Hypospadias is one of the most common malformations in males, with an estimated incidence of 3 to 8 per 1,000 newborn males in Europe (Nordenvall et al., Reference Nordenvall, Frisen, Nordenstrom, Lichtenstein and Nordenskjold2014; Pierik et al., Reference Pierik, Burdorf, Nijman, de Muinck Keizer-Schrama, Juttmann and Weber2002; Virtanen et al., Reference Virtanen, Kaleva, Haavisto, Schmidt, Chellakooty, Main and Toppari2001). In hypospadias, the urethral opening is displaced along the ventral side of the penis. The phenotype is defined by the position of the urethral meatus in anterior (glandular or coronal), middle (penile), and posterior (penoscrotal, scrotal, or perineal) hypospadias (van der Zanden et al., Reference van der Zanden, van Rooij, Feitz, Franke, Knoers and Roeleveld2012). Hypospadias is the result of an incomplete fusion of the urethral folds, which occurs between the 8th and 14th week of gestation (Manson & Carr, Reference Manson and Carr2003). The earlier the fusion fails in the embryonic period, the more severe the type of hypospadias (i.e., posterior hypospadias).
The etiology of isolated hypospadias is considered to be multifactorial: genetic, endocrine, environmental, and clinical factors have been implicated (van der Zanden et al., Reference van der Zanden, van Rooij, Feitz, Franke, Knoers and Roeleveld2012). A family history of hypospadias increases the risk by seven- to 10-fold, suggesting an important genetic component (Brouwers et al., Reference Brouwers, Feitz, Roelofs, Kiemeney, de Gier and Roeleveld2007; Fredell et al., Reference Fredell, Kockum, Hansson, Holmner, Lundquist, Lackgren and Nordenskjold2002; van Rooij et al., Reference van Rooij, van der Zanden, Brouwers, Knoers, Feitz and Roeleveld2013).
Association between low birth weight and hypospadias is well established (Fredell et al., Reference Fredell, Kockum, Hansson, Holmner, Lundquist, Lackgren and Nordenskjold2002; Weidner et al., Reference Weidner, Moller, Jensen and Skakkebaek1999). In particular, being small-for-gestational-age (SGA) has been shown to be an important risk factor for hypospadias (Akre et al., Reference Akre, Lipworth, Cnattingius, Sparen and Ekbom1999; Ghirri et al., Reference Ghirri, Scaramuzzo, Bertelloni, Pardi, Celandroni, Cocchi and Boldrini2009; Jensen et al., Reference Jensen, Wilcox, Olsen, Bonde, Thulstrup, Ramlau-Hansen and Henriksen2012; Nordenvall et al., Reference Nordenvall, Frisen, Nordenstrom, Lichtenstein and Nordenskjold2014). Studies suggest that intra-uterine growth restriction (IUGR) in itself is a determining risk factor for hypospadias rather than the absolute birth weight (Hussain et al., Reference Hussain, Chaghtai, Herndon, Herson, Rosenkrantz and McKenna2002; Jensen et al., Reference Jensen, Wilcox, Olsen, Bonde, Thulstrup, Ramlau-Hansen and Henriksen2012; Yinon et al., Reference Yinon, Kingdom, Proctor, Kelly, Salle, Wherrett and Chitayat2010).
Furthermore, twinning is a risk factor consistently associated with hypospadias (Brouwers et al., Reference Brouwers, Feitz, Roelofs, Kiemeney, de Gier and Roeleveld2007; Jensen et al., Reference Jensen, Wilcox, Olsen, Bonde, Thulstrup, Ramlau-Hansen and Henriksen2012; Nordenvall et al., Reference Nordenvall, Frisen, Nordenstrom, Lichtenstein and Nordenskjold2014; van Rooij et al., Reference van Rooij, van der Zanden, Brouwers, Knoers, Feitz and Roeleveld2013). In a large case-control study about structural birth defects in twins, hypospadias occurred more often in twins compared with singletons (adjusted odds ratio (OR) 2.1 [95% CI: 1.5–3.1]; Rider et al., Reference Rider, Stevenson, Rinsky and Feldkamp2013). In addition, hypospadias in birth weight discordant twins is more frequently found in the twin with the lowest birth weight (Chambers et al., Reference Chambers, Castilla, Orioli and Jones2006; Fredell et al., Reference Fredell, Lichtenstein, Pedersen, Svensson and Nordenskjold1998). Since this was determined in monozygotic twin pairs, genetic factors were excluded, suggesting the importance of environmental etiological risk factors for hypospadias in these twins (Fredell et al., Reference Fredell, Lichtenstein, Pedersen, Svensson and Nordenskjold1998). However, in the study by Fredell et al. (Reference Fredell, Lichtenstein, Pedersen, Svensson and Nordenskjold1998) no information was provided about chorionicity. To date, the rate of hypospadias in monochorionic (MC) and dichorionic (DC) twins has not been reported in literature. Therefore, the objective of this study was to determine the incidence of hypospadias in MC and DC male twins. Furthermore, the possible correlation between hypospadias and SGA-discordant twins was investigated.
