Empirical documentation that investments in agriculture improve child nutrition has been difficult to demonstrate in developing countries(Reference Alderman1–Reference Waage, Cornelsen and Dangour9). Agriculture production interventions, while generally successful in increasing participant wealth and socio-economic status, do not necessarily result in improved child nutritional status, as increased household income and assets are not always directed towards optimizing child diet or growth. Nutrition education has also been endorsed as an important tactic to improve child outcomes(Reference Bhutta, Das and Rizvi4,Reference Masset, Haddad and Cornelius5) . While educational efforts directed towards breast-feeding promotion and timely introduction of complementary feedings have had varying success(Reference Bhutta, Das and Rizvi4), overall, nutrition education, given alone, has had limited success. A systematic review published in 2008 concluded that nutrition education alone had only a modest effect size for child weight (mean effect size = 0·28; range = −0·06 to 0·96) and linear growth (mean effect size = 0·20, range = 0·04 to 0·64)(Reference Dewey and Adu-Afarwuah10). More recent evidence clearly indicates that nutrition education is not sufficient, particularly in food-insecure households(Reference Bhutta, Das and Rizvi4). Nevertheless,in non-research settings, much effort and time are expended in providing such training(11,12) .
Nutrition education can take many forms, from targeting a specific nutritional issue (e.g. complementary feeding; promotion of breast-feeding; need for supplementation of vitamin A, Fe, etc.) for a specific age group, to a more general approach. Some of these education programmes do show a positive effect on growth (reviewed by Bhutta et al.(Reference Bhutta, Das and Rizvi4)), but understanding of the impact of community-based programmes in family nutrition remains limited. Clearly, a ‘more nuanced and tailored approach to the implementation of nutrition interventions’(Reference Vaivada, Gaffey and Das13) is needed.
Undoubtedly, the context in which interventions are provided is of considerable importance. Nutrition education is sometimes combined with other interventions, including provision of supplementary foods, micronutrients, cash transfers, deworming or agriculture production activities (e.g. Rivera et al.(Reference Rivera, Sotres-Alvarez and Habicht14)). With several notable exceptions, few organizations provide household-level nutrition training in the context of multisectoral interventions framed around agriculture(Reference Olney, Pedehombga and Ruel15,Reference Rawlins, Pimkina and Barrett16) and only a few studies have attempted to disaggregate the impact of various components of such programmes on child nutrition and growth(Reference Bhutta, Das and Rizvi4,Reference Imdad, Yakoob and Bhutta17) . In particular, relatively little is known about the efficacy of combining nutrition education and livestock-based livelihoods training on child nutritional outcomes. Moreover, the impact of further adding community empowerment and social capital development programmes to the success of community-based educational programmes is unknown.
We previously evaluated the impact of a community-based, livestock-focused intervention (conducted by Heifer International Nepal) on child growth and diet outcomes in rural Nepal(Reference Miller, Joshi and Lohani18,Reference Miller, Joshi and Lohani19) . Although the intervention did not include nutrition education or any emphasis on child-specific outcomes, duration of exposure to the Heifer intervention was associated with improved child diet diversity and child growth (specifically, reduction in prevalence of stunting, wasting and particularly underweight). This suggested that community empowerment and social capital development could provide a supportive context for a nutrition education programme. Others have found that community mobilization, particularly when delivered via women’s groups, may facilitate training(Reference Olney, Pedehombga and Ruel15,Reference Rosato, Laverack and Grabman20) .
We hypothesized that nutrition education provided in the context of social capital development and community mobilization would strengthen the capacity of participants to make fundamental choices related to household practices and childcare, resulting in better child growth and nutrition outcomes. Therefore, we designed a longitudinal investigation to disaggregate the impact of a comprehensive community development intervention including nutrition and livestock training v. the impact of such training alone. The primary outcomes were child growth and diet diversity, and secondary outcomes were household wealth and hygiene practices.
Methods
Study design
This longitudinal-control impact evaluation(Reference Habicht, Victora and Vaughan21) was conducted in Banke district in western Nepal. This area is largely populated by low-income subsistence farmers. Heifer field activities are provided to specific areas at the request of local non-governmental organizations formed within rural communities. A waiting list of interested communities is maintained for each district. For the purposes of the present study, eighteen villages in this district were matched for sociodemographic characteristics including geographic location, altitude, population size, local natural resources, employment opportunities, availability of health care and type of agriculture practised. Six villages were randomly assigned to each treatment arm (i.e. Full Package, Partial Package or Control). Due to logistic considerations in delivery of this community-based intervention, the six villages in each arm were adjacent to each other, but geographically separate from the other groups of villages to avoid contamination across arms.
The village groups were randomly assigned to one of three conditions: (i) full Heifer intervention package, including community development, livestock training and nutrition education (Full Package); (ii) livestock training and nutrition education only (Partial Package); or (iii) Control (no inputs). Partial Package and Control communities were promised to receive the Heifer full intervention package after the research period concluded (Fig. 1), as required by the local ethics committee and in accordance with the demand of the communities as a condition for accepting the randomization scheme.

Fig. 1 Schema showing the different activities in the three different groups: Full Package, Partial Package and Control, as well as the timing of data collection in the communities. Households with children aged 1–60 months from Banke, western Nepal, were randomized to receive: (i) multisectoral community development activities (Full Package), (ii) nutrition education and livestock management training alone (Partial Package) or (iii) no intervention (Control). The study was conducted between August 2013 and August 2017
In each group of villages, local leaders were invited to serve on an advisory panel and as liaisons to the population about the project activities. Five rounds of data collection were performed by a local field research non-governmental organization (Valley Research Group), not connected to Heifer. Field supervisors monitored the performance and activities of the field enumerators and conducted daily reviews of the data collection. This allowed rapid identification and correction of errors and omissions. Enumerators were trained at the beginning of the project with 7 d of orientation to the project, field practice testing in two villages not included in the project sites, and ongoing quality control activities to monitor and maintain interobserver reliability. A 5 d refresher training, including field practice, was conducted prior to each subsequent field visit.
Interventions
The Full Package consisted of an intensive 12-month programme of participatory community development activities led by Heifer Nepal field staff, followed by another 24 months of less concentrated supervision. The Heifer training curriculum aims to provide tools for poverty alleviation, citizen empowerment and community development, with a strong emphasis on optimization of livestock management(Reference Miller, Joshi and Lohani19). These activities were delivered in women’s self-help groups, which met biweekly with a trained facilitator to discuss local and personal issues in the context of values training, gender and family issues, social mobilization, group strengthening, microcredit, enterprise development and livestock management. Nutrition education, delivered concurrently, was based on a formal curriculum, focused on healthy eating practices for infants, young children and expectant mothers, targeting understanding of basic nutrients and their benefits, diversification of food production and intake, dietary needs of children from conception to 2 years of age (and the importance of this for their cognitive and physical development) and safe, hygienic preparation of food. Additionally, the nutrition education promoted the production and consumption of animal foods by all family members and equitable distribution of food within the family.
At the conclusion of the 12-month curriculum, each household in the Full Package group received a female goat, with the proviso that the first offspring of the animal be passed on to a participating neighbour. (This obligation ensures the sustainability of this approach as well as strengthening community ties and personal responsibility.)
Households assigned to the Partial Package group received identical training in livestock management and child/expectant mother nutrition, and received a goat after training was completed, without the accompanying community development components. Control households received no inputs other than the five household visits from the enumerators.
