Introduction
Conservation measures focused on farmland birds rely heavily on knowledge of habitat associations of individual species and are of crucial importance due to the large-scale declines of European farmland bird populations (Donald et al. Reference Donald, Sanderson, Burfield and van Bommel2006, Voříšek et al. Reference Voříšek, Jiguet, van Strien, Škorpilová, Klvaňová and Gregory2010). However, habitat selection of farmland birds can be a complex process that largely depends on specific landscape features at various spatial scales and availability of suitable habitats that may substantially differ in individual regions and landscapes (e.g. Šálek et al. Reference Šálek, Chrenková, Dobrý, Kipson, Grill and Václav2016a). Therefore it is important to understand the habitat flexibility of individual species within various landscapes that are subjected to different, temporally changing, environmental pressures and thus may be heavily affected by these habitat modifications. Moreover, some farmland birds show broad habitat flexibility in nesting and foraging requirements, which may result in population and region-specific habitat associations (Fuller Reference Fuller2012). For example, many farmland birds extensively use farmed habitats for foraging, whereas for nesting they require variety of non-cropped habitat features, such as hedgerows, shrubby vegetation, forest edges or large isolated trees and bushes (Vickery and Arlettaz Reference Vickery, Arlettaz and Fuller2012, Šálek et al. Reference Šálek, Kučera, Zimmermann, Bartůšková, Plátek, Grill and Konvička2015). Furthermore, some populations of farmland birds, even of special conservation interest, can inhabit exclusively non-farmland habitats without an affinity for agricultural areas (Fuller et al. Reference Fuller, Hinsley and Swetnam2004).
Agricultural intensification and landscape abandonment that lead to a decrease in habitat heterogeneity and loss of suitable nesting and foraging habitats are assumed to be crucial factors behind declines of farmland birds (e.g. Suarez-Seoane et al. Reference Suarez-Seoane, Osborne and Baudry2002, Donald et al. Reference Donald, Sanderson, Burfield and van Bommel2006). The Ortolan Bunting Emberiza hortulana is a long-distance migratory farmland species, whose European populations have suffered marked population declines during recent decades (Menz and Arlettaz Reference Menz and Arlettaz2012). For example the results of Pan-European Common Bird Monitoring Scheme have shown that the Ortolan Bunting is one of the most rapidly declining farmland bird species with an 89% population decline during 1980–2014 (PECBMS 2015). Population declines of this species have been reported from 21 European countries and the most massive population reduction and range contractions are reported from northern and western Europe (Vepsäläinen et al. Reference Vepsäläinen, Pakkala, Piha and Tiainen2005, Menz and Arlettaz Reference Menz and Arlettaz2012, Jiguet et al. Reference Jiguet, Arlettaz, Bauer, Belik, Copete, Couzi, Czajkowski, Dale, Dombrovski, Elts, Ferrand, Hargues, Kirwan, Minkevicius, Piha, Selstam, Skierczynski, Siblet and Sokolov2016), but several central European countries also report steep population declines in recent decades (Jiguet et al. Reference Jiguet, Arlettaz, Bauer, Belik, Copete, Couzi, Czajkowski, Dale, Dombrovski, Elts, Ferrand, Hargues, Kirwan, Minkevicius, Piha, Selstam, Skierczynski, Siblet and Sokolov2016). For example, the Ortolan Bunting has a long-term unfavourable population trend in the Czech Republic, and the proportion of occupied quadrats with breeding occurrence declined there by about 60% during 1973–2003 with a total population estimate of 80–160 singing males during 2001–2003 (Šťastný et al. Reference Šťastný, Bejček and Hudec2006). A further population decline has been recorded at the beginning of this century, and the current distribution of Ortolan Buntings in the Czech Republic is highly fragmented, with population size reaching 75–100 singing males (Šálek et al. Reference Šálek, Beran, Hanzlíková, Kipson, Molitor, Praus, Procházka, Šimeček, Vít and Zeman2016b). The progressively negative trend for this farmland bird led to its inclusion to the national red list as a critically endangered species (Šťastný and Bejček Reference Šťastný, Bejček, Plesník, Hanzal and Brejšková2003).
