Management Implications
Road verges, essential for conserving native grassland vegetation, may be taken over by invasive plant species through seeds found in contaminated soil masses used during their construction and maintenance. Our study provides valuable insights into practical strategies for more effective prevention and management of invasive plants, with a specific focus on Lupinus polyphyllus (garden lupine), which is a prolific seed producer easily dispersed during soil transport.
Hot steam treatment of soil masses holds significant promise for controlling L. polyphyllus seeds; at a temperature of 97 C for 10 to 17 min, >99% of the seeds were killed. By conducting a comprehensive evaluation of various heat eradication methods, our research identifies the most efficient approach tailored to the unique challenges posed by this invasive species. This method is particularly suitable for authorities responsible for and engaged in road construction and maintenance that involves moving contaminated soil. It is important to note that the method is not intended for use as a control method for existing lupine populations but rather for treating excavated soil masses that are to be relocated. These findings offer practical guidance for field practitioners and land managers. The application of hot steam treatments represents a rapid solution to safeguard native plant communities in vulnerable areas such as road verges. Our research encourages the adoption of this method as a powerful tool in the ongoing battle against invasive plant species. It empowers practitioners with the knowledge to make informed decisions and actively contribute to the better prevention and management of these threats, thereby preserving the ecological integrity of these crucial landscapes.
Introduction
Habitat destruction, overexploitation, pollution, and invasion of alien species are major drivers of biodiversity loss (Cardinale et al. Reference Cardinale, Duffy, Gonzalez, Hooper, Perrings, Venail, Narwani, Mace, Tilman, Wardle and Kinzig2012; Dirzo and Raven Reference Dirzo and Raven2003). The ability to identify places that provide refuge for affected native species is a key strategy for conservation biologists (Selwood and Zimmer Reference Selwood and Zimmer2020). Road verges constitute important complementary habitats for many plant species usually found in semi-natural grasslands (Auestad et al. Reference Auestad, Rydgren and Austad2011; Cousins Reference Cousins2006; Dániel-Ferreira et al. Reference Dániel-Ferreira, Bommarco, Wissman and Öckinger2020; Lennartsson and Gylje Reference Lennartsson and Gylje2009). Road verges are regularly mown for traffic safety purposes, and this maintenance contributes to creating open areas resembling traditional semi-natural grasslands with similar disturbance regimes (Jantunen et al. Reference Jantunen, Saarinen, Valtonen and Saarnio2007). Green infrastructure with well-developed, species-rich road verges offers great prospects for compensating the loss of grassland ecosystem services such as control of agricultural pests, soil stability, water quality, carbon sequestration, and nutrient cycling (Phillips et al. Reference Phillips, Bullock, Osborne and Gaston2020). However, as linear habitats, road verges increase connectivity, which also makes them prone to colonization by nonnative species (Lázaro-Lobo and Ervin Reference Lázaro-Lobo and Ervin2019). As disturbed areas, mainly due to road construction, road verges are thus highly susceptible to the establishment of alien invasive plant species (Christen and Matlack Reference Christen and Matlack2006; Hansen and Clevenger Reference Hansen and Clevenger2005). This is currently seen as the greatest threat to species richness along roads (Tschan Reference Tschan2018; Wissmann et al. Reference Wissman, Norlin and Lennartsson2015).
Garden lupine (Lupinus polyphyllus Lindl.), native to western North America and initially introduced as an ornamental flower in different parts of the world, is now considered invasive in parts of Europe, New Zealand, Australia, and Chile (Eckstein et al. Reference Eckstein, Welk, Klinger, Lennartsson, Wissman, Ludewig, Hansen and Ramula2023; Hejda Reference Hejda2013). In Sweden, L. polyphyllus is primarily found along roads, and its presence has negative effects on biodiversity and the conservation of native flora (Lindqvist et al. Reference Lindqvist, Johansson, Ek, Adelsköld, Borlid, Karlsson and Röstell2012; Sjölund and Lindqvist Reference Sjölund and Lindqvist2012). As a tall perennial herb capable of fixing atmospheric nitrogen and increasing soil fertility, L. polyphyllus has a competitive advantage against low-growing and nitrogen-sensitive plants (Eckstein et al. Reference Eckstein, Welk, Klinger, Lennartsson, Wissman, Ludewig, Hansen and Ramula2023; Hiltbrunner et al. Reference Hiltbrunner, Aerts, Bühlmann, Huss-Danell, Magnusson, Myrold, Reed, Sigurdsson and Körner2014; Thiele et al. Reference Thiele, Isermann, Otte and Kollmann2010). Each plant can produce up to 2,000 seeds (Aniszewski et al. Reference Aniszewski, Kupari and Leinonen2001) that are spread ballistically up to 5.5 m from the mother plant (Volz Reference Volz2003). Like many legumes, L. polyphyllus has seeds with hard seed coats, preventing water uptake and leading to physical dormancy (Westerman et al. Reference Westerman, Hildebrandt and Gerowitt2012). However, the hardness of seed coat varies among L. polyphyllus seeds, resulting in asynchronous germination (Klinger et al. Reference Klinger, Eckstein, Horlemann, Otte and Ludewig2020), and the extent of dormancy may vary due to environmental and genetic factors (Finch-Savage and Footitt Reference Finch-Savage and Footitt2017). Lupinus polyphyllus disperses at the landscape scale with movement of soil masses during road construction, ditching, or road maintenance (Wissman et al. Reference Wissman, Norlin and Lennartsson2015). Seeds can remain dormant in the soil, serving as potential contaminants during transportation to new locations, and the handling of soil masses is characterized by a lack of legal regulations and monitoring (Eckstein et al. Reference Eckstein, Welk, Klinger, Lennartsson, Wissman, Ludewig, Hansen and Ramula2023; Lennartsson et al. Reference Lennartsson, Wissman, Eckstein and Helldin2021).