Participants
All members of each participating family were enrolled in the study. Children aged 1–60 months residing in participating families were assessed in detail (described below). Child age was determined by inspection of the birth or the vaccine certificate. Children with physical or neurological handicaps that prevented ingestion of a normal diet for age or children with severe intercurrent illnesses at the time of survey were excluded. Children who ‘aged in’ to the entry criteria were enrolled in the study at the first visit at which they were eligible.
Field procedures
Each family was visited five times over 36 months. Intervals varied from 6 to 12 months, due to natural disasters and political unrest (Fig. 1); three rounds were pre-harvest and two were post-harvest. Field enumerators travelled in pairs to conduct the visits during which a 145-item questionnaire was completed with the female head of household or her designee; a supervisor was also present for part of each visit. The questionnaire was based on the Nepal Demographic and Health Survey (DHS)(22)and included dietary information (described below). Anthropometric measurements and diet information on all enrolled children were also collected, along with diet information for ‘anyone else in the household’ (described below).
Anthropometry
Child growth was assessed at each field visit. Weight (in kilograms, to two decimal places) was measured with a Seca 835 electronic scale (Hamburg, Germany), calibrated before each measurement. Standing height in centimetres (children aged >2 years) was measured with a portable Seca 213 stadiometer; supine height in centimetres (children aged <2 years) was measured with a Seca BabyMat 210. Head circumference was measured in centimetres with a disposable paper tape at the maximum occipito-frontal measurement. Mid-upper arm circumference (MUAC) was measured in centimetres with a disposable insertion tape (Harlow Ltd, South Shields, UK). Height, head circumference and MUAC were measured to one decimal place. Each measurement was obtained twice and results averaged. If results were >5 % discrepant, then a third measurementwas obtained. Results were converted to Z-scores using WHO Anthro(23). The prevalence of underweight, stunting and wasting was determined according to WHO standards(23).
Diet
Respondents were asked if each child in the household aged 1–60 months had consumed any of sixteen specific foods/food groups within the past 24 h(Reference Kennedy, Ballard and Dop24). A diet diversity score (DDS) was constructed by aggregating the food groups into eight categories: (i) starchy staples (grains and white potatoes); (ii) vitamin A-rich fruits and vegetables; (iii) other fruits and vegetables; (iv) offal, meat and fish; (v) eggs; (vi) legumes, nuts and seeds; (vii) milk and dairy products; and (viii) oils, as defined by the US Agency for International Development’s Food and Nutrition Technical Assistance Project specifically for young children(Reference Swindale and Bilinsky25). Each of these food categories was categorized as ‘yes’ or ‘no’; thus, the maximum DDS for each round was 8. In addition, the number of animal-source food (ASF) categories (meat, fish, offal, eggs, milk, other dairy products; maximum score 6) consumed in the previous 24 h by each child was recorded(Reference Ruel26,Reference Steyn, Nel and Nantel27) .
To determine if each food item was available for consumption in the household, whether or not the child consumed it, we asked if ‘anyone else in the household’ had consumed it during the last 24 h. However, the individuals who consumed these items were not specified.
The child’s DDS and ASF sum were then compared with those of other household members and categorized as ‘worse’ or ‘the same or better’ to determine if preferential or disadvantageous feeding practices were directed towards these young children. Calculations of child ASF consumption and dietary diversity were limited to children >6 months of age to exclude children who were (for the most part) exclusively breast-fed.
Household characteristics
Demographics
Demographic information was collected on each household, including socio-economic status, animal ownership, annual income and amount of land owned. Socio-economic status score (wealth score) was constructed using household asset data via a principal component analysis, calculated using DHS-Nepal guidelines(22). Household assets including possessions (such as a refrigerator, bicycle, chair and bed) as well as quality of housing (such as availability of electricity, type of water supply and toilet) were considered. This measure has been used in many DHS and other country-level surveys to indicate inequalities in household characteristics and serves as an indicator of level of wealth that is consistent with expenditure and income measures(22). Animal ownership (converted to a standardized score using FAO Global Livestock Units(28)), annual income per household and per capita (in NPR, Nepali rupees) and amount of land owned (in square metres) were also collected as additional indicators of household wealth.
Maternal education
Previous work indicated the important influence of the level of mother’s education on child outcomes(Reference Miller, Joshi and Lohani29); therefore, this variable was incorporated into the analysis. The educational status of the mothers was classified as: (i) none or simple literacy classes; (ii) some or completed primary school; and (iii) some or completed secondary school (or beyond).
Hygiene practices
Household hygiene practices were assessed by determining the number of uses of soap cited by the respondents, without prompting. Soap availability was also verified.
Statistical analysis
The original sample size was calculated to detect a difference of >0·25 in mean weight-for-age Z-score (WAZ), with a power of 80 % and a two-sided significance level of <0·05. Data were entered and analysed with JMP 13.1 and the statistical software package Stata version 12.0. Analysis was conducted at the community, household and individual levels, starting with a descriptive analysis of the variables, including t tests and ANOVA with Bonferroni post hoc tests to correct for multiple comparisons, followed by a series of χ 2 tests and correlations to assess collinearity. Dependent variables were evaluated with histograms to verify normal distribution. Anthropometric Z-scores (height-for-age Z-score (HAZ), WAZ, weight-for-height Z-score (WHZ), MUAC Z-score (MUACZ), head circumference Z-score (HCZ)) were calculated at each round independently. Mixed-effect regression modelling (using the Stata command ‘xtmixed’) was used to estimate the impact of the intervention. We performed a regression with survey round, treatment group and group-by-round interaction as a fixed effect (with the interaction being the term of interest) and child as a random effect (to control for household-level clustering). A linear mixed-effect regression was used to estimate the impact on anthropometric Z-scores, household wealth score and total soap use. A mixed-effect Poisson regression was used for count outcomes, for example number of food groups consumed and number of ASF consumed. To evaluate the effects of group assignment over time, we then calculated and plotted adjusted marginal predictions from linear mixed-effects regression models for anthropometry and Poisson mixed-effects regression for the diet indicators using post-estimation commands in Stata version 15. All models were adjusted for child factors (age, gender, baseline anthropometry) and household factors (household animal and wealth score, land ownership, household per capita income, mother’s educational attainment). The adjustment for baseline anthropometry was mandated by the fact that there were some significant differences in these measures at baseline. Collinearity was assessed by measuring variance inflation factors for household variables; these were <1·5.
Results
Participants
At baseline, 6692 individuals in 960 families were enrolled (Fig. 2). Some of these households subdivided over the course of the study; thus, the total number of households visited was 974. Communities were randomized to Control (304 households), Full Package (290 households) or Partial Package (366 households). Communities were similar for many important characteristics (Table 1); however, differences were noted in the amount of land owned and the calculated animal score. Regression analyses adjusted for these factors. Retention over the 36-month course of the study was 90·3 % overall (Full Package 88 %, Partial Package 91 %, Control 91 %; Fig. 2).