Within its European distribution, the Ortolan Bunting inhabits various breeding habitats including montane landscapes with open shrubland, steppe-like habitats, or heterogeneous farmland with mosaics of non-agricultural elements and low-intensity farmland (Hagemeijer and Blair Reference Hagemeijer and Blair1997, Menz and Arlettaz Reference Menz and Arlettaz2012). More specifically, in the temperate zone in western and central Europe the bunting breeds in areas characterised by structurally diverse farmlands with the presence of non-cropped habitats, shrubby patches and forest edges surrounded by low-intensity arable field cultivations (Deutsch and Sudbeck Reference Deutsch, Sudbeck and Bernardy2009, Šimeček Reference Šimeček and Bernardy2009, Menz and Arlettaz Reference Menz and Arlettaz2012). The crucial feature of its habitat selection is the preference for sparsely vegetated areas that are important for catching arthropod prey species (Menz and Arlettaz Reference Menz and Arlettaz2012). Thus, within farmland landscapes, the species may select breeding habitats within cultivated fields with a higher availability of bare ground (Vepsäläinen et al. Reference Vepsäläinen, Pakkala, Piha and Tiainen2005, Morelli Reference Morelli2012, Brambilla et al. Reference Brambilla, Gustin, Vitulano, Negri, Bogliani, Falco and Celada2017). In contrast, in non-farmland landscapes the Ortolan Bunting may inhabit several early successional and temporary habitats such as forest clear-cuts or burned areas (Dale and Olsen Reference Dale and Olsen2002, Brotons et al. Reference Brotons, Herrando and Pons2008, Menz et al. Reference Menz, Brotons and Arlettaz2009a). For example, in the Czech Republic, the species also inhabits non-farmland habitats of the post-mining landscape created by surface mining of brown coal that are characterised by extensive areas of early successional habitats. The affinity of the Ortolan Bunting for this ecologically highly dynamic anthropogenic landscape is unique within its European range, though the species’ occurrence was documented for other anthropogenic habitats such as quarries, gravel pits and sandpits (Dale and Manceau Reference Dale and Manceau2003, Šálek et al. Reference Šálek, Beran, Hanzlíková, Kipson, Molitor, Praus, Procházka, Šimeček, Vít and Zeman2016b).
In this study we present the first comprehensive results on the habitat associations of the Ortolan Bunting breeding in two ecologically distinct landscapes in the Czech Republic, namely farmland and post-mining landscapes. The main objectives of this study were to identify habitat features which are crucial for Ortolan Buntings within the two contrasting landscapes and to see how habitat associations differ at different spatial scales. Our results bring important insights about a species which shows a rapid decline even despite its remarkable habitat flexibility, thus offering important implications for conservation of ecologically similar species.
Methods
Study area
We studied habitat associations of Ortolan Buntings in all currently known breeding populations of the species in the Czech Republic, that are mainly located in northern and central Bohemia and in northern Moravia (see Šálek et al. Reference Šálek, Beran, Hanzlíková, Kipson, Molitor, Praus, Procházka, Šimeček, Vít and Zeman2016b for further details; Fig. 1a). The species’ individual populations inhabit markedly contrasting landscape types that differ in habitat composition, structure and land-use history.
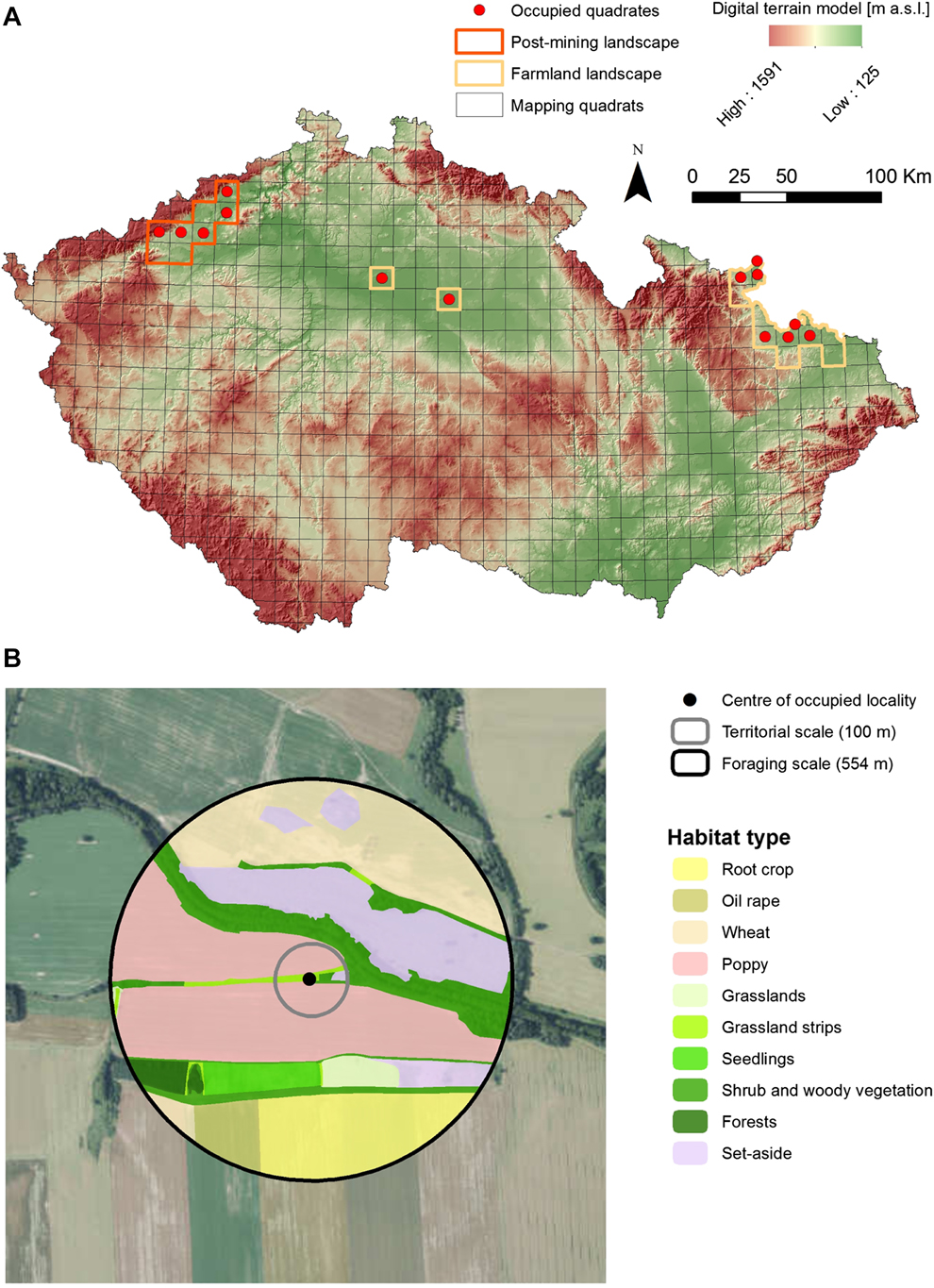
Figure 1. Breeding distribution of the Ortolan Bunting Emberiza hortulana in the Czech Republic in 2015 with (A) the distribution of study regions involving farmland and post-mining landscapes and (B) an example of classification of land use characteristics at the territorial and the foraging spatial scales.