Several studies investigated how single types of heat treatment, such as hot air (Blomqvist Reference Blomqvist2021), steam (Bitarafan et al. Reference Bitarafan, Kaczmarek-Derda, Brandsæter and Fløistad2021), or composting (Hassani et al. Reference Hassani, Vallius, Rasi and Sormunen2021) affect seed germination of L. polyphyllus. Generally, they all suggest that seeds of L. polyphyllus are rather tolerant to heat due their hard seed coat. For example, after being heated in a drying oven at 70 C for 15 min, 81 ± 7% (mean ± SD) of the seeds were viable (Blomqvist Reference Blomqvist2021). Similarly, pre-germination treatment of L. polyphyllus seeds in a drying oven at 80 C for 7 min had no inhibitory effect on germination (Elliott at al. Reference Elliott, Fischer and LeRoy2011). Water has a higher thermal conductivity than air, and as a result, replacing air with water provides a significant improvement in heat conduction (Dong et al. Reference Dong, McCartney and Lu2015). Consequently, exposure of dry L. polyphyllus seeds in soil to hot steam (Bitarafan et al. Reference Bitarafan, Kaczmarek-Derda, Brandsæter and Fløistad2021) seems to be an effective method to kill seeds; only 4.7% of the seeds germinated after steam treatment at 98 C for 3 min.
The hard and impermeable seed coat protects the embryo from damage caused by temperature and humidity variations, and a coat-imposed dormancy occurs as a result of the seed coat’s impermeability to water (Kelly et al. Reference Kelly, Van Staden and Bell1992; Mohamed-Yasseen et al. Reference Mohamed-Yasseen, Barringer, Splittstoesser and Costanza1994). Heat tolerance may be lost, however, after the seed coat has become permeable to water and physical dormancy is broken. The latter may occur as a result of mechanical impact on the seed coat, such as different types of scarification (Mohamed-Yasseen et al. Reference Mohamed-Yasseen, Barringer, Splittstoesser and Costanza1994; Westerman et al. Reference Westerman, Hildebrandt and Gerowitt2012). The seedbank population of L. polyphyllus in the field will probably consist of seeds that vary in their degree of physical dormancy, ranging from dry and fully dormant to imbibed and physiologically active. However, to our knowledge, no one has yet studied whether and how much dry and imbibed seeds differ in their sensitivity high-temperature treatments in terms of germination.
Additionally, given the large variation in germination of L. polyphyllus seeds between source regions (19% to 25.8%; Sober and Ramula Reference Sober and Ramula2013) and neighboring populations (10%; Volz Reference Volz2003), we also tested the response of different seed batches to heat treatments.
This study aims at comprehensively comparing different heat eradication methods for L. polyphyllus seeds. By evaluating the efficacy of dry heat and steam treatments, we seek to identify the most efficient seed eradication approach. Additionally, we explore variations in germination patterns and heat tolerance among different seed batches from various regions and collection years. Also, understanding the responses of both dry and physiologically active seeds will provide valuable insights into the differential effects of heat treatments on seed germination. This knowledge will contribute to our understanding of seed behavior and inform effective strategies for seed management and conservation efforts. This research aims to offer recommendations for managing invasive species and safeguarding native plant communities in disturbed areas like road verges. During this study, we conducted four experiments that addressed the following questions:
-
1. Do the effects on germination of L. polyphyllus seeds differ between dry heat and steam?
-
2. Which combination of temperature and exposure time to dry heat and steam is needed to kill >95% of the L. polyphyllus seeds?
-
3. Do the effects of dry heat and steam on germination differ between dry and physiologically active (imbibed) seeds?
-
4. Is there variation in seed germination after heat treatment between different seed batches?
Materials and Methods
Plant Material Collection and Seed Preparation
The L. polyphyllus seeds used in our experiments were collected from several plants in July 2020 (seed batch 2020: Blekinge County, Sweden: 56.335194°N, 15.119833°E) and July 2022 (seed batch 2022: Värmland County, Sweden: (59.378333°N, 13.504167°E). Seeds were initially stored at room temperature in paper bags. After being cleaned, seeds were transferred to plastic containers and refrigerated at 8 C until they were used in the experiments.
Only intact and ripe seeds were selected for the experiments. Seeds of L. polyphyllus show physical dormancy, which results in high mean germination time (average of 114 d; Klinger et al. Reference Klinger, Eckstein, Horlemann, Otte and Ludewig2020) and high asynchrony of germination in climate chambers. Therefore, we decided to break dormancy by manual scarification to be able to assess the immediate response (germination vs. nongermination) to our experimental treatments. Scarification involved scraping the seed with a scalpel to weaken the seed coat, with the primary aim of enabling rapid imbibition and, consequently, accelerating the germination process. Experiments 1, 2, and 4 included comparisons of dry (dormant) seeds, which were heat treated before scarification, and imbibed (physiologically active) seeds, which were scarified before treatment. For Experiment 3, only dry non-scarified seeds were used. To standardize the preparation of imbibed seeds across all experiments, physiological activity was induced by placing seeds in petri dishes and covering them with 4 ml of distilled water. They were then placed in a growing chamber Panasonic MLR-352 (Panasonic Industry Co., Osaka 571-8506, Japan) with a constant temperature of 20 C for a duration of 72 h. To mimic the natural light cycle, illumination varied with light (12,200 lux) between 0800 and 2200 hours and darkness between 2201 and 0759 hours per day. Thereafter, the seeds were manually scarified by scraping the seed coat, and another 2 ml of water was added after scarification. The imbibed seeds were kept in the growing chamber until the experimental heat treatment the next day (approx. 24 h). Dry seeds were exposed to the same experimental treatments as imbibed seeds (temperatures and exposure times), but in a dormant state. To facilitate imbibition and germination, dry seeds also underwent the same manual scarification 1 d posttreatment in all experiments. Because dormant or quiescent dry seeds rapidly start a variety of metabolic activities after initial imbibition (Bewley Reference Bewley1997), we assume that differences in response to heat treatments between dry and imbibed seeds are a consequence of differences in physiological activity and not simply a result of variation in scarification. The control groups of seeds were selected from the same seed batch as indicated in Table 1, and stored in the growing chamber without undergoing heat treatment. Simultaneously with the dry heat–treated seeds, the control seeds were scarified and given 2 ml of water.