Fig. 2 Number of households (HH), individuals and children aged <60 months included in the five surveys. HH with children aged 1–60 months from Banke, western Nepal, were randomized to receive: (i) multisectoral community development activities (Full Package), (ii) nutrition education and livestock management training alone (Partial Package) or (iii) no intervention (Control); the study was conducted between August 2013 and August 2017
Table 1 Household characteristics at baseline according to study group
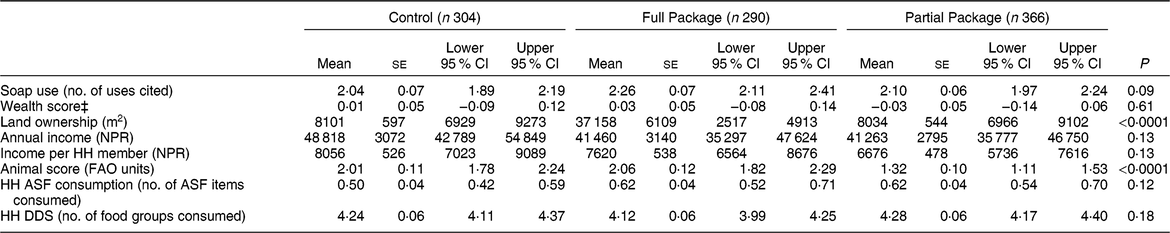
NPR, Nepali rupees; HH, household; ASF, animal-source food; DDS, dietary diversity score.
Households with children aged 1–60 months from Banke, western Nepal, were randomized to receive: (i) multisectoral community development activities (Full Package), (ii) nutrition education and livestock management training alone (Partial Package) or (iii) no intervention (Control); the study was conducted between August 2013 and August 2017.
‡Wealth score determined by principal components analysis, using method of the Nepal Demographic and Health Surveys(22).
Mother’s education
The educational achievements of the mothers varied widely: 64 % reported no education, 15 % had completed only basic non-formal literacy classes, 11 % had some primary school, while only 2 % had completed secondary school or beyond. Educational achievements for mothers differed significantly among the three groups, with less-educated women in the Partial Package group (χ 2 = 124·79, P < 0·0001).
Participation in the intervention activities
Participation in the intervention activities was approximately 100 % for the Full Package programme. Despite efforts of Heifer field staff, Partial Package households participated in about 70 % of the offered training activities.
Child characteristics
Growth
Anthropometric measurements and diet quality indicators (ASF consumption and dietary diversity) were collected on all children at baseline (Table 2). However, unexpectedly, at baseline Control group children had significantly lower mean HAZ, WAZ, HCZ and MUACZ than the other two groups; WHZ did not differ among the three groups. Regression analyses were adjusted to take baseline growth status into account.
Table 2 Child characteristics at baseline according to study group (children aged <60 months; for dietary indicators, only children aged >6 and <60 months were included)

ASF, animal-source food; DDS, dietary diversity score; HAZ, height-for-age Z-score; WAZ, weight-for-age Z-score; WHZ, weight-for-height Z-score; HCZ, head circumference Z-score; MUACZ, mid-upper arm circumference Z-score.
Households with children aged 1–60 months from Banke, western Nepal, were randomized to receive: (i) multisectoral community development activities (Full Package), (ii) nutrition education and livestock management training alone (Partial Package) or (iii) no intervention (Control); the study was conducted between August 2013 and August 2017.
Over the 36 months of the study, the growth status of children (aged >6 and <60 months) generally improved. Compared with baseline values, the percentage of stunting and microcephaly did not change significantly at endline (respectively, from 48 to 51 % and from 20 to 18 %). In contrast, the percentage of wasted and underweight children declined significantly (respectively, from 17 to 9 % and from 43 to 33 %). However, mean WAZ, WHZ, HCZ and MUACZ increased significantly more in the Full Package group than in the other two groups (across-group comparison, P value by anthropometric outcome respectively P = 0·006, P = 0·0002, P = 0·0001 and P = 0·0001). HAZ was a notable exception, with significantly less improvement in this parameter among children in the Full Package group compared with the other two groups (P = 0·009; Fig. 3(a)). Changes in growth were examined using a mixed-effects regression model, with survey round, treatment group and group-by-round interaction as a fixed effect (with the interaction being the term of interest) and child as a random effect (to control for household-level clustering). The model was adjusted for child factors (age, gender, baseline anthropometry) and household factors (household animal and wealth score, land ownership, household per capita income, mother’s educational attainment). When these results were examined using group-by-round interactions (Table 3), children in the Full Package group showed significantly better WHZ (Rounds 2–3–4–5), WAZ (Rounds 4–5), HCZ (Rounds 2–4–5) and MUACZ (Rounds 4–5) compared with Control children. Except for MUACZ, these did not occur in a ‘dose-dependent’ fashion over time. Children in the Partial Package group also showed significantly better WHZ (Rounds 2–3–4–5), WAZ (Rounds 2–3–4), HCZ (Rounds 2–3–4) and MUACZ (Rounds 2–4–5). Adjusted marginal predictions from linear mixed-effects regression models showed significant improvements over time in the Full Package group with regard to MUACZ, WHZ and WAZ (Fig. 3(b)).

Fig. 3 (a) Change in child growth indicators from baseline to endline in the Control (![]() ), Full Package (
), Full Package (![]() ) and Partial Package (
) and Partial Package (![]() ) groups. Mean WAZ, WHZ, HCZ and MUACZ increased significantly more in the Full Package group than in the other two groups; HAZ improved in the Partial Package and Control groups, but not in the Full Package group. Across-group comparison by anthropometric outcome: **P < 0·01, †P < 0·0001. (b) Adjusted marginal predictions from linear mixed-effects regression models, with their standard errors represented by vertical bars, showing significant improvements over time in the Full Package group with regard to MUAC, WHZ and WAZ (
