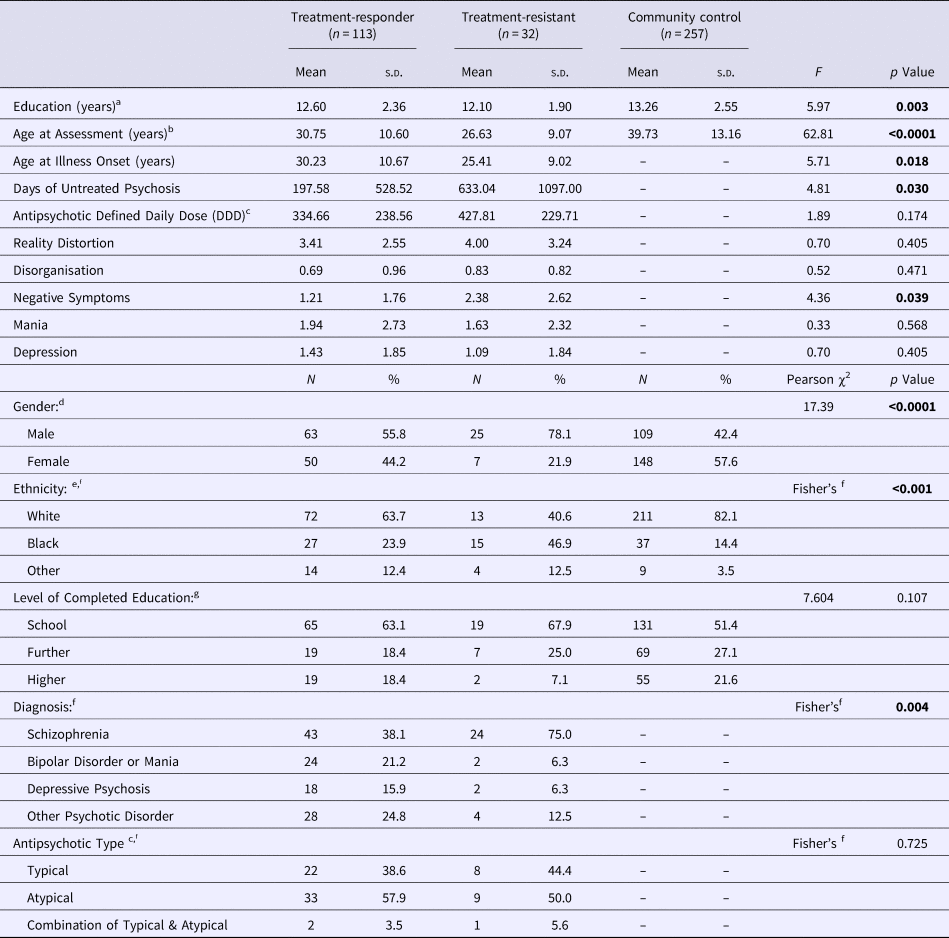
Bold denote significance level for p values.
a TR, non-TR<CC.
b TR, non-TR<CC, TR<non-TR.
c Data was available for a subset of 75 cases (52%).
d Proportion Male: TR, non-TR>CC; TR>non-TR.
e Proportion Black: TR, non-TR>CC; TR>non-TR.
f As the statistical assumptions for the χ2 test were violated, Fisher's Exact Test was performed.
g Level of completed education was missing for 16 participants (4%).
Missing data
The level of completeness of sociodemographic and clinical information at the time of the baseline neuropsychological assessment was high (96–100% for socio-demographic variables and 82–100% for clinical variables). The high level of completeness at baseline is partly attributable to the temporal proximity of the sociodemographic, clinical and neuropsychological assessments, which were typically completed within a few days of each other. Antipsychotic medication history was re-constructed in detail at the 10-year follow up. Exact recording of neuropsychological testing dates at baseline was available only in a subset of cases, allowing us to map the participants’ complex medication histories onto the dates of their neuropsychological testing in a subgroup of 75 cases (52%, 57 non-TR, 18 TR). Detailed information on the medication received by this subgroup is presented in online Supplementary Table S2.