The Northern Bohemian population inhabits the post-mining landscape resulting from surface brown-coal mining at altitudes of 250–350 m a.s.l. The study area is composed of post-mining habitats (57%), agricultural land (24.6%), forests (14.2%), human settlements (2.6%), and water bodies (1.4%). The post-mining habitats are represented by active opencast mining combined with heaped soil, grassland or herbaceous vegetation that are either left for spontaneous succession or technically reclaimed. The areas of spontaneous succession are characterised by topographic heterogeneity and mosaics of sparsely vegetated patches that are covered by early successional plant and herb communities. In contrast, the most common technique of technical reclamation is terrain smoothing, surface covering with fertile topsoil, resowing with grass mixtures and subsequently afforestation with same-age trees (forest reclamation) or using the land for agricultural production (agricultural reclamation).
The study areas in central Bohemia and northern Moravia are situated within farmland landscapes that are characterised by predominantly flat or gently rolling topography with altitudes of 200–400 m. The study areas are covered with the mosaics of agricultural landscape (73.4%), forests (16.7%), human settlements (4.8%) and water bodies (0.6%). Agricultural land is mainly used for cultivation of cereals (wheat, barley, oat), oilseed rape, maize, root crops, or poppy, and grassland mainly comprises intensively used hayfields and, to a lower extent, semi-natural grasslands. Forest habitats are represented by deciduous forests, mixed stands or shrubland vegetation. The forest-farmland edges are usually composed of broadleaved trees from adjacent forest and structurally diverse shrubby mantles.
Bird surveys
Data on Ortolan Bunting breeding distribution came from a nation-wide species monitoring programme. However, this monitoring was mainly focused on core areas of Ortolan Bunting distribution in the Czech Republic. A probable occurrence of the species was reported from one site outside the focal study areas (see Šálek et al. Reference Šálek, Beran, Hanzlíková, Kipson, Molitor, Praus, Procházka, Šimeček, Vít and Zeman2016b). Monitoring of Ortolan Buntings within individual study areas was determined by the locations of singing males (Bibby et al. Reference Bibby, Burgess and Hill2000, Gregory et al. Reference Gregory, Gibbons, Donald, Sutherland, Newton and Green2004), which is an effective method of determining the presence of a bird species within an area (e.g. Vepsäläinen et al. Reference Vepsäläinen, Pakkala, Piha and Tiainen2005). In all study areas, we systematically monitored potentially suitable breeding habitats for breeding territories, with the exception of the interior of dense and compact forests and urban habitats that do not provide suitable breeding habitat for the species (Šťastný et al. Reference Šťastný, Bejček and Hudec2006; Menz and Arlettaz Reference Menz and Arlettaz2012). During 2015, we performed two field visits, the first one from 15 May to 30 May (covering the period of Ortolan Bunting arrival and territory acquisition) and the second one from 30 May to 20 June (covering the nesting period). The monitoring was carried out during optimal weather conditions (without heavy rain and strong wind) within the period of their highest vocal activity (three hours after sunrise), but sometimes extended to the afternoon hours. Accurate locations of individual singing males were plotted on satellite orthophoto maps (scale 1:1000) and recorded on GPS devices.