) groups. Mean WAZ, WHZ, HCZ and MUACZ increased significantly more in the Full Package group than in the other two groups; HAZ improved in the Partial Package and Control groups, but not in the Full Package group. Across-group comparison by anthropometric outcome: **P < 0·01, †P < 0·0001. (b) Adjusted marginal predictions from linear mixed-effects regression models, with their standard errors represented by vertical bars, showing significant improvements over time in the Full Package group with regard to MUAC, WHZ and WAZ (![]() , Control;
, Control; ![]() , Full Package;
, Full Package; ![]() , Partial Package). Households with children aged 1–60 months from Banke, western Nepal, were randomized to receive: (i) multisectoral community development activities (Full Package), (ii) nutrition education and livestock management training alone (Partial Package) or (iii) no intervention (Control); the study was conducted between August 2013 and August 2017 (HAZ, height-for-age Z-score; WAZ, weight-for-age Z-score; WHZ, weight-for-height Z-score; HCZ, head circumference Z-score; MUACZ, mid-upper arm circumference Z-score; R1, Round 1 survey (baseline); R2–R5, Round 2 to Round 5 surveys)
, Partial Package). Households with children aged 1–60 months from Banke, western Nepal, were randomized to receive: (i) multisectoral community development activities (Full Package), (ii) nutrition education and livestock management training alone (Partial Package) or (iii) no intervention (Control); the study was conducted between August 2013 and August 2017 (HAZ, height-for-age Z-score; WAZ, weight-for-age Z-score; WHZ, weight-for-height Z-score; HCZ, head circumference Z-score; MUACZ, mid-upper arm circumference Z-score; R1, Round 1 survey (baseline); R2–R5, Round 2 to Round 5 surveys)
Table 3 Mixed-effect linear regression showing coefficient (β) and se for anthropometric measurements, household wealth score and total soap use (hygiene measure). Results are shown by survey round, treatment group and group-by-round interaction as a fixed effect adjusted for child factors (age, gender, baseline anthropometry) and household factors (household animal and wealth score, land ownership, household per capita income, mother’s educational attainment)
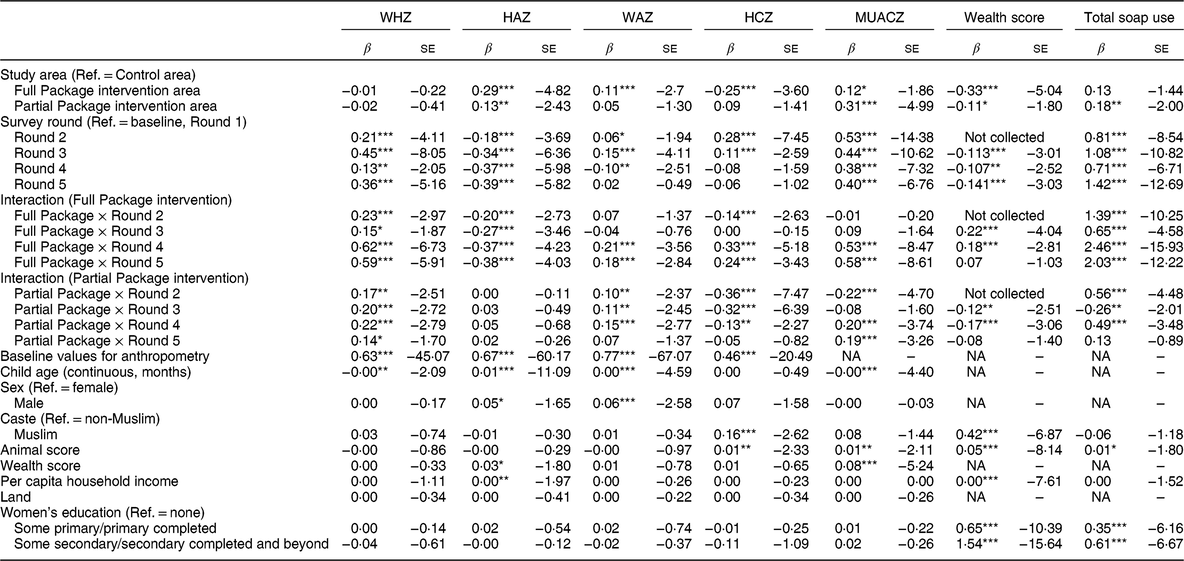
WHZ, weight-for-height Z-score; HAZ, height-for-age Z-score; WAZ, weight-for-age Z-score; HCZ, head circumference Z-score; MUACZ, mid-upper arm circumference Z-score; Ref., reference category; NA, not applicable.
Households with children aged 1–60 months from Banke, western Nepal, were randomized to receive: (i) multisectoral community development activities (Full Package), (ii) nutrition education and livestock management training alone (Partial Package) or (iii) no intervention (Control); the study was conducted between August 2013 and August 2017.
*P < 0·10, **P < 0·05, ***P < 0·01.
Child diet
Child dietary diversity
Child dietary diversity differed at baseline; Control children consumed a more diverse diet (mean (sd): 3·92 (0·07) Full Package and 3·94 (0·05) Partial Package v. 4·18 (0·06) Control, P = 0·01). This pattern was maintained for the first three rounds of data collection, but by Round 4 and Round 5, the Full Package children were consuming a significantly more diverse diet (mean (sd): 5·38 (0·08) Full Package, 4·90 (0·06) Partial Package and 4·74 (0·07) Control at Round 4, P < 0·0001; 5·34 (0·08) Full Package, 4·84 (0·06) Partial Package and 4·91 (0·06) Control Round 5, P < 0·0001; Fig. 4(a)). The mean (sd) increase in DDS was greater among the Full (+1·81 (0·17)) and Partial Package children (+1·76 (0·13)) compared with the Control children (+1·12 (0·14), P = 0·002; matched pairs for individuals in each group, baseline to endline). When examined using Poisson regressions and group-by-round interaction (adjusted for child factors (age, gender, baseline anthropometry, baseline dietary intake) and household factors (household animal and wealth score, land ownership, household per capita income, mother’s educational attainment); Table 4), diet diversity was less in children in the Full Package group in Rounds 2 and 3 compared with Control (respectively, 18 and 44 % lesslikely to eat one more food group than Control). However, in Rounds 4 and 5, Full Package group children showed a trend to more likely having better diet diversity than Control (respectively, 13 % more likely to eat one additional food group, P < 0·05, and 8 %, NS). In contrast, diet diversity among children in the Partial Package group generally did not differ significantly from Control (except at Round 3). Adjusted marginal predictions from Poisson mixed-effects regression models showed improvements over time in the Full Package group with regard to diet diversity (Fig. 4(b)).
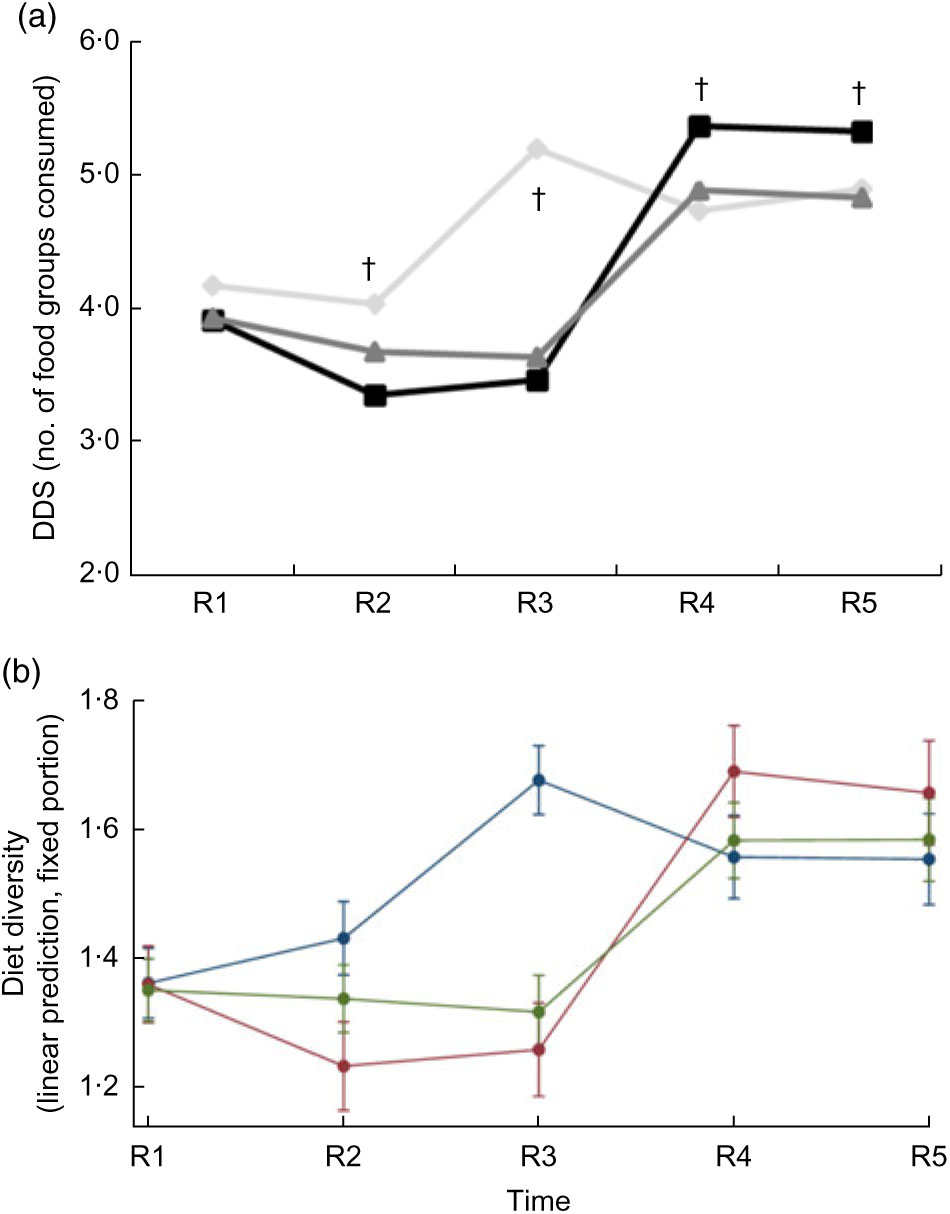
Fig. 4 (a) Dietary diversity scores (DDS) for children did not differ at baseline among the three groups (![]() , Control;
, Control; ![]() , Full Package;