Results
Representativeness of the analytic cohort
Of the 286 cases who were classified in terms of treatment response at follow-up (see Methods), 136 cases (48%) lacked neuropsychological data at baseline, and 5 (2%) had IQ<70, leaving 113 non-TR cases, 32 TR cases (of whom 26: 81% were TRO), and 257 community controls in the present analysis. There were no notable differences in age, gender, DUP or diagnosis between the patient analytic cohort (n = 145), and those who lacked neuropsychological data at baseline or met exclusion criteria (N = 141). However, the patient analytic cohort (treatment-responders and TR cases combined) comprised a lower proportion of black ethnic minorities (29%) than the cases who lacked neuropsychological data or who met exclusion criteria (51%; χ2 = 14.563, p = 0.001).
Sociodemographic and clinical characteristics
The sociodemographic and clinical characteristics of the analytic cohort are presented in Table 1. Compared with controls, both patient groups were younger, had fewer years of education, and a higher proportion of male and Black participants (p < 0.01–0.0001). Compared with treatment-responders, TR cases were younger, had a higher proportion of male and Black participants, a higher score on the negative symptom dimension, and a longer duration of untreated psychosis (p < 0.05–0.0001) (Table 1). The two patient groups did not differ statistically significantly in illicit substance use [positive lifetime history present in 27 (25%) non-TR- and in 4 (12.5%) TR participants; χ2 = 2.237, p = 0.135].
Data reduction and estimation of composite neuropsychological scores
The PCA gave rise to a three-component solution (eigenvalues 1.20–5.78) accounting for 0.65% of the variance. The results of the promax rotation of the solution are presented in Table 2. NART IQ, WAIS-R Vocabulary, WAIS-R Comprehension, Phonological Verbal Fluency and Semantic Verbal Fluency showed primary loadings (0.306–0.520) on Component 1, which was labelled Verbal Intelligence and Fluency. WAIS-R Block Design, WAIS-R Digit Symbol and Trail Making (A & B) showed primary loadings (0.332–0.565) on Component 2, which was labelled Visuospatial Ability and Executive Function. The immediate and delayed recall trials of the RAVLT showed primary loadings (0.598–0.698) on Component 3, which was labelled Verbal Memory and Learning. The Kaiser-Meyer Olkin measure of sampling adequacy indicated that the sample had very high factorability (KMO = 0.875). Composite neuropsychological scores (PCA scores) were generated for the three components and used in the remaining analyses.
Table 2. Obliquely rotated component loadingsa for 13 neuropsychological variables in the analytic cohortb (n = 402)
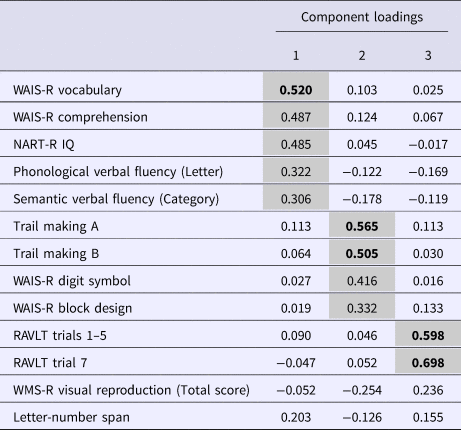
NART-R, National Adult Reading Test-Revised; RAVLT, Rey Auditory Verbal Learning Test; WAIS-R, Wechsler Adult Intelligence Scale-Revised.
a Variables with primary loadings of >0.30 are set against a grey background, and high loadings of >0.50 are highlighted in bold font.
b The sample included participants who had undergone neuropsychological testing at baseline, had IQ ⩾70, and could be classified (in the case of patients, retrospectively, i.e. at the 10-year follow-up) as treatment responders (n = 113), treatment-resistant (n = 32), or community controls (n = 257).