Environmental analysis
In order to determine habitat associations of Ortolan Buntings, we performed comprehensive habitat mapping within occupied versus unoccupied sites. This approach is a standard methodological procedure in determining species habitat associations (e.g. Šálek et al. Reference Šálek, Červinka, Banea, Krofel, Ćirović, Selanec, Penezić, Grill and Riegert2014, Reference Šálek, Chrenková, Dobrý, Kipson, Grill and Václav2016a) and was successfully applied earlier in studies on Ortolan Buntings (Berg Reference Berg2008, De Groot et al. Reference De Groot, Kmelcl, Figelj, Figelj, Mihelič and Rubinič2010, Morelli Reference Morelli2012) and other bunting species (Whittingham et al. Reference Whittingham, Swetnam, Wilson, Chamberlain and Freckleton2005, Sánchez et al. Reference Sánchez, Václav and Prokop2009) in various European landscapes. We determined the centre of the occupied site as the exact male singing post or where any signs of the breeding behaviour (e.g. alarm calls, birds carrying nesting material) were recorded (see also Berg Reference Berg2008, Sánchez et al. Reference Sánchez, Václav and Prokop2009). If singing posts of singing males were less than 100 m apart during subsequent field visits, we treated this as one individual/pair and chose the middle point for the territory (Dale and Olsen Reference Dale and Olsen2002, Sánchez et al. Reference Sánchez, Václav and Prokop2009). Two individual territories that were closer than 100 m were only considered if individual males were heard singing simultaneously. Within the monitoring areas of individual populations, we produced a grid (200 m x 200 m), which we used to randomly generate the centre points of unoccupied sites, i.e. sites where we did not record Ortolan Buntings (Berg Reference Berg2008). The analyses of habitat preferences of Ortolan Buntings were studied on two spatial scales: territory scale (100 m radius from the centre of occupied sites) that presents the core area of the Ortolan Bunting territory and foraging scale (554 m radius from the centre of occupied sites) which represents approximate wider home range where the majority of foraging behaviour occurs (Cramp and Perrins Reference Cramp and Perrins1994, Dale and Olsen Reference Dale and Olsen2002, Berg Reference Berg2008, De Groot et al. Reference De Groot, Kmelcl, Figelj, Figelj, Mihelič and Rubinič2010; Fig. 1b). In order to compare habitat attributes between occupied and unoccupied patches, we used the same two spatial scales for the unoccupied sites. Due to extremely dissimilar habitats in the study areas (i.e. farmland vs post-mining landscape), it was not possible to record the same habitat features in the two areas. Yet, most habitat features selected have been identified previously as potentially important predictors of Ortolan Bunting presence (Vepsäläinen et al. Reference Vepsäläinen, Pakkala, Piha and Tiainen2005, Berg Reference Berg2008, Deutsch and Sudbeck Reference Deutsch, Sudbeck and Bernardy2009). In particular, we estimated cover of 17 and seven agricultural and non-agricultural habitats for farmland and post-mining landscapes, respectively (Table 1). In addition, we also determined habitat heterogeneity at both spatial scales with respect to edge density and the Shannon habitat diversity index that represent important measures of compositional and configurational heterogeneity (Table 1). Furthermore, for the post-mining landscape, we also evaluated slope steepness and terrain ruggedness of habitat patches at the territory scale classified based on six main categories of mean slope (for more details see Appendix S1 in the online supplementary material). Terrain ruggedness was previously mentioned as an important factor for Ortolan Bunting presence in post-mining landscape (Šálek et al. Reference Šálek, Červinka, Banea, Krofel, Ćirović, Selanec, Penezić, Grill and Riegert2014). Habitat characteristics in the farmland landscape were recorded following the bird monitoring period (mid-June), conducting in situ habitat mapping with the aid of satellite images, which were subsequently vectorised using GIS tools (ESRI 2014, QGIS Development Team 2014). For the study sites located in post-mining landscapes, we conducted ex situ habitat mapping using recent (May 2015) and extremely accurate aerial drone pictures.
Table 1. Environmental characteristics used for evaluation of Ortolan Bunting breeding habitat associations in two contrasting landscapes (post-mining and farmland).

Data analyses
Habitat data acquired in this study imposed several analytical limitations. Specifically, variation in habitat features was generally very low due to zero inflation for most habitat attributes recorded. In addition, for some habitat attributes, marked heterogeneity existed in the frequency of zero values between occupied and unoccupied patches for the same habitat attribute. While these issues prevented examination of several habitat attributes in generalised linear models on the probability of Ortolan Bunting occurrence, it would be incorrect to discard such data from the study. Consequently, we provide two sets of results describing habitat features of occupied vs unoccupied habitat patches. First, we examine for each habitat attribute differences between occupied and unoccupied habitat patches, using the Wilcoxon rank sum test. The likelihood ratio (G) test was used to examine a difference in slope characteristics, as defined by six categories, between occupied and unoccupied habitat patches. Second, we model the probability of Ortolan Bunting occurrence for both farmland (n = 46 occupied and 45 unoccupied habitat patches) and post-mining (n = 45 occupied and 45 unoccupied habitat patches) landscapes using generalised linear mixed models (GLMM) with binomial error distribution. We modelled the bunting occurrence for both landscapes across all spatial scales because breeding habitat selection essentially is a multiscale process (e.g. Jedlikowski et al. Reference Jedlikowski, Chibowski, Karasek and Brambilla2016). Also, predictors were evaluated across all spatial scales because the fit of the model at a specific scale may be biased due to spatial autocorrelation in predictors (e.g. Bradter et al. Reference Bradter, Kunin, Altringham, Thom and Benton2013). In order to explicitly address spatial dependence in Ortolan Bunting distribution data, we used GLMM via Penalized Quasi-Likelihood (glmmPQL) with a spatial correlation structure, based on geographical coordinates, specified within the random component of the model (Dormann et al. Reference Dormann, McPherson, Araújo, Bivand, Bolliger, Carl, Davies, Hirzel, Jetz, Kissling, Kuhn, Ohlemuller, Peres-Neto, Reineking, Schroder, Schurr and Wilson2007). Gaussian, linear, exponential, spherical and rational quadratic spatial correlation structures were considered in GLMMs; the specific structure was selected based on variograms depending on how well it captured spatial correlation. Only habitat attributes showing sufficient variability and approximately normal data distribution for both occupied and unoccupied habitat patches were considered for model building. All such predictors were scaled and centred (z-transformed). We considered curvilinear relationships between response and predictor variables by including quadratic terms in GLMMs. Since glmmPQL output does not include a deviance component, it is not possible to assess the model’s performance based on criteria such as AIC. Consequently, we adopted the approach used by Kissling and Carl (Reference Kissling and Carl2008), and first evaluated how well a model captured spatial correlations using variograms and then assessed the model’s fit based on R2. Models were built from less to more complex by using a forward stepwise approach, starting with predictors that captured spatial structure and variance in the data most accurately. Model building stopped when a new predictor did not contribute significantly to explaining the variance in our data. We addressed the problem of collinearity among significant predictors in GLMMs by inspecting variance inflation factors (VIF) and considered exclusion of predictors with VIF > 2 from models. GLMMs were calculated with glmmPQL function from the MASS package (Venables and Ripley Reference Venables and Ripley2002) and R2 were calculated with the r2glmm package (Jaeger Reference Jaeger2017). Wilcoxon rank sum tests were performed with the stats package in R (R Core Team 2015).