, Full Package; ![]() , Partial Package). At R2 and R3, the DDS was significantly greater in the Control children than in the other two groups. However, this pattern then changed, and by R4 and R5, the Full Package children were consuming a significantly more diverse diet than the remainder of children (mean (sd): 5·38 (0·08) Full Package, 4·90 (0·06) Partial Package and 4·74 (0·07) Control at R4, P < 0·0001; 5·34 (0·08) Full Package, 4·84 (0·06) Partial Package and 4·91 (0·06) Control at R5, P < 0·0001). Across-group comparison: †P < 0·0001. (b) Adjusted marginal predictions from Poisson mixed-effects regression models, with 95 % confidence intervals represented by vertical bars, showing significant improvements over time in the Full Package group for child dietary diversity (
, Partial Package). At R2 and R3, the DDS was significantly greater in the Control children than in the other two groups. However, this pattern then changed, and by R4 and R5, the Full Package children were consuming a significantly more diverse diet than the remainder of children (mean (sd): 5·38 (0·08) Full Package, 4·90 (0·06) Partial Package and 4·74 (0·07) Control at R4, P < 0·0001; 5·34 (0·08) Full Package, 4·84 (0·06) Partial Package and 4·91 (0·06) Control at R5, P < 0·0001). Across-group comparison: †P < 0·0001. (b) Adjusted marginal predictions from Poisson mixed-effects regression models, with 95 % confidence intervals represented by vertical bars, showing significant improvements over time in the Full Package group for child dietary diversity (![]() , Control;
, Control; ![]() , Full Package;
, Full Package; ![]() , Partial Package). Households with children aged 1–60 months from Banke, western Nepal, were randomized to receive: (i) multisectoral community development activities (Full Package), (ii) nutrition education and livestock management training alone (Partial Package) or (iii) no intervention (Control); the study was conducted between August 2013 and August 2017 (R1, Round 1 survey (baseline); R2–R5, Round 2 to Round 5 surveys)
, Partial Package). Households with children aged 1–60 months from Banke, western Nepal, were randomized to receive: (i) multisectoral community development activities (Full Package), (ii) nutrition education and livestock management training alone (Partial Package) or (iii) no intervention (Control); the study was conducted between August 2013 and August 2017 (R1, Round 1 survey (baseline); R2–R5, Round 2 to Round 5 surveys)
Table 4 Mixed-effect Poisson regression showing relative risk (RR) and se for the number of food groups and number of animal-source foods (ASF) consumed by children in the project areas. The model was adjusted for child factors (age, gender, baseline anthropometry, baseline dietary intake) and household factors (household animal and wealth score, land ownership, household per capita income, mother’s educational attainment)
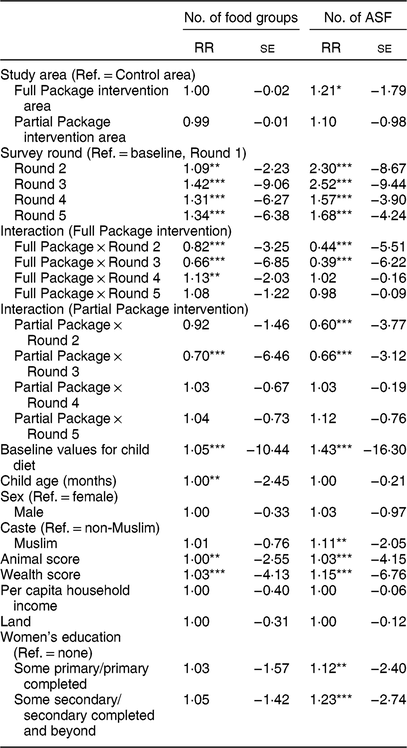
Ref., reference category; NA, not applicable.
Households with children aged 1–60 months from Banke, western Nepal, were randomized to receive: (i) multisectoral community development activities (Full Package), (ii) nutrition education and livestock management training alone (Partial Package) or (iii) no intervention (Control); the study was conducted between August 2013 and August 2017.
*P < 0·10, **P < 0·05, ***P < 0·01.
The percentage of children achieving minimum dietary diversity (≥4 food groups) was the same in all three groups at baseline (Full Package 38 %, Partial Package 34 %, Control 30 %, NS) and at endline (respectively, 4, 7 and 5 %, NS). Although the percentage of children exceeding minimum dietary diversity (>5 food groups) was the same in all three groups at baseline (33 % Full Package, 35 % Partial Package, 39 % Control, NS), by endline more children in the Full Package group exceeded minimum dietary diversity (80 % Full Package, 65 % Partial Package, 67 % Control, P < 0·0004).
Child consumption of animal-source foods
The mean number of ASF consumed by children was the same at baseline in all three groups (Fig. 5(a)). Child consumption of ASF was limited; at baseline only about 40 % of children had consumed any ASF within the prior 24 h. At endline, this had increased to 62–70 % of children. ASF consumption in the Control group initially increased (Round 2 and Round 3) but then declined; by Round 4 and Round 5, the children in the Full Package consumed significantly more ASF than Partial Package and Control children (mean (sd), respectively: 1·00 (0·07) v. 0·78 (0·05) and 0·69 (0·06) at Round 4, P = 0·0003; 1·29 (0·08) v. 0·81 (0·06) and 0·96 (0·06) at Round 5, P < 0001). When consumption at baseline and endline were analysed by matched pairs (of individuals), both the mean-mean and the mean difference for the Full Package group were greater than those of the other two groups (mean-mean: 0·97 Full Package v. 0·69 Partial Package and 0·71 Control, P < 0·0001; mean difference: 0·78 Full Package v. 0·31 Partial Package and 0·54 Control, P < 0·0001). When examined using Poisson regressions and group-by-round interaction (adjusted for child factors (age, gender, baseline anthropometry, baseline dietary intake) and household factors (household animal and wealth score, land ownership, household per capita income, mother’s educational attainment); Table 4), children in both the Full and Partial Package groups ate fewer ASF than Control children in Round 2 and Round 3, but by Round 4 and Round 5, this measure did not differ from Control children. Adjusted marginal predictions from Poisson mixed-effects regression models showed improvements over time in the Full Package group for ASF consumption (Fig. 5(b)).