Comparison of composite neuropsychological scores across study groups
Tables 3 and 4 present the means and standard deviations of the composite neuropsychological scores in the three study groups, as well as the results and effect sizes of selected group comparisons. Figure 1 presents the distribution of the composite scores in Verbal Intelligence and Fluency in the full analytic cohort divided by study group. Both patient samples were impaired in all composite scores compared with controls (p ⩽0.001) (Table 3), with moderate to large effect sizes in the treatment-responsive patients and with large to very large effect sizes in the TR cohort.
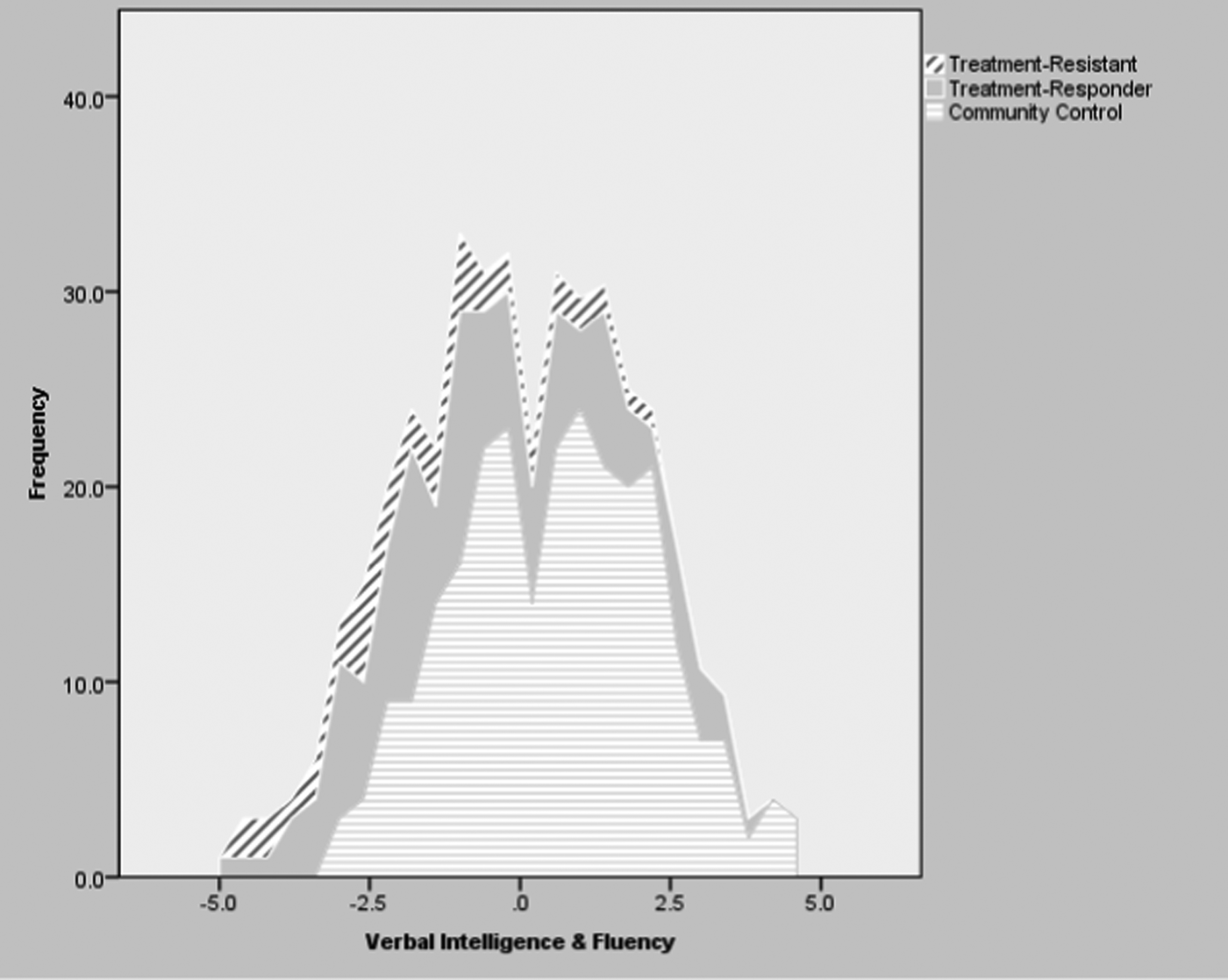
Fig. 1. Distribution of Composite Verbal Intelligence & Fluency Scores in the Complete Analytic Cohorts of Treatment-Resistant, Treatment-Responder and Community Control Participants.