Results
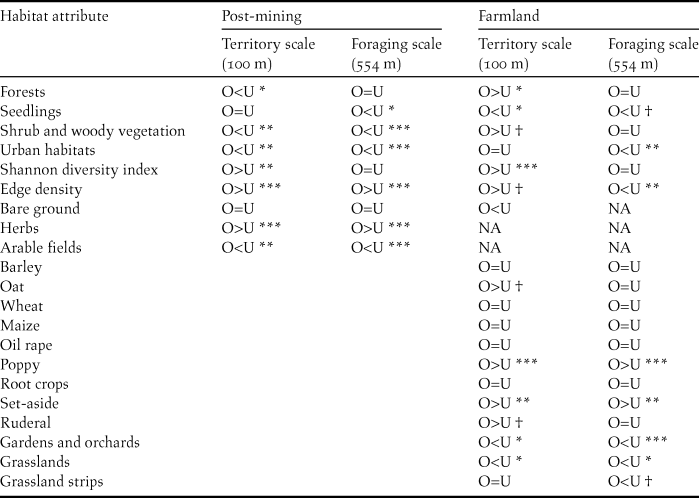
Examining values of median cover for habitat attributes in occupied and unoccupied breeding habitat patches in farmland and post-mining landscapes reveals a high degree of habitat flexibility of Ortolan Buntings in the Czech Republic (Table 2, Appendix S2). Specifically, while in the farmland landscape the cover of shrub and woody vegetation at the territorial scale was higher for occupied than unoccupied habitat patches (Fig. S2A), differences for this habitat attribute were the opposite for the post-mining landscape (Fig. S2C). Also, for some habitat attributes differences between occupied and unoccupied habitat patches occurred only at specific spatial scales (Table 2). For example, while edge density at the territory scale was higher in occupied than unoccupied patches for both landscape types (Fig. S2A, C), this was not true for the foraging spatial scale (Table 2, Fig. S2B, D). Nevertheless, we also identified common habitat features for farmland and post-mining landscapes. At the foraging scale, the cover of seedlings and the cover of urban habitats were smaller for occupied than unoccupied patches in both landscapes (Fig. S2B, D). In turn, at the territory spatial scale, the Shannon diversity index and edge density were consistently higher for occupied than unoccupied patches for both landscapes (Fig. S2A, C). Finally, the likelihood ratio test revealed a significant difference in slope characteristics between occupied and unoccupied patches (G5 = 66.01, P < 0.001). Specifically, Ortolan Buntings were associated with habitat patches showing higher mean slope and terrain ruggedness. Concerning the habitat attributes assessed exclusively for farmland or post-mining landscape, occupied habitat patches showed at both spatial scales a higher cover of herbs (post-mining), poppy fields and agricultural set-asides (farmland). In contrast, occupied habitat patches showed at both spatial scales a smaller cover of arable fields for the post-mining landscape and a smaller cover of grasslands and gardens for the farmland landscape (Table 2, Appendix S2).
Table 2. Wilcoxon rank sum tests on the differences in habitat attributes between occupied (O) and unoccupied (U) breeding habitat patches at territory (100 m) and foraging range scales (554 m) by Ortolan Buntings in post-mining (n = 90 habitat patches) and farmland (n = 91 habitat patches) landscapes.
† 0.05 < P < 0.1, * P < 0.05, ** P < 0.01, *** P < 0.001
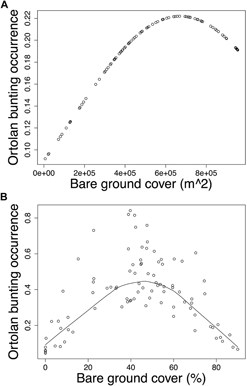
Modelling Ortolan Bunting occurrence for the post-mining landscape, the probability of occurrence was curvilinearly associated with the cover of bare ground at the foraging spatial scale (Table 3a). Specifically, probability of Ortolan Bunting occurrence increased with the cover of bare ground, but it levelled off for habitat patches with the highest bare ground cover (Fig. 2a). For the farmland landscape, the probability of Ortolan Bunting occurrence increased with the Shannon diversity index and was highest for habitat patches with intermediate values of bare ground cover at the territory spatial scale (Table 3b, Fig. 2b). Moreover, the probability of Ortolan Bunting occurrence increased with the cover of shrub and woody vegetation at the foraging spatial scale (Table 3b). GLMM results suggest that Ortolan Bunting distribution was spatially correlated up to 1,324 and 2,374 m for post-mining and farmland landscapes, respectively (Table 3).