Table 1. Overview of experiments performed using dry heat or steam

a The combination of dry seeds × 95 C × 10 min was used as a comparison to the treatment combination in Experiment 3.
b Seeds collected in different years and locations (2020 in Blekinge County, 2022 in Värmland County).
Experiments
Experiment 1: Dry Heat
To determine the effects of different temperatures and exposure times of dry heat on the germination of dry and imbibed seeds of L. polyphyllus, the experiment in a laboratory drying oven (Termaks series 8000, Termaks AS, Bergen 5057, Norway) tested temperatures of 88, 93, 98, and 103 C and exposure times of 1, 3, 5, and 10 min, resulting in 32 treatment combinations with five to eight replicates (Table 1). Each replicate consisted of 50 seeds per petri dish.
The respective exposure time started as soon as a temperature sensor inside the drying oven reached the treatment temperature. After the heat treatment, dry seeds underwent scarification. Subsequently, all seeds (dry and imbibed) received 4 ml of water and were placed in a growing chamber at 20 C and 12 h of light (12,200 lux) per day until they germinated or until they were checked for viability (day 11 post–heat treatment). To ensure optimal germination conditions, dry seeds were watered with 4 ml and 2 ml on day 4 and day 7, respectively, post–heat treatment; imbibed seeds were only watered on day 7 posttreatment (2 ml). Germination of seeds was checked after 8 d post–heat treatment, and all germinated seedlings were removed. Then, 2 ml of water was added to the remaining seeds, and they were checked again after day 11 post–heat treatment. We performed a tetrazolium test to determine seed viability. Seeds that did not germinate until day 11 post–heat treatment were transferred to petri dishes with a 1% solution of 2,3,5-triphenyl-tetrazolium-chloride (Thermo Fisher Scientific, Waltham, MA 02451, USA) and kept at 30 C for 24 h in darkness. Thereafter, the entire seeds were visually checked for viability (red color). In summary, all remaining, nongerminating seeds after treatment in our experiments were classified as dead.
Experiment 2: Steam Heat Using SoilSteam International AS Steaming Prototype Device
In the second experiment, we used the SoilSteam International AS Steaming Prototype Device (as in Bitarafan et al. Reference Bitarafan, Kaczmarek-Derda, Brandsæter and Fløistad2021; Figure 1A) to test the effects of hot steam treatments on germination of dry and imbibed seeds in soil, at temperatures of 85, 90, and 95 C and exposure times of 3 and 5 min (Table 1). The non-scarified dry and scarified imbibed seeds were placed in empty tea bags (50 seeds per bag). Three subsamples per treatment combination were put inside a laundry bag (30 by 40 cm) that was then placed in a plastic container with holes (60 by 40 by 20 cm) and covered with 7 cm of compost soil. The plastic container was exposed to steam in an experimental steaming prototype device (test box) made by SoilSteam International AS (Stokke 3160, Norway). As described by Bitarafan et al. (Reference Bitarafan, Kaczmarek-Derda, Brandsæter and Fløistad2021), the device has dimensions of 190 by 144 by 88 cm and an effective steaming area of 120 by 80 cm. The steam generator has a production capacity of 250 kg h−1 and a calorific rating of 167,000 kcal h−1, with 0.5-bar working pressure. The steam was distributed through the soil in a non–air proof chamber, and excess steam was released from the top of the chamber in case of any imbalance between steam supply and demand. Soil temperature was monitored using PT1000 sensors, which were connected to a cRIO-9073 data logger (National Instruments, Austin, TX 78759-3504, USA). In addition, 10 thermocouples were placed in the plastic container carrying the soil-submerged samples; steaming and vacuum were shut off when at least five of the sensors had reached the target soil temperature. Thereafter, soil temperatures decreased; the average temperatures across the exposure times of the different treatments are summarized in Table 2. The plastic container was removed from the steaming test box after the respective post-steaming exposure times, and the samples were taken out of the soil. After the soil steam treatment, dry seeds were scarified, and all seeds (dry and imbibed) were handled following the posttreatment procedure described for Experiment 1. Additionally, a test box steam treatment of 95 C and 10 min duration was applied to dry seeds only (scarified after steaming) to serve as a comparison to the steam treatment in Experiment 3 (S30 SoilSteam machine; Table 1).

Figure 1. (A) The steaming prototype device (test box) used in the second experiment. (B) The prototype steaming machine (S30) used in the third experiment. Photos: (A) Ewa Orlikowska; (B) Soil Steam International AS.
Table 2. The average maximum and mean soil temperatures and their respective standard deviations reached in the SoilSteam AS test box during Experiment 2 a
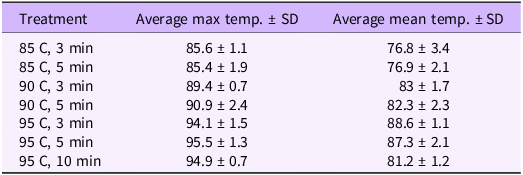
a The calculations are based on the mean values of the six replicates in each treatment group.