Table 3. Comparisona of composite neuropsychological scores across the treatment-responder, treatment-resistant and community control groups
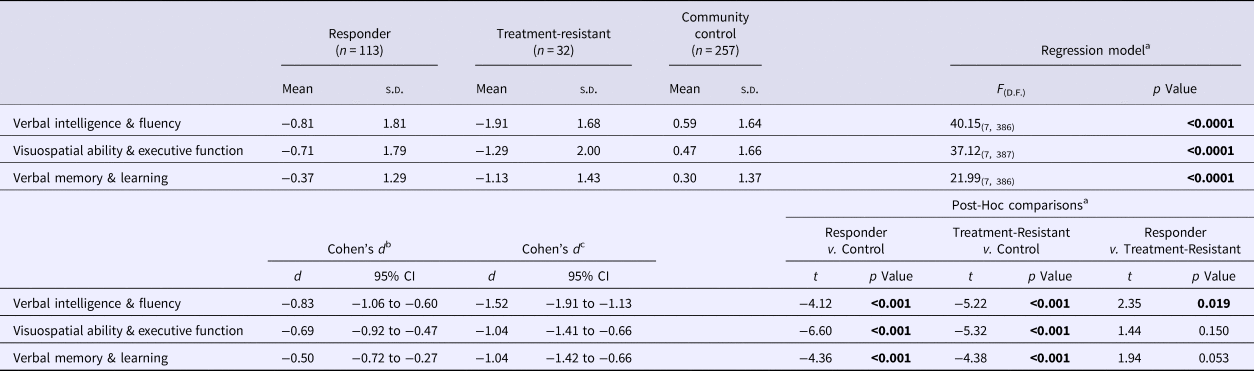
Bold denote significance level for p values.
a The effect of Group (Treatment Responder, Treatment-Resistant, Community Control) on each Composite Score was examined using multivariable regression analysis with robust standard errors, co-varying for demographic variables that emerged as significant (p < 0.05) or suggestive (p < 0.1) predictors of each Composite Score in preliminary univariable linear regression analyses (online Supplementary Table S1): Age, Ethnicity, Years of Education (all Composite Scores) and Gender (Verbal Intelligence & Fluency; Verbal Memory & Learning).
b Standardised mean difference the between treatment-responder and community-control groups.
c Standardised mean difference between the treatment-resistant and community-control groups.
Table 4. Comparison of composite neuropsychological scores between the treatment-responder and treatment-resistant groups
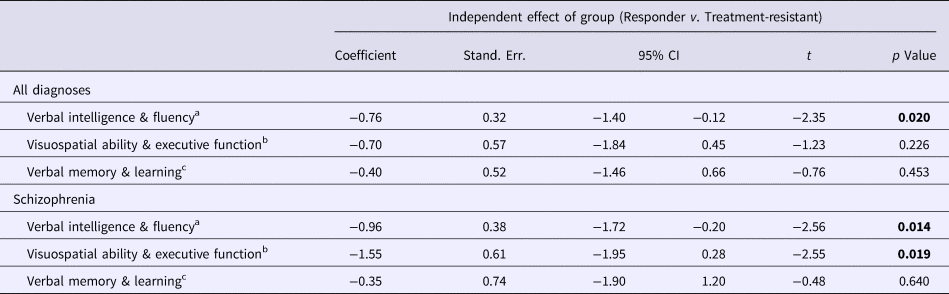
Bold denote significance level for p values.