Material and Methods
All MC and DC male twins admitted to the neonatal intensive care unit at the Leiden University Medical Centre, the Netherlands, between January 2004 and August 2013 were included in this study. Data were extracted from the hospital's patient database, and all collected data were anonymized. The following perinatal data were recorded: birth weight, gestational age at birth, SGA (defined as a birth weight of <10th centile for gestational age according to Dutch references; Kloosterman, Reference Kloosterman1969), and the presence and type of hypospadias. We excluded cases with major congenital anomalies. Chorionicity was determined by prenatal ultrasound, and after birth by macroscopic examination of the placenta and the inter-twin membrane.
Statistics
Results from MC and DC twin groups were compared using an independent t-test for categorical data and the chi-square test for dichotomous data. Univariate logistic regression analysis was performed to investigate the possible relationship between hypospadias, type of chorionicity, and SGA. The multivariate logistic regression model included all variables that showed significant association in univariate analysis. Results are expressed as OR with 95% confidence interval (CI). All analyses were conducted using the generalized estimated equation module to account for the effect that observations within twins are not independent. A p-value of <.05 was considered to indicate statistical significance. Statistical data were analyzed using SPSS statistic version 20.0 (SPSS Inc. Chicago, IL).
Results
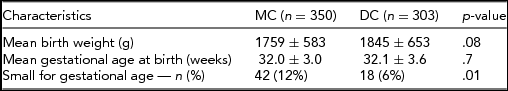
Between January 2004 and August 2013, a total of 360 MC and 306 DC male twins were admitted to our neonatal nursery. Thirteen patients were excluded because of major congenital birth defects. Therefore, analysis was performed, including 350 MC and 303 DC male twins. Baseline characteristics of the study population are presented in Table 1.
TABLE 1 Baseline Characteristics of MC and DC Twin Groups
A total of 17 patients with hypospadias were identified in our study cohort: 14 of 350 (4%) in the MC group and 3 of 303 (1%) in the DC group (p = .016). Table 2 shows different phenotypes of hypospadias in both groups. In two MC twin pairs, both twin brothers had hypospadias, while the other 10 twin pairs were discordant for hypospadias. In nine of the 12 (75%) MC twin pairs, the twin with the lowest birth weight had hypospadias. In one pair, the birth weight was identical in both infants. In DC twins, all hypospadias occurred in the same-sex twin pairs. All three DC twins were discordant for hypospadias, with two having a lower birth weight than their unaffected brother and one having higher birth weight. Overall, in 11 of the 15 (73%) twin pairs, the twin with the lowest birth weight had hypospadias.
TABLE 2 Hypospadias Phenotype

In the MC group, in total, 42 males (12%) were born SGA and 18 (6%) in the DC group (p = .008). The rate of hypospadias in SGA and non-SGA groups was 10% (6/60) and 2% (11/593; p = .002) respectively. Of the 14 MC twins with hypospadias, six were SGA at birth, whereas none of the DC twins with hypospadias was born SGA. In a multivariate logistic regression model (see Table 3), monochorionicity was found to be an independent risk factor for hypospadias (OR 4.1, 95% CI: 1.1–14.7, p = .032) as well as SGA (OR 6.1, 95% CI: 2.2–17.2, p = .001).