Table 3. Generalised linear mixed models (GLMM) on the probability of Ortolan Buntings breeding occurrence across territory (100 m) and foraging (554 m) scales in (a) post-mining and (b) farmland landscapes. GLMMs accounted for spatial autocorrelation based on geographical coordinates of each habitat patch. Degrees of freedom are 87 and 86 for post-mining and farmland landscapes, respectively. In addition to predictors contained in optimal models, the following predictors also were considered in model building: post-mining landscape – bare ground cover 100 m, herbs cover 100 and 554 m, Shannon diversity index 100 and 554 m, edge density 100 and 554 m; farmland landscape – Shannon diversity index 554 m, edge density 100 and 554 m, wheat cover 554 m.
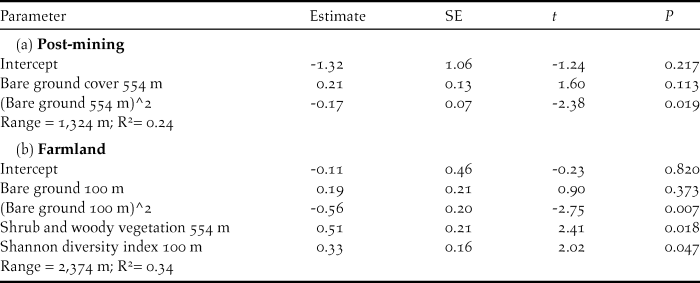
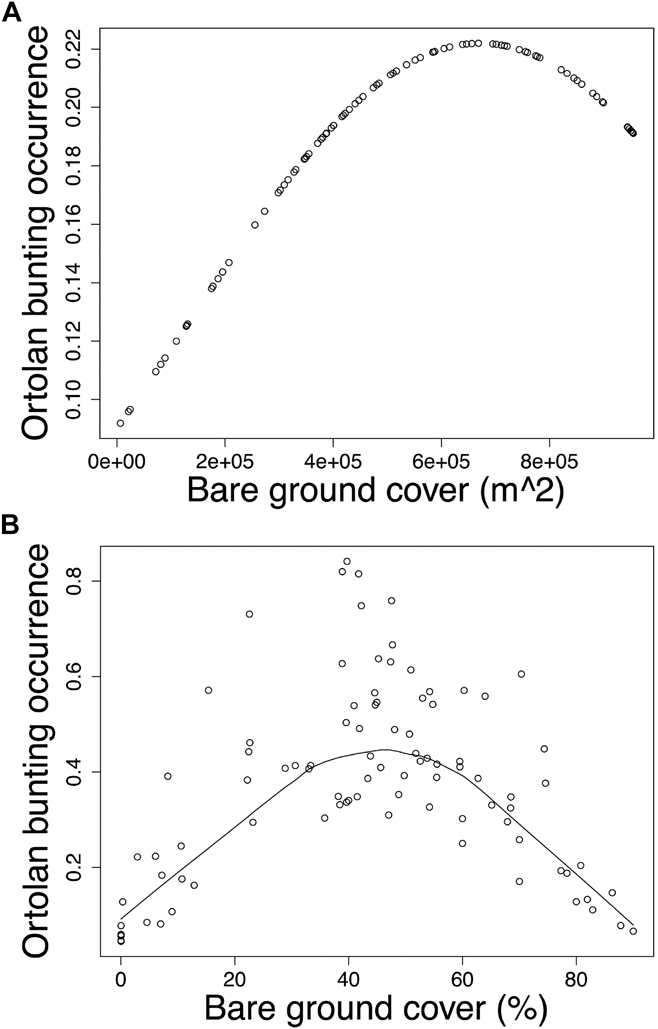
Figure 2. The probability of occurrence of Ortolan Bunting Emberiza hortulana as a function of bare ground cover in (A) the post-mining and (B) the farmland landscapes. Ortolan bunting occurrence was back transformed and is shown on the probability scale; (A) bare ground cover in m2 within the radius of 554 m and (B) the proportion of bare ground within the radius of 100 m are shown for raw untransformed values.