a The effect of Group (Treatment Responder v. Treatment Resistant) on Verbal Intelligence & Fluency was examined using multivariable regression analysis with robust standard errors, co-varying for demographic and clinical variables that emerged as significant (p < 0.05) or suggestive (p < 0.1) predictors of Verbal Intelligence & Fluency in preliminary univariable linear regression analyses (online Supplementary Table S1): Age, Gender, Ethnicity, Years of Education, Negative Symptoms, Mania and Depression. The analysis for ‘All Diagnoses’ additionally co-varied for ‘Diagnosis’.
b The effect of Group (Treatment Responder v. Treatment Resistant) on Visuospatial Ability & Executive Function was examined using multivariable regression analysis with robust standard errors, co-varying for demographic and clinical variables that emerged as significant (p < 0.05) or suggestive (p < 0.1) predictors of Visuospatial Ability & Executive Function in preliminary univariable linear regression analyses (online Supplementary Table S1): Age, Ethnicity, Years of Education, Age at Illness Onset, Negative Symptoms, Mania, Medication Dose (expressed in Defined Daily Dose units) and Illicit Substance Use [positive/negative lifetime history of, based on information collected from relatives or carers, the Schedules for Clinical Assessment in Neuropsychiatry (SCAN; WHO, 1992b), clinical case notes and an extended version of the WHO Life Chart Schedule (WHO, 1992c)]. The analysis for ‘All Diagnoses’ additionally co-varied for ‘Diagnosis’.
c The effect of Group (Treatment Responder v. Treatment Resistant) on Verbal Memory & Learning was examined using multivariable regression analysis with robust standard errors, co-varying for demographic and clinical variables that emerged as significant (p < 0.05) or suggestive (p < 0.1) predictors of Verbal Memory & Learning in preliminary univariable linear regression analyses (online Supplementary Table S1): Age, Gender, Ethnicity, Years of Education, Duration of Untreated Psychosis, Reality Distortion, Negative Symptoms, Mania and Medication Dose (expressed in Defined Daily Dose units). The analysis for ‘All Diagnoses’ additionally co-varied for ‘Diagnosis’.
Compared to non-TR patients, the TR cases performed worse in Verbal Intelligence and Fluency in all main analyses (p < 0.05) (Tables 3 and 4; Figure 1), sub-analyses (p < 0.01–0.001) (excluding DOTR case: online Supplementary Tables S3 and S4), and sensitivity analyses (p < 0.05) (excluding non-UK-born participants: online Supplementary Tables S5 and S6).
In the schizophrenia subgroup, TR cases performed worse than non-TR patients in Visuospatial Ability and Executive Function (p < 0.05) (Table 4), but the finding did not persist in our sub-analyses or sensitivity analyses (online Supplementary Tables S3–S6).
Discussion
Summary of findings
Our analysis of baseline data from the ÆSOP-10 longitudinal, population-based study of FEP provides a snapshot of the neuropsychological function at the earliest stages of TR psychosis. Both TR and treatment-responsive patients with schizophrenia and other psychoses showed generalised neuropsychological deficits in verbal intelligence and fluency, visuospatial abilities and executive function, and verbal memory and learning compared with community controls. Furthermore, TR patients showed, on average, impairments in verbal intelligence and fluency compared with treatment responders, replicating previous findings (Joober et al., Reference Joober, Rouleau, Lal, Dixon, O'Driscoll, Palmour, Annable, Bloom, Lalonde, Labelle and Benkelfat2002; Frydecka et al., Reference Frydecka, Beszlej, Goscimski, Kiejna and Misiak2016). This differential was an enduring finding of our analyses – evident for individuals with schizophrenia, those with any psychoses, psychotic and schizophrenia patients born in the UK, and after excluding cases with delayed-onset treatment resistance.