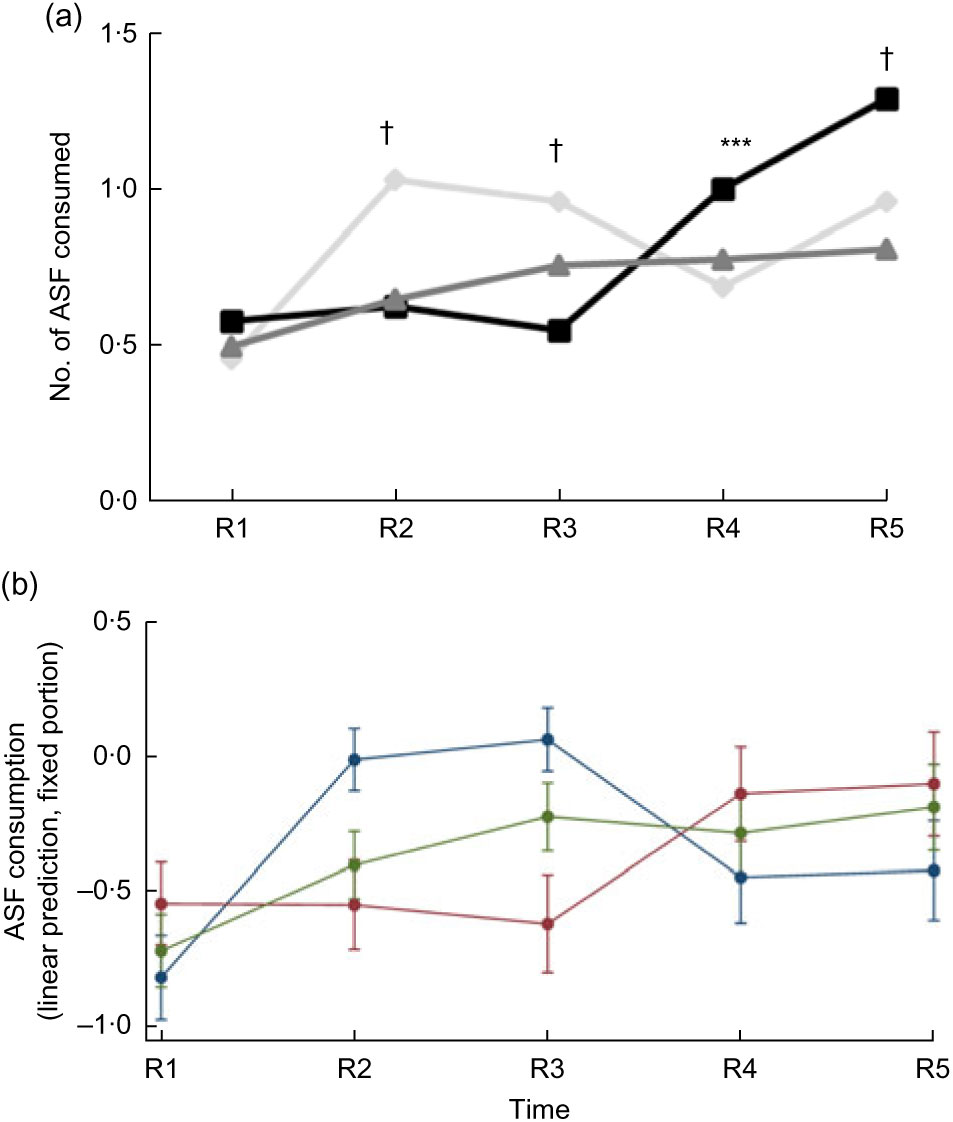
Fig. 5 (a) Animal-source food (ASF) consumption by children did not differ significantly at baseline between the three groups (![]() , Control;
, Control; ![]() , Full Package;
, Full Package; ![]() , Partial Package). After an initial increase in ASF consumption noted in the Control group at R2 and R3, this declined, and by R4 and R5, the children in the Full Package consumed significantly more ASF than Partial Package and Control children (mean (sd): 1·00 (0·07) Full Package v. 0·78 (0·05) Partial Package and 0·69 (0·06) Control at R4, P = 0·0003; 1·29 (0·08) Full Package v. 0·81 (0·06) Partial Package and 0·96 (0·06) Control at R5, P < 0·0001). Across-group comparison: ***P < 0·001, †P < 0·0001. (b) Adjusted marginal predictions from Poisson mixed-effects regression models, with 95 % confidence intervals represented by vertical bars, showing significant improvements over time in the Full Package group for child ASF consumption diversity (
, Partial Package). After an initial increase in ASF consumption noted in the Control group at R2 and R3, this declined, and by R4 and R5, the children in the Full Package consumed significantly more ASF than Partial Package and Control children (mean (sd): 1·00 (0·07) Full Package v. 0·78 (0·05) Partial Package and 0·69 (0·06) Control at R4, P = 0·0003; 1·29 (0·08) Full Package v. 0·81 (0·06) Partial Package and 0·96 (0·06) Control at R5, P < 0·0001). Across-group comparison: ***P < 0·001, †P < 0·0001. (b) Adjusted marginal predictions from Poisson mixed-effects regression models, with 95 % confidence intervals represented by vertical bars, showing significant improvements over time in the Full Package group for child ASF consumption diversity (![]() , Control;
, Control; ![]() , Full Package;
, Full Package; ![]() , Partial Package). Households with children aged 1–60 months from Banke, western Nepal, were randomized to receive: (i) multisectoral community development activities (Full Package), (ii) nutrition education and livestock management training alone (Partial Package) or (iii) no intervention (Control); the study was conducted between August 2013 and August 2017 (R1, Round 1 survey (baseline); R2–R5, Round 2 to Round 5 surveys)
, Partial Package). Households with children aged 1–60 months from Banke, western Nepal, were randomized to receive: (i) multisectoral community development activities (Full Package), (ii) nutrition education and livestock management training alone (Partial Package) or (iii) no intervention (Control); the study was conducted between August 2013 and August 2017 (R1, Round 1 survey (baseline); R2–R5, Round 2 to Round 5 surveys)
Effect of child and household factors on child diet
Notably, gender did not impact child diet. Not surprisingly, slight increases in diet diversity and ASF consumption were found with increasing child age (Table 4). However, maternal education significantly impacted child diet, particularly ASF consumption. Children of mothers with primary education or secondary education were respectively 19 or 26 % more likely to eat one additional ASF item than children of mothers with no education.
Household diet
Household dietary diversity
Household dietary diversity was limited at baseline, with only about 30 % of households consuming four or more food groups (33 % Full Package, 27 % Partial Package, 31 % Control, NS), with mean DDS for the household equal among all three groups (mean (sd): 4·12 (1·18) Full Package, 4·28 (1·17) Partial Package and 4·24 (1·13) Control, NS). By endline, only 2–3 % of households reported consumption of fewer than four food groups. Notably, the DDS for the household increased nearly twice as much in the Full Package group as in the other two groups (mean (sd) increase: +1·33 (0·09) Full Package, +0·71 (0·07) Partial Package and +0·73 (0·08) Control, P = 0·0001).
Household consumption of animal-source foods
In general, ASF were not widely available in these households, although this generally improved over time. At baseline, more than half of households reported no ASF consumption (no significant difference among the three groups). At endline, however, the number of households with no ASF consumption declined in all three groups, with the greatest reduction in the Full Package households (Full Package from 55 to 28 %, Partial Package from 54 to 36 % and Control from 62 to 39 %; χ 2 = 8·098, P = 0·01). Correspondingly, mean (sd) ASF consumption did not differ significantly among the three groups at baseline (0·62 (0·04) Full Package, 0·50 (0·04) Partial Package and 0·62 (0·04) Control), while at endline, households in the Full Package group consumed more ASF (0·94 (0·04) Full Package, 0·79 (0·03) Partial Package and 0·75 (0·04) Control, P = 0·005).