TABLE 3 Analysis of Potential Risk Factors for Hypospadias

Discussion
Hypospadias is known to occur more frequently in twins in comparison with singletons (Nordenvall et al., Reference Nordenvall, Frisen, Nordenstrom, Lichtenstein and Nordenskjold2014; Rider et al., Reference Rider, Stevenson, Rinsky and Feldkamp2013). This is the first study showing that the incidence of hypospadias among MC twins is significantly higher than in DC twins (4% vs. 1% respectively). Our findings are in line with a previous study describing approximately a two-fold higher incidence of congenital anomalies in MC compared with DC twins (Glinianaia et al., Reference Glinianaia, Rankin and Wright2008). In that specific study, hypospadias, however, was not reported. The association between monozygosity and hypospadias had been previously suggested by Fredell et al. (Reference Fredell, Kockum, Hansson, Holmner, Lundquist, Lackgren and Nordenskjold2002). In their study, the zygosity status could, however, only be established in 83% of the twins (33/40; 67% monozygotic and 33% dizygotic), and chorionicity was not determined. In a different study, the rate of hypospadias was similar for the same-sex twins (a mixture of monozygotic and dizygotic twins) and the opposite-sex twins (purely dizygotic; Rider et al., Reference Rider, Stevenson, Rinsky and Feldkamp2013). Once more, zygosity and chorionic status was not determined. Since MC twins are by definition monozygotic, 82% (14/17) of twins with hypospadias in our study were thus monozygotic. For our DC group, data about zygosity were not available; however, in general, around 90% of the DC group is dizygotic and only 10% is monozygotic (Cameron, Reference Cameron1968). Our data, showing an increased risk of hypospadia in MC twins, is clinically relevant since chorionicity in contrast to zygosity is easily determined during pregnancy with antenatal ultrasound in the first trimester.
In our study, in nine of the 10 MC twin pairs discordant for hypospadias, it occurred only in the smaller twin. This was also the case for two of the three cases in the DC twin pairs. A previous study had also shown that in 16 of the 18 monozygotic twin pairs the twin with the lowest birth weight indeed had hypospadias (Fredell et al., Reference Fredell, Lichtenstein, Pedersen, Svensson and Nordenskjold1998). In addition, in three MC and two DC twin pairs, all hypospadias were reported in the smaller pair of discordant IUGR twins (Yinon et al., Reference Yinon, Kingdom, Proctor, Kelly, Salle, Wherrett and Chitayat2010). Chambers et al. (Reference Chambers, Castilla, Orioli and Jones2006) described an association of IUGR and hypospadias in like-sex twins. However, zygosity or chorionicity status could not be determined. Furthermore, in singletons, hypospadias occurs more frequently in SGA infants (Gatti et al., Reference Gatti, Kirsch, Troyer, Perez-Brayfield, Smith and Scherz2001; Hussain et al., Reference Hussain, Chaghtai, Herndon, Herson, Rosenkrantz and McKenna2002). This correlation between hypospadias and SGA may suggest an association between placental insufficiency and abnormal genital development. Hypothetically, nutrients and gonadotropins may be insufficient for the normal development of the two pairs of male gonads in twin pregnancies due to inadequate placentation or unequal placental sharing (Fredell et al., Reference Fredell, Lichtenstein, Pedersen, Svensson and Nordenskjold1998). The smaller twin would be more sensitive to relative deficit in hormones and would therefore be at increased risk of developing hypospadias.
Interestingly, the incidence of hypospadias in MC twins compared with DC twins cannot be explained by a higher incidence of SGA in MC twins because SGA was identified as an independent risk factor for hypospadias in our study. It could be that the above-mentioned environmental nutrient deficiency plays a larger role in MC twins due to sharing of single placenta. Furthermore, it can be speculated that a possible etiological explanation might lie in a factor or mechanism at the timing of monozygotic twinning event. For example, post-zygotic unequal distribution of blastomeres to both embryos might result in structural malformations and growth deficiency (Machin, Reference Machin1996).
Care should be taken when interpreting the results of our study due to some limitations. First, the retrospective design is dependent on charted data. Second, an ascertainment bias might have occurred, since only admitted infants to our tertiary level neonatal intensive care unit are recorded in the database. This is, for example, the cause for the lower than expected gestational age for twins. However, the gestational age at birth was similar for both MC and DC groups (32.0 vs. 32.1 weeks respectively) and this should not have influenced our results. Lastly, the study comprises a relative small sample size, hence a small number of hypospadias. Nevertheless, with regard to the studies described in twins in literature, including data about zygosity and/or chorionicity, our number is relatively large.
In conclusion, MC and DC twins have an increased risk for hypospadias with the incidence being four-fold higher in MC twins than in DC twins. Monochorionicity and SGA were identified to be independently associated with hypospadias.