Experiment 3: Steam Heat Using SoilSteam International AS Prototype Steaming Machine (S30)
Here, we tested the effects of hot steam on germination of dry L. polyphyllus seeds in a stationary soil steaming machine S30 (SoilSteam International AS; Figure 1B). The machine sterilizes soil using superheated steam and is used in Norway for commercial soil sanitation. The soil was fed into the container via a conveyor system, where it was exposed to superheated steam—specifically, a predetermined temperature of 97 C in a mixing chamber (Table 1). In the first section of the mixing chamber, the soil was heated to the desired temperature. Once the correct temperature was reached, the soil was transported to a space where it remained, maintained at the desired temperature as confirmed by the S30 machine sensors. The soil, having undergone treatment for approximately 10 to 17 min (including the heating-up time), was then extracted from the machine using another conveyor system and transferred to a storage location. In total, 40 tea bags containing 50 non-scarified dry seeds each were used in this experiment. To improve posttreatment detection of the samples, several color plastic zip ties were attached to each tea bag, and only 10 bags were placed in the machine at a time. One bag went missing, and one bag was torn during the soil steaming process. Therefore, seed germination was monitored for the remaining 38 replicates. After the treatment, seed germination was checked as in Experiment 1.
Experiment 4: Dry Heat—2020 and 2022 Seed Batches
Here we tested whether germination in response to different temperatures and exposure times (Table 1) differed between seeds collected in 2020 in Blekinge County (southern Sweden) and in 2022 in Värmland County (central Sweden). Non-scarified dry seeds and scarified imbibed seeds from both batches were exposed to 88 or 98 C for 3 or 5 min in a laboratory drying oven (Termaks series 8000), resulting in 12 treatment combinations with three replicates. As in Experiment 1, the respective exposure time started as soon as the temperature sensor inside the drying oven reached the treatment temperature. After the heat treatment, dry seeds underwent scarification, and all seeds (dry and imbibed) were processed following the posttreatment procedure outlined in Experiment 1.
Statistical Analyses
All experiments followed a factorial design (Table 1), and we used the cumulative percentage of seeds over the experiment (11 d posttreatment) as the response variable. To test for over/underdispersion in the data, the Pearson chi-square goodness-of-fit test was employed. We used generalized linear models (GLMs) with quasi-binomial distribution and logit function to analyze the germination data from all experiments, as they accommodate both under- and overdispersion and are appropriate for proportional data that do not meet the assumptions of a binomial distribution (Gómez-Déniz et al. Reference Gómez-Déniz, Gallardo and Gómez2020). We included seed status (dry or imbibed), temperature, and exposure time as fixed factors in the model. In Experiment 4, beyond the fixed factors noted, we also included seed batch as a fixed factor. We performed ANOVAs using an F-test to test the significance of each factor in the GLMs. We conducted post hoc pairwise comparisons using estimated marginal means (emmeans) with Bonferroni correction, as implemented in the R package emmeans (Lenth et al. Reference Lenth, Singmann, Love, Buerkner and Herve2019). All analyses were performed using the statistical software R v. 4.2.2. (R Core Team 2022). In Experiment 1, there was great variation in some treatment combinations (maximum coefficient of variation: 94.7%), while we had a floor effect (i.e., no seed germination and no variation at all; Ruxton and Colegrave Reference Ruxton and Colegrave2011) for 103 C at 10 min. Because the latter also resulted in a spurious three-way interaction, we excluded the 10-min treatment level from the analyses. In Experiment 2, there was a floor effect for imbibed seeds, which showed no germination at 90 and 95 C and only 0.5% germination (± 0.4 SE) at 85 C. Because it was neither meaningful nor necessary to do any statistical analyses on imbibed seeds, we only analyzed dry seeds in Experiment 2.
Results and Discussion
Experiment 1: Dry Heat (Drying Oven)
We found that no germination occurred after treatment with 103 C for 10 min in the case of dry seeds (imbibed seeds 10.8% ± 1.4% SE), indicating that the highest temperature and longest exposure time combined were highly effective. The floor effect caused by no seed germination for some treatment combinations created statistical issues, and therefore, we chose to exclude all 10-min treatments from further analysis. With the 10-min treatments excluded, there were significant interactions between time by temperature (F(6, 150) = 4.69, P < 0.001) and seed status by temperature (F(3, 156) = 4.59, P = 0.004), but not between seed status by time (F(6, 159) = 0.05, P = 0.95). For dry seeds, germination did not differ between temperature treatments (Figure 2), whereas average germination of imbibed seeds decreased significantly with increasing temperatures. Post hoc analysis for the time by temperature interaction revealed that longer treatment duration (across seed status) generally reduced seed germination, but there was considerable variation within treatment combinations (Figure 3).