Methodological considerations
This is the first longitudinal, population-based study of FEP that compared baseline neuropsychological function across patients with longitudinally-defined TR- and non-TR psychosis and community controls. The study draws on unique methodological advantages: The epidemiological source and robust size of the combined patient and community control groups increased the representativeness of our cohort and generalisability of our findings, whilst facilitating stringent statistical controls (including a sensitivity analysis) for a wide array of clinical, sociodemographic, and language confounders. Analysing neuropsychological data from the first episode minimised information bias (i.e. examiners did not know the participants’ treatment response status at the time of testing) and reduced differentiation in clinical features between the two patient groups. Specifically, in contrast to all previous neuropsychological studies of TRS (Joober et al., Reference Joober, Rouleau, Lal, Dixon, O'Driscoll, Palmour, Annable, Bloom, Lalonde, Labelle and Benkelfat2002; Bourque et al., Reference Bourque, Lakis, Champagne, Stip, Lalonde, Lipp and Mendrek2013; de Bartolomeis et al., Reference de Bartolomeis, Balletta, Giordano, Buonaguro, Latte and Iasevoli2013; Anderson et al., Reference Anderson, McIlwain, Kydd and Russell2015; Frydecka et al., Reference Frydecka, Beszlej, Goscimski, Kiejna and Misiak2016; Iasevoli et al., Reference Iasevoli, Giordano, Balletta, Latte, Formato, Prinzivalli, De Berardis, Tomasetti and de Bartolomeis2016), our patient groups did not seem to differ notably in treatment profiles or medication doses at the time of testing (based on a subgroup analysis). These aspects served to further reduce confounding in our analysis.
Methodological limitations of our study include loss to follow up; limitations on clinical data accuracy associated with case history reconstruction; the availability of baseline neuropsychological testing dates for only a subgroup of patients; the lack of screening for family history of psychosis in community controls; and the moderate size of the TR cohort. A deficit in verbal intelligence and fluency in TR patients compared with responders was a highly consistent finding across our main analyses, sub-analyses and sensitivity analyses. However, we cannot exclude the possibility that additional deficits are integral to TR psychosis, particularly in relation to schizophrenia (discussed below). Our selected cut-off for factor loadings (0.30) is among the lowest reported in the literature (Peres-Neto et al., Reference Peres-Neto, Jackson and Somers2003), and may have reduced the clarity of the PCA components. Using a 0.40 cut-off would not have changed the pattern of findings (data available upon request). Finally, our analyses included baseline diagnoses. As with the extended ÆSOP sample (Heslin et al., Reference Heslin, Lomas, Lappin, Donoghue, Reininghaus, Onyejiaka, Croudace, Jones, Murray, Fearon, Dazzan, Morgan and Doody2015), most schizophrenia patients (77%) and 40% of those with ‘other’ diagnoses in our analytic cohort were classified as having schizophrenia at 10 years. Using diagnostic classifications at follow up, 31.5% of schizophrenia patients would have been classified as TR compared with 35.8% using baseline diagnoses.
TR psychosis as a severe neurodevelopmental variant of psychosis
The neurodevelopmental theory of schizophrenia posits the existence of a neurodevelopmental subtype of schizophrenia, which is the end product of aberrant neurodevelopmental processes unfolding from conception or early life (Murray et al., Reference Murray, O'Callaghan, Castle and Lewis1992). It has been suggested that primary TRS has a distinct neurodevelopmental origin, while secondary TRS may arise through the induction of dopamine super-sensitivity, or after periods of relapse, although a later emergence of an intrinsic treatment resistance, or a combination of underlying factors cannot be ruled out (Lally et al., Reference Lally, Ajnakina, Di Forti, Trotta, Demjaha, Kolliakou, Mondelli, Reis Marques, Pariante, Dazzan, Shergil, Howes, David, MacCabe, Gaughran and Murray2016; Demjaha et al., Reference Demjaha, Lappin, Stahl, Patel, MacCabe, Howes, Heslin, Reininghaus, Donoghue, Lomas, Charalambides, Onyejiaka, Fearon, Jones, Doody, Morgan, Dazzan and Murray2017; Gillespie et al., Reference Gillespie, Samanaite, Mill, Egerton and MacCabe2017). The clinical and demographic profiles of the TR cases in the present study largely encapsulate the defining features of ‘neurodevelopmental schizophrenia’ – younger, ‘more male’, with an earlier age of onset, more severe negative symptoms, more severe cognitive impairment and a longer duration of untreated psychosis (Murray et al., Reference Murray, O'Callaghan, Castle and Lewis1992). Three further observations suggest a pathogenic origin for the observed verbal deficit in the TR group compared with treatment-responsive individuals. Firstly, the impairment was established by the first episode, arguing against the deficit being caused by non-remitting psychosis. Secondly, Vocabulary and NART-R, two tasks with primary loadings on Verbal Intelligence and Fluency, are reliable tests of premorbid ability, and are both resistant to brain pathological changes (Bright et al., Reference Bright, Jaldow and Kopelman2002; de Oliveira et al., Reference de Oliveira, Nitrini, Yassuda and Brucki2014). Finally, the pattern of deficits in verbal intelligence and fluency was accentuated (deficits were significant at a lower level of statistical significance) after removing cases with secondary treatment resistance.