Discussion
For conservation measures to be effective across large spatial scales, it is crucial to determine spatial pattern and scale differences in species-habitat associations. This study documents a high degree of flexibility in habitat selection of rapidly declining Ortolan Buntings, thus emphasizing the importance of within-species variation in habitat affinity. Overall, our results conclusively point to habitat affinity of the Ortolan Bunting for areas with a higher spatial diversity of landscape features, especially at the territory scale. The effect of compositional (i.e. Shannon diversity index) and configurational (i.e. edge density) habitat heterogeneity on Ortolan Bunting breeding occurrence in both landscape types was demonstrated by comparisons of occupied and unoccupied habitat patches at the territory scale. This also was corroborated by GLMM for the farmland landscape, revealing that the probability of the species’ occurrence increased with the Shannon diversity index at the territory scale. A more heterogeneous landscape pattern, comprising the fine-scale mosaic of different agricultural and non-agricultural habitats with different management types, provides a high diversity of foraging and nesting resources (Benton et al. Reference Benton, Vickery and Wilson2003, Vickery and Arlettaz Reference Vickery, Arlettaz and Fuller2012). Landscape heterogeneity is known as a crucial factor in supporting populations of Ortolan Buntings (Vepsäläinen et al. Reference Vepsäläinen, Pakkala, Piha and Tiainen2005, Deutsch and Sudbeck Reference Deutsch, Sudbeck and Bernardy2009, Berg 2012, Brambilla et al. Reference Brambilla, Gustin, Vitulano, Negri, Bogliani, Falco and Celada2017) and other declining farmland birds (Haslem and Bennet 2008, Vickery and Arlettaz Reference Vickery, Arlettaz and Fuller2012, Šálek et al. Reference Šálek, Červinka, Banea, Krofel, Ćirović, Selanec, Penezić, Grill and Riegert2014).
Differences in habitat associations of the Ortolan Bunting between two landscapes with respect to shrub and woody vegetation and forest habitats are an interesting finding of this study. In particular, Ortolan Bunting occurrence in the farmland landscape was positively associated with the cover of shrub and woody vegetation and forests, whereas the opposite was true for the post-mining landscape. Indeed, our analyses showed that Ortolan Buntings inhabiting farmland select for a heterogeneous landscape with a higher representation of shrub and woody vegetation (e.g. tree alleys, shrubs, hedges, woody corridors, and small woodlots) that provide song-posts, foraging and nesting places (cf. Berg, Reference Berg2008, De Groot et al. Reference De Groot, Kmelcl, Figelj, Figelj, Mihelič and Rubinič2010, Brambilla et al. Reference Brambilla, Gustin, Vitulano, Negri and Celada2016, Reference Brambilla, Gustin, Vitulano, Negri, Bogliani, Falco and Celada2017). Shrub and woody vegetation within the agricultural landscape is an important habitat attribute for farmland wildlife (Hilty et al. Reference Hilty, Lidicker and Merenlender2006). Unlike for farmland, the territories of Ortolan Buntings in the post-mining landscape were characterised by a minimal cover of shrub and forest vegetation, with habitat openness being further exemplified by a relatively higher cover of herbs at the territory and foraging scales. Another typical feature of occupied habitat patches in the post-mining landscape is greater slope steepness and terrain ruggedness. In particular, the majority of singing males were recorded on the top of soil heaps (M. Šálek unpubl. data), which indicates that in the absence of shrub or tree vegetation such elevated structures may represent important perching and song posts. Moreover, the patches with steeper slopes and soil heaps also are more prone to soil erosion which may in turn increase the surface of bare ground and thus provide suitable foraging habitats for Ortolan Buntings (Brambilla et al. Reference Brambilla, Gustin, Vitulano, Negri and Celada2016; and see below). Alternatively, the elevated posts may facilitate predator detection from longer distances (Sánchez et al. Reference Sánchez, Václav and Prokop2009).
Importantly, the habitat attributes of breeding habitat patches of Ortolan Buntings in the post-mining landscape are typical for disturbed environments in early stages of spontaneous succession (see Appendix S3). Such environments serve as important regional refugia for farmland bird specialists of conservation concern, such as Tawny Pipit Anthus campestris, Northern Wheatear Oenanthe oenanthe, Whinchat Saxicola rubetra, and Corn Bunting Miliaria calandra (Šálek, Reference Šálek2012, Šálek, unpubl. data), i.e. species rapidly declining or disappearing throughout intensively-used farmlands in central Europe. Moreover, early successional habitats, represented by recently burnt forests or forest clear-cuts, have been identified as crucial Ortolan Bunting breeding habitats in many regions across southern and northern Europe (Dale and Olsen Reference Dale and Olsen2002, Brotons et al. Reference Brotons, Herrando and Pons2008, Menz et al. Reference Menz, Brotons and Arlettaz2009a).