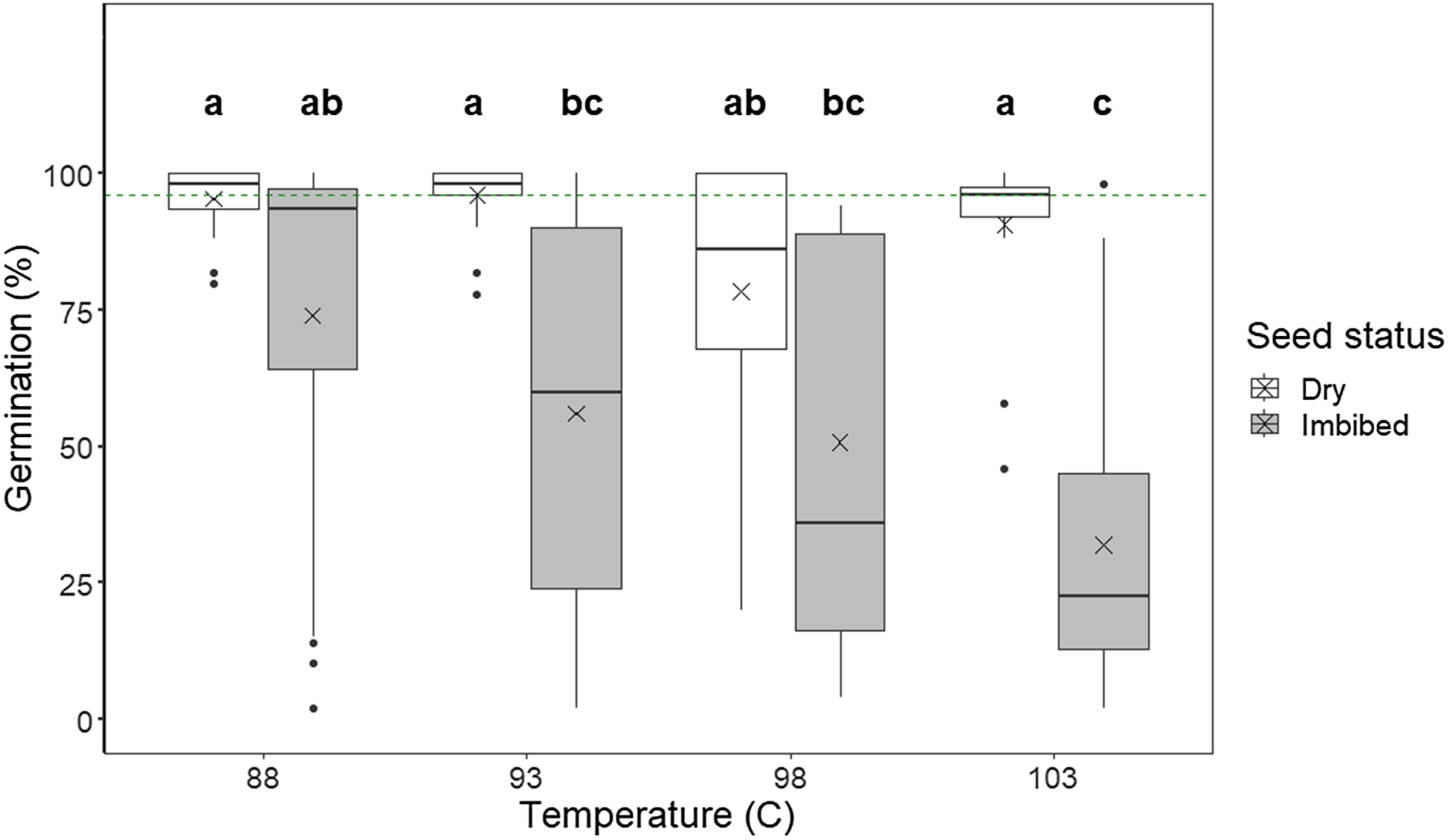
Figure 2. Germination percentage of Lupinus polyphyllus seeds exposed to dry heat in a laboratory drying oven (Termaks series 8000) at temperatures of 88, 93, 98, and 103 C. The germination percentage of controls is indicated by the green dashed line. The boxes represent upper and lower quartiles; the whiskers are the minimum and maximum of the data, except for the outliers, which are shown as dots. The thick line in each bar represents the median value; the crosses depict the mean values. Letters indicate homogeneous groups based on post hoc tests conducted on the significant interaction seed status × temperature (Bonferroni correction).
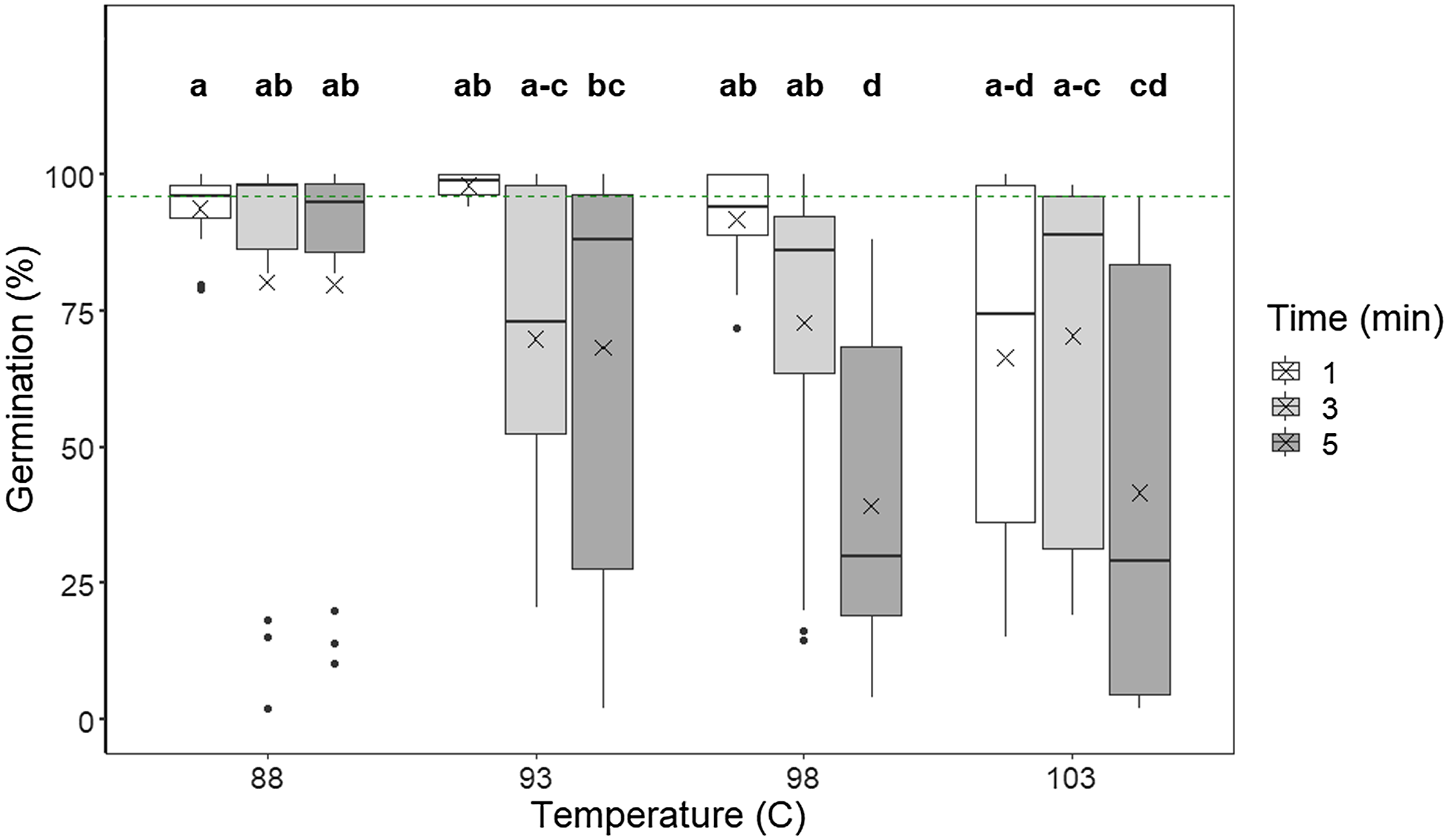
Figure 3. Germination percentage of Lupinus polyphyllus seeds exposed to dry heat in a laboratory drying oven (Termaks series 8000) at temperatures of 88, 93, 98, and 103 C for 1, 3, or 5 min. The germination percentage of controls is indicated by the green dashed line. The boxes represent upper and lower quartiles; the whiskers are the minimum and maximum of the data, except for the outliers, which are shown as dots. The thick line in each bar represents the median value; the crosses depict the mean values. Letters indicate homogeneous groups based on post hoc tests conducted on the significant interaction exposure time × temperature (Bonferroni correction), and hyphens are used to include all the letters between the two written letters.
Experiment 2: Steam Heat (SoilSteam AS Test Box)
Due to the floor effect for imbibed seeds (no germination at 90 and 95 C, and only 0.5% germination [± 0.4] at 85 C), robust statistical analyses were not feasible. For dry seeds, there was a main effect of temperature (F(2, 32) = 28.15, P < 0.001; Figure 4), but not of time (F(1, 34) = 1.7, P = 0.2). Seed germination was highest after steam treatment at 85 C (61.7% ± 5.2%), but we observed no significant difference in germination after treating seeds at 90 C (25% ± 3.2%) and 95 C (12.4% ± 5.1%).

Figure 4. Germination percentage of dry (left) and imbibed (right) Lupinus polyphyllus seeds exposed to steam heat in the test box (SoilSteam AS) at temperatures of 85, 90, and 95 C. Post hoc tests for homogeneous groups were conducted for dry seeds (Bonferroni correction). Germination of control treatment is indicated by the green dashed line. The boxes represent upper and lower quartiles; the whiskers are the minimum and maximum of the data, except for the outliers, which are shown as dots. The thick line in each bar represents the median value; the crosses depict the mean values.
Experiment 3: Steam Heat (SoilSteam AS S30)