Child diet compared with household diet
Child diet diversity relative to diet diversity available in the household
The share of children with the same or better DDS compared with household-level DDS was significantly greater in the Control group at baseline (78 % Full Package and 75 % Partial v. 85 % Control, P = 0·0003). This was accounted for by a larger percentage of Control households where child and household-level DDS were equivalent at baseline (74 %, v. 67 % of Full and 62 % of Partial Package households). However, over the 36 months of surveys, there was a steady increase in the percentage of children in the Full and Partial Package groups whose DDS equalled or exceeded the household DDS (Fig. 6(a)), while the Control group percentage remain relatively unchanged.

Fig. 6 (a) The share of children with the same or better dietary diversity score (DDS) compared with household-level DDS was significantly greater in the Control group at baseline (78 % Full Package, 75 % Partial Package v. 85 % Control, P = 0·0003; ![]() , Control;
, Control; ![]() , Full Package;
, Full Package; ![]() , Partial Package). However, by simple comparison, over the 36 months of surveys, there was a steady increase in the percentage of children in the Full Package and Partial Package groups whose DDS was the same as or better than the household DDS (R2 P = 0·03, R3 P = 0·05, R4 P = 0·01, R5 P = 0·0001). (b) Regarding difference in animal-source foods (ASF) consumed by the child and any other household member from baseline to endline, children in the Full Package and Partial Package significantly increased their consumption of ASF relative to what was available in the household. Across-group comparison: **P < 0·01. Households with children aged 1–60 months from Banke, western Nepal, were randomized to receive: (i) multisectoral community development activities (Full Package), (ii) nutrition education and livestock management training alone (Partial Package) or (iii) no intervention (Control); the study was conducted between August 2013 and August 2017 (R1, Round 1 survey (baseline); R2–R5, Round 2 to Round 5 surveys)
, Partial Package). However, by simple comparison, over the 36 months of surveys, there was a steady increase in the percentage of children in the Full Package and Partial Package groups whose DDS was the same as or better than the household DDS (R2 P = 0·03, R3 P = 0·05, R4 P = 0·01, R5 P = 0·0001). (b) Regarding difference in animal-source foods (ASF) consumed by the child and any other household member from baseline to endline, children in the Full Package and Partial Package significantly increased their consumption of ASF relative to what was available in the household. Across-group comparison: **P < 0·01. Households with children aged 1–60 months from Banke, western Nepal, were randomized to receive: (i) multisectoral community development activities (Full Package), (ii) nutrition education and livestock management training alone (Partial Package) or (iii) no intervention (Control); the study was conducted between August 2013 and August 2017 (R1, Round 1 survey (baseline); R2–R5, Round 2 to Round 5 surveys)
Child animal-source food consumption relative to animal-source food availability in the household
Over all five rounds of data collection, child ASF consumption matched what was available in the household for 82–89 % of children. Of the remaining children, about half ate more ASF than other household members and about half ate less. However, the consumption of ASF by children relative to the availability of this food item in the household improved significantly more from baseline to endline in the Full and Partial Package groups, compared with Control group children (matched pairs comparison: +0·24 Full Package, P = 0·003, +0·16 Partial Package, P = 0·01 and +0·07 Control, NS; Fig. 6(b)).
Household outcomes
Figure 7 presents results for household outcomes.

Fig. 7 From baseline to endline, household wealth score, hygiene practices (number of uses of soap cited) and dietary diversity score (DDS, number of food groups consumed) all improved significantly more in the Full Package group compared with the other two groups (respectively, P < 0·0001, P < 0·001 and P < 0·0001; ![]() , Control;
, Control; ![]() , Full Package;
, Full Package; ![]() , Partial Package). Across-group comparison: ***P < 0·001, †P < 0·0001. Households with children aged 1–60 months from Banke, western Nepal, were randomized to receive: (i) multisectoral community development activities (Full Package), (ii) nutrition education and livestock management training alone (Partial Package) or (iii) no intervention (Control); the study was conducted between August 2013 and August 2017
, Partial Package). Across-group comparison: ***P < 0·001, †P < 0·0001. Households with children aged 1–60 months from Banke, western Nepal, were randomized to receive: (i) multisectoral community development activities (Full Package), (ii) nutrition education and livestock management training alone (Partial Package) or (iii) no intervention (Control); the study was conducted between August 2013 and August 2017
Household wealth
In the Control group there was minimal change in household wealth over time. Group-by-round interactions (Table 3) showed that compared with Control, household wealth increased significantly in the Full Package group (Rounds 3–4) but decreased in the Partial Package group (Rounds 3–4). (This measure was not collected at Round 2.) Over the 36 months, the net changes in household wealth score (matched-pairs t test, mean difference (se)) were +0·10 (0·03) Full Package (P = 0·01), −0·07 (0·03) Partial Package (P = 0·04) and −0·05 (0·03) Control (NS).
Hygiene practices
At baseline, the mean number of soap uses cited was 2·13 (range 0–6); 9 % of respondents were unable to name a single use, with no differences by group (data not shown). By Round 5, the mean number of soap uses cited was 4·02 (range 1–9), with significantly more cited by the Full Package group (mean (sd): 5·69 (1·12) v. 3·61 (0·92) Partial Package and 3·53 (0·68) Control, P < 0·0001). Group-by-round interactions (Table 3) showed that compared with Control, soap use increased significantly in the Full Package group (Rounds 2–3–4–5) and to a lesser extent in the Partial Package group (Rounds 2–4–5).
Discussion
Agriculturally oriented, multisectoral programmes aimed at improving child nutrition are complicated, time-consuming and costly to implement. However, we previously found that a comprehensive, agriculture livelihoods and community development programme had a favourable impact on child nutritional status (especially with longer duration of programme exposure), even when this outcome was not specifically addressed by the intervention(Reference Miller, Joshi and Lohani18,Reference Miller, Joshi and Lohani19) . The present study asked the question whether similar results could be accomplished via a straightforward nutrition training programme combined with training on animal husbandry and management practices, or if the community mobilization activities were an essential component to improving child nutritional status. We sought to disaggregate the impact of didactic training alone (Partial Package) v. identical training provided in the context of a multisectoral community development programme implemented over 12 months (Full Package).
The results were striking. Compared with children living in Control or Partial Package households, those in the Full Package group had better growth. After adjustments for child and household factors, WAZ, WHZ, HCZ and MUACZ all increased more in the Full Package than in the Control group children. Adjusted marginal prediction models showed significant improvements over time in the Full Package group with regard to growth parameters and diet. Children in the Partial Package households also had improvement in growth parameters, but to a lesser extent. For both of these groups, changes were most notable in Rounds 4 and Round 5. Hygiene practices showed a similar pattern of improvement in the Full Package households. In contrast, the largest increase in wealth score occurred earlier (Round 3; this was not collected in Round 2) in Full Package households. These findings emphasize the benefits of long periods of follow-up(Reference Miller, Joshi and Lohani18), especially to assess child outcomes.
In addition, children in the Full Package households benefited from preferential feeding practices, consuming significantly more diverse diets and ASF than other members of their households. Again, these changes became more evident in the later phases of data collection.
Training focused on child feeding practices is the cornerstone of many programmes directed towards improving child nutritional outcomes. Such training is sometimes coupled with additional concrete interventions (such as provision of micronutrient supplements or complementary foods). These programmes may indeed improve child growth, but when training is given alone, results have been inconsistent and generally disappointing(Reference Imdad, Yakoob and Bhutta17,Reference Bhandari, Mazumder and Bahl30) . Moreover, the sustainability of such approaches is questionable. In contrast, the efficacy of didactic training programmes related to improving agricultural practices is more readily demonstrable (e.g. Rawlins, Davis, Argenta and their co-workers(Reference Rawlins, Pimkina and Barrett16,Reference Davis, Nkonya and Kato31,Reference Argenta, Augsburg and Rasulc32) ).