In accordance with other studies performed across various regions and landscape types (Berg Reference Berg2008, Menz et al. Reference Menz, Mosimann-Kampe and Arlettaz2009b, Brambilla et al. Reference Brambilla, Gustin, Vitulano, Negri and Celada2016, Reference Brambilla, Gustin, Vitulano, Negri, Bogliani, Falco and Celada2017), our study also revealed that the probability of Ortolan Bunting occurrence was significantly affected by the cover of bare ground, however, the importance of this attribute was scale-specific for the two landscape types. Specifically, in the post-mining landscape, the probability of Ortolan Bunting occurrence increased concavely with the cover of bare ground at the foraging scale, whereas in farmland a similarly shaped association occurred at the territory scale. We suggest that these differences could be related to the overall availability of bare ground in farmland and post-mining landscapes. The post-mining landscape, especially early successional stages with the majority of Ortolan Bunting nests, offer a high availability of bare ground patches throughout the breeding season. In contrast, the cover of bare ground is more dynamic in farmland, decreasing as the vegetation season progresses. Importantly, our result pointing out a non-linear relationship between the bunting occurrence and the cover of bare ground suggests that extensive surfaces of bare ground within farmland are not preferred by the species. Sparsely vegetated patches within the agricultural landscape provide enhanced foraging opportunities in terms of a higher availability of invertebrate prey and they were identified as the key foraging habitats also for other bunting species (e.g. Yellowhammer Emberiza citrinella – Douglas et al. Reference Douglas, Vickery and Benton2009, Corn Bunting Emberiza calandra – Altewischer et al. Reference Altewischer, Buschewski, Ehrke, Fröhlich, Gärtner, Giese, Günter, Heitmann, Hestermann, Hoffmann, Kleinschmidt, Kniepkamp, Linke, Mayland-Quellhorst, Pape, Peterson, Schendel, Schwieger, Wadenstorfer and Fischer2015) and ground-foraging insectivorous farmland birds, such as Eurasian Hoopoe Upupa epops (Tagmann-Ioset et al. Reference Tagmann-Ioset, Schaub, Reichlin, Weisshaupt and Arlettaz2012), Eurasian Wryneck Jynx torquilla (Weisshaupt et al. Reference Weisshaupt, Arlettaz, Reichlin, Tagmann-Ioset and Schaub2011), or Little Owl Athene noctua (Šálek and Lövy Reference Šálek and Lövy2012). We suggest that it is the Ortolan Bunting preference for intermediately vegetated habitat patches that may explain an intriguing selection of poppy fields and set-asides in farmland, both containing sparse vegetation throughout the whole vegetation season.
In the agricultural landscapes of central Europe, the species is primarily associated with arable habitats, whereas grassland and orchards are generally avoided (Lang et al. Reference Lang, Bandorf, Dornberger, Klein and Mattern1990, Deutsch and Sudbeck Reference Deutsch, Sudbeck and Bernardy2009), which also was documented in our study. More specifically, we found that occupied habitat patches at both spatial scales had a lower cover of grasslands, gardens and orchards in the farmland landscape. Finally, our results also reveal that in both landscape types, Ortolan Bunting occurrence at the foraging range scale was negatively associated with the cover of seedlings/young forest plantations and urban habitats. These results suggest a potentially negative impact of afforestation and urbanisation on species occurrence in central Europe (see also De Groot et al. Reference De Groot, Kmelcl, Figelj, Figelj, Mihelič and Rubinič2010, Menz and Arlettaz Reference Menz and Arlettaz2012).
In conclusions, our results reveal important fine-scale habitat associations of the Ortolan Bunting common to both farmland and post-mining landscapes. Consequently, we propose that conservation activities in the farmland landscape be focused on enhancing habitat heterogeneity, promoting non-agricultural elements and crop diversity, thus increasing the availability of suitable breeding and foraging habitats. This measure may benefit the Ortolan Bunting, but also other declining farmland birds/taxa with complex requirements, i.e. associated with a combination of different habitat types (Benton et al. Reference Benton, Vickery and Wilson2003, Morelli et al. Reference Morelli, Santolini and Sisti2012, Vickery and Arlettaz Reference Vickery, Arlettaz and Fuller2012, Brambilla et al. Reference Brambilla, Gustin, Vitulano, Negri, Bogliani, Falco and Celada2017, Pavliska et al. Reference Pavliska, Riegert, Grill and Šálek2018, Šálek et al. Reference Šálek, Hula, Kipson, Daňková, Niedobová and Gamero2018). In addition, increasing the representation of set-aside and sparsely growing cultivations in farmland may be an effective conservation measure not only for Ortolan Buntings, but also for farmland birds of conservation concern (Henderson et al. Reference Henderson, Vickery and Fuller2000, Berg Reference Berg2008). In the post-mining landscape, we propose that the conservation priority should be to prevent the loss and degradation of suitable habitats, particularly the patches in early stages of spontaneous succession. In contrast to technical reclamation represented by forest and agricultural reclamation, spontaneous succession provides greater topographic and structural habitat diversity, including a higher availability of bare ground. This type of habitat is of crucial importance for various rare and endangered birds and invertebrates inhabiting post-mining landscapes in central Europe (Šálek Reference Šálek2012, Tropek et al. Reference Tropek, Kadlec, Hejda, Kočárek, Skuhrovec, Malenovský, Vodka, Spitzer, Baňař and Konvička2012). However, in the long term, Ortolan Bunting breeding habitat suitability at post-mining sites depends on regulating the rate of succession, e.g. by clipping disturbance areas.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270918000060
Acknowledgements
We would like to thank to M. Kipson, M. Hanzlíková, P. Vít, P. Horák, V. Beran, L. Kadava, L. Praus, P. Molitor, J. Círl for their help with fieldwork and S. Grill for his assistance with geographic information system and map preparation. This work was supported by EEA and Norway Grants in the Czech Republic (grant MGSII – 52:”Zpracování odborných podkladů pro rozhodnutí o ZPZCHD pro druhy sýček obecný (Athene noctua) a strnad zahradní (Emberiza hortulana)” and the research aim of Czech Academy of Sciences (RVO 68081766).