The treatment in S30 was applied to 1,900 seeds, resulting in a germination rate of only 0.47% (± 0.2%). Specifically, germination was observed in just 9 out of 1,900 total seeds. Comparisons of the germination percentage between the test box and S30 was not feasible due to technical differences. Specifically, maintaining the target soil temperature throughout the treatment period was not possible in the test box, whereas removing the seeds after an exactly specified exposure time was not feasible in S30.
Experiment 4: Dry Heat (Drying Oven)—2020 and 2022 Seed Batches
We observed a significant three-way interaction (F(1, 35) = 2.54, P = 0.016) among seed batch by seed status by temperature, indicating that the response to these treatments varied between seed batches. Specifically, the older batch (2020) exhibited greater sensitivity to most temperature treatments compared with the younger batch (2022; Figure 5). The other three-way interactions were not significant (F < 2.59, P > 0.12). Significant two-way interactions were found for seed status by temperature (F(1, 38) = 9.95, P = 0.003) and exposure time by temperature (F(1, 37) = 9.64, P = 0.004). Regarding the interaction between exposure time and temperature, there were no large differences between exposure times in terms of germination at the lower temperature (88 C). However, a clear difference was observed at 98 C, with lower seed germination after longer exposure times.

Figure 5. Germination percentage of two batches (2020, 2022) of dry and imbibed Lupinus polyphyllus seeds exposed to dry heat treatments in a laboratory drying oven (Termaks series 8000) at temperatures of 88 and 98 C. Germination of control treatments is indicated by the dashed line (2020 seed batch) and the solid line (2022 seed batch). The boxes represent upper and lower quartiles; the whiskers are the minimum and maximum of the data. The line in each bar represents the median value; the crosses represent the mean value. Post hoc tests for homogeneous groups were conducted for the three-way interaction seed batch × seed status × temperature (Bonferroni correction).
We conducted four experiments to investigate the heat sensitivity of L. polyphyllus seed germination under different conditions with the aim of developing effective heat eradication methods for seeds in soil masses. Our results suggest that steam had stronger negative effects on seed germination than dry heat. Germination decreased to <5% at temperatures of >90 C with steam, while temperatures >100 C were needed for the same effect in case of dry heat. Dry seeds were less sensitive to heat treatments than imbibed seed, that is, physiologically active seeds; older seeds were more sensitive to heat than seeds collected in the same year as the experiment was conducted.