Black participants were over-represented among TR patients. Although Black ethnicity is not a defining feature of ‘neurodevelopmental schizophrenia’ (Murray et al., Reference Murray, O'Callaghan, Castle and Lewis1992), the finding is in keeping with evidence that treatment resistance is associated with early first contact with psychiatric services (<20 years), and more so in Black (OR 3.71) than in White (OR 1.60) patients (Lally et al., Reference Lally, Ajnakina, Di Forti, Trotta, Demjaha, Kolliakou, Mondelli, Reis Marques, Pariante, Dazzan, Shergil, Howes, David, MacCabe, Gaughran and Murray2016). Indeed, a closer look at our data revealed that only 9.4% of White patients, but 21.4% of Black patients had the first contact with psychiatric services before age 20, which may have increased disproportionately the outcome of treatment resistance in the Black ethnic group.
TR schizophrenia
An additional deficit in visuospatial ability and executive function emerged in TR- compared with non-TR patients in the schizophrenia subgroup. This finding did not generalise to the ‘all-diagnoses’ group and did not persist in our sub-analyses or sensitivity analyses. The finding is consistent with evidence that deficits in executive function, processing speed and verbal memory, albeit less salient in other diagnostic categories of psychosis, are prototypical of schizophrenia (Kravariti et al., Reference Kravariti, Morgan, Fearon, Zanelli, Lappin, Dazzan, Morgan, Doody, Harrison, Jones, Murray and Reichenberg2009a, Reference Kravariti, Reichenberg, Morgan, Dazzan, Morgan, Zanelli, Lappin, Doody, Harrison, Jones, Murray and Fearon2009b; Zanelli et al., Reference Zanelli, Reichenberg, Morgan, Fearon, Kravariti, Dazzan, Morgan, Zanelli, Demjaha, Jones, Doody, Kapur and Murray2010). As the size of the schizophrenia subgroup in the present study was modest, it is important to explore the significance of executive function and verbal memory deficits in TR schizophrenia in larger studies.