For some outcomes, such as maternal and newborn health, participatory action training is more effective than passive training programmes, according to a recent meta-analysis(Reference Prost, Colbourn and Seward33). However, training programmes to improve child outcomes are often provided to passive recipient communities(Reference Rosato, Laverack and Grabman20). Even when using such facilitator-led ‘action-learning cycles’, the impact of training is often minimal in the absence of broader social change(Reference Manandhar, Osrin and Shrestha34).
Training related to household practices may function in a qualitatively different manner when mobilization efforts enable the community itself to become an active agent of change(Reference Alderman1,Reference Rosato, Laverack and Grabman20) . Ideally, communities become actively engaged with identification and resolution of problems and embrace the action of working together to collectively change their circumstances(Reference Brehm and Rahn35). Within this milieu, individuals may more readily alter socio-environmental risk factors including home care practices and household decision making, with subsequent benefits to the children in the household. However, participation in women’s groups alone may not be sufficient to produce change(Reference Gram, Morrison and Saville36).
Such complexities underlie in part the difficulties in linking agricultural interventions and child growth outcomes (recently reviewed elsewhere(Reference Webb and Kennedy8,Reference Haddad37–Reference Webb Girard, Self and McAuliffe39) )(Reference Ruel and Alderman7). Recent work has suggested the importance of understanding the role of food access, care practices and the health environment in linking agriculture to nutrition outcomes(Reference Herforth and Ballard40). As many sociodemographic factors influence child care and feeding practices, enhanced income alone is not sufficient to address the problems of malnutrition. Behaviour change communication directed towards feeding practices can contribute to improved child nutritional status; however, women’s educational level and empowerment are important determinants of the efficacy of this approach(Reference Miller, Joshi and Lohani29). Regardless, both reductions in poverty and behaviour change take time after implementation of an intervention(Reference Miller, Joshi and Lohani18). Indeed, the time course of the changes we observed underline this point.
The causal mechanisms by which the community development pathway improves child outcomes are not known with certainty. Working as a community, individual households share knowledge and experience, collaborate, develop problem-solving skills and provide support to one another(Reference Rosato, Laverack and Grabman20,Reference Tripathy, Nair and Barnett41) . Community mobilization has the potential to improve capability to deal with various difficulties related to poverty and social inequalities(Reference Tripathy, Nair and Barnett41) including low self-esteem and limited assertiveness(Reference Jacobs, Ir and Bigdeli42). When community mobilization efforts are sustained and successful, there is the potential to produce a long-term and fundamental shift in village, family and gender power relations(Reference Rosato, Laverack and Grabman20). However, the evidence suggests that social mobilization alone is not sufficient to promote change(Reference Rosato, Laverack and Grabman20,Reference Woolcock and Narayan43) . Hypothetically, improved psychological well-being, gender empowerment, coping ability and economic circumstances may contribute to better living conditions, including better access to and utilization of food resources, knowledge networks and health services(Reference Bhutta, Das and Rizvi4). These pathways may improve food security, reduce childhood illness and increase the family’s ability to care for the child.
In our study, the outcomes of the Partial Package group were also informative. While child growth improved somewhat, child diet and household wealth essentially remained unchanged. This emphasizes the importance of unmeasured social, economic, cultural and psychological variables that may have been impacted by the Full Package programme. Although families in the Partial Package programme received identical nutritional and livestock management training as those in the Full Package, it may take time for this knowledge to be adopted. Such training may be necessary but not sufficient to produce real change, as the interplay between motivational factors and information processing is complex(Reference Worsley44). The exact mechanisms by which the multisectoral intervention increases household wealth are not known with certainty, but it is plausible that the combination of social capital development, enhancement of community ties, reinforcement of personal agency and women’s empowerment interact to provide a base by which the training is more readily adopted and incorporated into practice.
Our study had several strengths and limitations. The major strength was the longitudinal nature of the study, with high retention rate and participation in the Full Package. Important limitations included the unexpected differences in several characteristics of the three groups at baseline, particularly the lower WAZ and HAZ in the Control group children. We adjusted for these differences in our analyses, but these differences merit further investigation. As with any community-based trial, it is possible that there were important differences between the groups that were not collected or measured. The possibility exists of a systemic bias which may have influenced our results; the geographic grouping and selection of participants in the study may have made it more susceptible to such bias(Reference Manandhar, Osrin and Shrestha34). It is also possible that programmes conducted by other organizations active in western Nepal influenced the Control group households, although we have no specific knowledge that this occurred. An additional weakness was the lower participation rate of the Partial Package group. Despite efforts of field staff, only about 70 % of the offered instruction was attended. We were unable to adjust for individual household attendance in our analysis.
Our statistical approach also had several potential limitations. While collinearity of independent variables in our regression models may have been a problem, we found that the variance inflation factors were <1·5 for the variables we selected. Finally, due to the nature of the community-based intervention, we were unable to randomize by household, but rather used geographically defined village groups, randomly assigned to intervention status.
Nevertheless, our results suggest how strongly many community- and household-level factors are intertwined: without concurrent improvement in household wealth, social capital development and effective behaviour change communication, improvement in child outcomes may be limited. The improved child growth and diet parameters in the Full Package group suggest that behaviour change training in the context of community and social capital development was more likely to be integrated into household practices, compared with training provided in the absence of this supportive framework.
Conclusions
Our results provide empirical support for the positive impact of links of nutrition-sensitive programming that included livelihoods promotion and community empowerment(Reference Ruel and Alderman7). In this low-income, food-insecure context, a livestock-based programme integrated with other components, including nutrition training and community social capital development, was associated with better child growth and nutrition outcomes than isolated training programmes alone. Although more time-consuming and costly to administer, multisectoral programmes should be considered by organizations seeking measurable and sustainable improvements in child outcomes. Important facets of success in this setting were the key focus on community empowerment and mobilization, the delivery of the Full Package intervention via women’s groups and the sustained, in-depth involvement of the implementing organization (Heifer Nepal) during the first year of Full Package intervention. Further studies empirically exploring appropriate cost-effective packages of interventions, delivery options and component elements should be supported to expand on this important evidence base to guide policy makers and donors.
Acknowledgements
Acknowledgements: The authors gratefully acknowledge the participation of community families and the contribution of community leaders, Heifer Nepal field staff and Valley Research Group field enumerators. They are also thankful for the statistical assistance and expertise of Dr Breanne Langlois. Financial support: This work was supported by the Innovation Lab for Nutrition, which is funded under grant contract AID-OAA-L-10-00006 by the US Agency for International Development (USAID). USAID had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: L.C.M. designed and conducted the research, analysed the data, wrote the paper and had primary responsibility for the final content. Su.N. analysed the data. N.J. designed and conducted the research. M.L. designed the research. B.L.R. designed the research and reviewed the paper. Sh.N. conducted the research. S.G. reviewed the paper. P.W. reviewed the paper. All authors have read and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Nepal Health Research Council (reference number 1369, 1327) and Tufts University Institutional Review Board (#1305009, ClinicalTrials.gov identifier NCT03516396). Verbal informed consent was obtained from all participants. Verbal consent was witnessed and formally recorded.