The germination of seeds treated with dry heat in the drying oven was higher compared with seeds treated with steam, notwithstanding temperatures being constant in the drying oven, while the temperature (for procedural technical reasons) decreased with time in the steam test box. Thus, although seeds exposed to steam experienced lower average temperatures than seeds exposed to dry heat, they showed significantly lower germination percentages after the treatment. This is most probably due to higher thermal conductivity of water compared with air (Dong et al. Reference Dong, McCartney and Lu2015), which makes hot steam a promising source of energy for heat eradication methods.
Generally, seed germination decreased with increasing treatment temperatures and with increasing exposure times. However, we did not find a significant effect of exposure time on germination when applying steam in the test box. This is most probably due to the relatively small difference in average temperature between heat treatments inside the test box (Table 1) and between tested exposure times (3 vs. 5 min). While Bitarafan et al. (Reference Bitarafan, Kaczmarek-Derda, Brandsæter and Fløistad2021) suggest treating dry seeds of L. polyphyllus with hot steam at 98 C for 3 min (4.5% seed germination posttreatment), we found similar germination (4.3%) after steam treatment with 95 C for 5 min (corresponding to an average temperature of 87.3 C during the treatment). This suggests that higher temperatures might compensate for shorter exposure times and vice versa and still result in the same effects on posttreatment germination.
Lupinus polyphyllus seeds have hard coats, and previous studies have shown them to have a large heat tolerance (Bitarafan et al. Reference Bitarafan, Kaczmarek-Derda, Brandsæter and Fløistad2021; Blomqvist Reference Blomqvist2021; Hassani et al. Reference Hassani, Vallius, Rasi and Sormunen2021). However, this seems only to be true for dry seeds, as imbibed seeds showed significantly lower germination percentages irrespective of the heat type applied (dry heat vs. hot steam). This suggests that imbibed seeds are more sensitive to heat treatments, which most likely is related to the breaking of physical dormancy through scarification, followed by water uptake and subsequent vulnerability of the physiologically active embryo (Kelly et al. Reference Kelly, Van Staden and Bell1992; Mohamed-Yasseen et al. Reference Mohamed-Yasseen, Barringer, Splittstoesser and Costanza1994; Westerman et al. Reference Westerman, Hildebrandt and Gerowitt2012). Consequently, the physiological state of the seeds plays a crucial role in their response to heat treatments, with imbibed seeds being more susceptible to damage than dry seeds. Given the demonstrated effectiveness of soil steaming for eradication of other invasive plant species seeds, for example, ornamental jewelweed (Impatiens glandulifera Royle) and wild oat (Avena fatua L.) (Bitarafan et al. Reference Bitarafan, Kaczmarek-Derda, Brandsæter and Fløistad2021), it is reasonable to consider that a method that successfully kills hard-coated L. polyphyllus seeds should be a viable strategy for controlling other invasive plant species as well.
Our results show, however, that the seeds’ response to the treatments is very variable. We observed very low or no variation at all within certain treatment combinations. This was especially evident in cases where seed germination reached either 100% or 0%, which complicated the analyses. We believe this phenomenon may be attributed to the practical constraints of using a relatively small number of replicates for each treatment combination, but it could also be related to the condition of the seeds. We also found large differences in germination among the different seed batches that were subjected to the same treatment combinations. Previous studies have indicated that the germination percentages vary among regions (Sober and Ramula Reference Sober and Ramula2013; Volz Reference Volz2003) and with development stage (Klinger et al. Reference Klinger, Eckstein, Horlemann, Otte and Ludewig2020). Seed maturation includes a period of desiccation (Angelovici et al. Reference Angelovici, Galili, Fernie and Fait2010) during which seeds dry, shrink, and eventually enter dormancy. After the seed finally enters the state of physical dormancy, the aging of the seed coat begins, and it is likely that older seeds gradually lose physical dormancy. This matches with our results, which showed that the older seed batch (2020) was more sensitive to heat treatment than the younger batch (2022). However, we cannot separate the effects of age and location, as the batches were from different years and places, and more studies are needed to better understand the effects of age and locality on the response of seeds to heat treatments.
We used a scalpel for scarification to scrape the hard seed coats, which is an effective method to break coat-imposed dormancy and enable water uptake (Mohamed-Yasseen et al. Reference Mohamed-Yasseen, Barringer, Splittstoesser and Costanza1994; Westerman et al. Reference Westerman, Hildebrandt and Gerowitt2012). However, it is difficult to determine how much the seed coat has been affected by this mechanical stress in practice. Therefore, large variation of germination percentage within treatments in our experiment may partly be a consequence of variation in the impact of manual scarification, leading to faster or slower imbibition and physiological activation of the treated seeds. Beuthin (Reference Beuthin2012) mentions that in its native environment, L. polyphyllus germinates unevenly if not scarified before sowing; thus physical or chemical scarification is recommended to improve the uniformity of germination.
An important dispersal route of L. polyphyllus seeds at the landscape scale occurs through the movement of seed-containing soil masses related to road construction and maintenance (Wissman et al. Reference Wissman, Norlin and Lennartsson2015). It is possible that the transportation of soil masses contaminated with L. polyphyllus seeds could impose enough mechanical stress on the seed coats to break physical dormancy and induce rapid germination after the transport. This mechanical stress could be analogous to the scarification performed on imbibed seeds before heat exposure, as well as after heat exposure on dry seeds. To our knowledge, there is a lack of studies addressing these questions. However, some unpublished data suggest that shaking seeds of L. polyphyllus in a bottle with sand or gravel significantly increased seed germination after these treatments in comparison to a control (F Afzelius, personal communication). Because L. polyphyllus seeds exhibit highly asynchronous germination (Klinger et al. Reference Klinger, Eckstein, Horlemann, Otte and Ludewig2020), it can be assumed that the extent of dormancy varies among seeds in the soil. We observed that dry dormant seeds are less heat sensitive than physiologically active imbibed seeds. To maximize seed mortality during treatment, exposure time and temperature should be determined based on what is required for dry seeds.