Integrating neurodevelopmental and glutamatergic hypotheses of TR psychosis
Some recent findings implicate glutamate rather than dopamine as the primary neurotransmitter system impaired in TR schizophrenia (Demjaha et al., Reference Demjaha, Murray, McGuire, Kapur and Howes2012, Reference Demjaha, Egerton, Murray, Kapur, Howes, Stone and McGuire2014; Gillespie et al., Reference Gillespie, Samanaite, Mill, Egerton and MacCabe2017). Glutamate plays an important role in several language-related neurodevelopmental processes. This highlights the possibility that core deficits in verbal intelligence and fluency, a neurodevelopmental aetiology, and a primary glutamatergic dysfunction may converge in a single model of TR psychosis. Several lines of evidence support this possibility: pre-reading language abilities (e.g. phonological processing) show significant correlations with glutamate in the anterior cingulate of healthy preschool-aged children (Lebel et al., Reference Lebel, MacMaster and Dewey2016); microdeletions in glutamate receptors have been implicated in developmental delays predominantly affecting language and fine motor skills (Takenouchi et al., Reference Takenouchi, Hashida, Torii, Kosaki, Takahashi and Kosaki2014); the high-risk metabotropic glutamate receptor 3 (GRM3) haplotype is associated with schizophrenia, as well as with deficits in verbal fluency and verbal list-learning (Spangaro et al., Reference Spangaro, Bosia, Zanoletti, Bechi, Cocchi, Pirovano, Lorenzi, Bramanti, Benedetti, Smeraldi and Cavallaro2012); and poor-functioning subjects at ultra-high-risk for psychosis show a negative relationship between thalamic glutamate levels and prefrontal-striatal activation during a verbal fluency task (Allen et al., Reference Allen, Chaddock, Egerton, Howes, Barker, Bonoldi, Fusar-Poli, Murray and McGuire2015).
Clinical and research implications
Neuropsychological deficits weigh disproportionally on the psychosocial and functional toll of psychosis (Kaneda et al., Reference Kaneda, Jayathilak and Meltzer2010; Shamsi et al., Reference Shamsi, Lau, Lencz, Burdick, DeRosse, Brenner, Lindenmayer and Malhotra2011; Iasevoli et al., Reference Iasevoli, Giordano, Balletta, Latte, Formato, Prinzivalli, De Berardis, Tomasetti and de Bartolomeis2016). Encouragingly, verbal fluency and executive function deficits, which differentiated TR from non-TR patients in the present study, do not seem refractory to pharmacological interventions. Indeed, there is strong evidence that clozapine improves attention and verbal fluency, and moderate evidence that it improves some types of executive function (Meltzer and McGurk, Reference Meltzer and McGurk1999; Woodward et al., Reference Woodward, Purdon, Meltzer and Zald2005). In the only studies to report equivalent verbal performances in TRS- and non-TRS patients to date, TR cases were uniformly treated with clozapine (Bourque et al., Reference Bourque, Lakis, Champagne, Stip, Lalonde, Lipp and Mendrek2013; Anderson et al., Reference Anderson, McIlwain, Kydd and Russell2015). These findings re-iterate the necessity of timely detection and tailored pharmacological interventions as early as possible in the course of TR psychosis (Lally and MacCabe, Reference Lally and MacCabe2016). They further highlight the importance of neuropsychological constructs in designing multimodal research and clinical approaches to improving prognosis and personalised treatment (Gilbert et al., Reference Gilbert, Merette, Jomphe, Emond, Rouleau, Bouchard, Roy, Paccalet and Maziade2014).
Conclusion
A constitutional deficit in verbal intelligence and fluency, significantly exceeding – at a group level – the levels manifest in the general population of patients with psychoses, is a phenotypic indicator of TR psychosis. Our findings are in keeping with emerging evidence that TR psychosis is a pathogenically distinct and severe variant, embedded in aberrant neurodevelopmental processes.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718002957.
Acknowledgements
We are grateful to the ÆSOP-10 participants for making the study possible and for their valuable contribution of data to the present analysis. The ÆSOP study has received funding support by the UK Medical Research Council (grant number G0500817) and the Department of Health via the National Institute for Health Research (NIHR) Specialist Biomedical Research Centre for Mental Health award to South London and Maudsley NHS Foundation Trust (SLaM) and the Institute of Psychiatry at King's College London. R.M.M. has received honoraria from Janssen, Astra-Zeneca, Lilly, BMS, and Roche. C.M. and R.M.M. have received funding support from the Wellcome Trust (grant no. HEALTH-F2-2009-241909) (Project EU-GEI) and the European Union (European Community's Seventh Framework Program; grant no. HEALTH-F2-2009-241 909; Project EU-GEI). C.M. has further received funding from the Wellcome Trust (grant number WT087417). We thank the Stanley Medical Research Institute for their support.
Conflict of interest
There are no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.