Because handling of soil masses contributes significantly to the spread of invasive plants (Wissman et al. Reference Wissman, Norlin and Lennartsson2015), we tested experimentally whether seeds can be killed through soil steaming in the SoilSteam S30 machine that is currently used in Norway for a commercial soil sanitation. Here, we exclusively used dry, non-scarified dormant seeds to investigate their posttreatment germination, with scarification performed after the treatment. The results were very promising for practical applications, with <0.5% seeds germinating. Unlike the test box, where it was not possible to maintain the target soil temperature throughout the entire exposure period (see Table 2), in S30, the temperature remained constant. However, it was not possible to precisely control the duration of exposure in S30, whereas this could be done for the test box. This is because seed bags are mixed in and transported with large soil masses through the machine, resulting in some variation of exposure time. A factor that was not investigated in this study, but that could potentially enhance the effectiveness of soil steaming, is to adjust the treatment temperature and exposure time according to specific soil conditions. Melander and Kristensen (Reference Melander and Kristensen2011) found that moisture in the soil promotes heat penetration and improves the effectiveness of soil steaming for weed control. On the other hand, they found that coarse-structured soils, with large aggregates, can act as refuges for weed seeds and make it more challenging for heat to penetrate the soil effectively. An aspect to consider is that soil steaming will not only kill L. polyphyllus seeds, but it will also significantly reduce microbial biomass and change the soil structure (Dietrich et al. Reference Dietrich, Cesarz, Eisenhauer and Roscher2020). These negative effects of soil steaming must be weighed against the benefits of being able to safely store and reuse soil previously contaminated with L. polyphyllus without increasing the spread of the invasive species. Not treating contaminated soil will cause ecological consequences and negatively affect biodiversity when the soil is reused. There are indications that soil steaming has minimal long-term effects on microbial communities and quality of soil (Jäderlund et al. Reference Jäderlund, Norberg, Zackrisson, Dahlberg, Teketay, Dolling and Nilsson1998; Li et al. Reference Li, DiLegge, Minas, Hamm, Manter and Vivanco2019; Norberg et al. Reference Norberg, Dolling, Jäderlund, Nilsson and Zackrisson2001), but also that the choice of biological indicator is critical to characterize the status of the soil (Roux-Michollet et al. Reference Roux-Michollet, Czarnes, Adam, Berry, Commeaux, Guillaumaud, Le Roux and Clays-Josserand2008). Considering all the risks involved, soil steaming, with its impact on soil microbiota, may pose lower risk to the local ecosystem than the one associated with invasion of native grassland communities by L. polyphyllus. To our knowledge, there is no research examining the long-term effects of reusing soil from this type of steam treatment, which may be relevant for future management. Further studies under field conditions are necessary to validate these findings, including testing the effectiveness of the soil steaming machine on different types of soil. Although our experiment demonstrated the efficacy of the soil steaming machine in treating contaminated soils, it is important to investigate its performance in diverse soil conditions encountered in the field, not only to ensure high seed mortality but also to improve energy and cost-efficiency.
Developing effective heat eradication methods for L. polyphyllus seeds in soil masses is essential for preventing the spread of this invasive species along road verges. Our study provides valuable insights for practical eradication of L. polyphyllus seeds using soil heat treatments. We found that hot steam showed greater effectiveness in reducing seed germination compared with dry heat. This suggests that steam-based methods have great potential for controlling the spread of L. polyphyllus in road verges and other areas where soil masses are involved. To effectively eradicate L. polyphyllus seeds in soil, we recommend application of steam treatment at a constant temperature of 97 C for at least 10 min. This recommendation is based on the results obtained from the test conducted in the S30 machine, where the survival rate of dry seeds, which we found to be more resilient than imbibed seeds, was less than 0.5%.The technique of steaming soil masses containing L. polyphyllus seeds can primarily be applied during excavation and relocation of soil masses in road construction, ditching, or road maintenance. The excavated soil masses should be treated in a soil steaming machine to reduce the risk of species spread at the landscape level. Our results highlight the importance of considering the physiological state of the seeds. However, it is unclear how to practically break seed dormancy and physiologically activate seeds before the steam treatment. These aspects deserve further studies, considering that lower temperatures and less energy would be required to kill imbibed seeds.
Acknowledgments
We thank SoilSteam International (Norway), in particular Hans Kristian Westrum, Silje Eftang, and Tobias Glemming, for good collaboration on this project. Many thanks to Geni Zanol and Emelie Jernberg for their assistance during the laboratory and field parts of the project and to Yves P. Klinger for support during the statistical analyses. Thank you to the two anonymous reviewers for their comments that contributed to improving the manuscript.
Funding
This project (grant no. TRV 2021/14629) was funded by the Swedish Transport Administration (Trafikverket) to improve the management of invasive species in a joint effort with the Swedish Environmental Protection Agency (Naturvårdsverket), the Swedish Research Council Formas, and the Swedish Agency for Marine and Water Management (Havs- och vattenmyndigheten). Additional funds have been obtained through the research program TRIIAS of the Swedish Transport Administration through the Swedish University of Agricultural Sciences (SLU) Uppsala.
Competing interests
The authors declare no competing interests.










