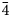Introduction
Stibiogoldfieldite, ideally Cu12(Sb2Te2)S13, is a recently defined member of the tetrahedrite group, described from the Mohawk mine, Goldfield mining district, Nevada, USA (Biagioni et al., Reference Biagioni, Sejkora, Musetti, Makovicky, Pagano, Pasero and Dolníček2022a). In the description of this new mineral, two specimens from the type locality were described. Holotype material has the chemical formula (Cu12.05Ag0.04Zn0.03Fe0.01)Σ12.13(Sb1.12As0.63Bi0.23Te2.02)Σ4.00(S12.99Se0.11)Σ13.10, whereas cotype material is richer in Te, with empirical chemical formula (Cu11.30Ag0.03)Σ11.33(Sb0.80As0.57Bi0.06Te2.57)Σ4.00(S12.83Se0.20)Σ13.03, close to the idealised formula (Cu11.40□0.60)Σ12.00(Sb0.80As0.55Bi0.05Te2.60)Σ4.00(S12.80Se0.20)Σ13.00. The higher Te content of this latter sample was coupled with a (Cu+Ag) deficit, in agreement with the substitution M (2)Cu+ + X (3)(Sb/As)3+ = M (2)□ + X (3)Te4+ (e.g. Shimizu and Stanley, Reference Shimizu and Stanley1991; Biagioni et al., Reference Biagioni, Sejkora, Musetti, Makovicky, Pagano, Pasero and Dolníček2022a). The occurrence of vacancies at the M(2) site in the crystal structure of synthetic Te-bearing tetrahedrites was first proposed by Kalbskopf (Reference Kalbskopf1974) and later confirmed on natural specimens by Dmitrieva et al. (Reference Dmitrieva, Yefremov and Kovalenker1987), using single-crystal X-ray diffraction data collected on arsenogoldfieldite, and by Pohl et al. (Reference Pohl, Ließmann and Okrugin1996), on the basis of Rietveld refinement performed on samples with composition Cu11.1(Te2.7Sb0.9As0.4Bi0.1)S12.0Se0.7. The resulting formula of the Te end-member goldfieldite is thus Cu10Te4S13, also agreeing with the theoretical model based on a Brillouin-model analysis (Johnson and Jeanloz, Reference Johnson and Jeanloz1983).
Holotype material of stibiogoldfieldite has been fully characterised, both chemically and structurally; however the cotype material has only been studied chemically (Biagioni et al., Reference Biagioni, Sejkora, Musetti, Makovicky, Pagano, Pasero and Dolníček2022a). In order to fill this gap, a single-crystal X-ray diffraction study was performed on the Te-rich stibiogoldfieldite. Moreover, an associated and still unidentified Ag–Bi–(S,Se) phase was investigated. This latter species was observed in both holotype and cotype material of stibiogoldfieldite (Biagioni et al., Reference Biagioni, Sejkora, Musetti, Makovicky, Pagano, Pasero and Dolníček2022a) and chemical data indicated it could belong to the pavonite homologous series (unpublished data). The single-crystal X-ray diffraction results (below) confirm this hypothesis, allowing its identification as the N = 6 homologue of the pavonite series, namely dantopaite. Further, this sample showed a peculiar chemical composition, being Se-enriched, hence its crystal structure was refined, in order to understand the behaviour of Se in this compound.
This study has consequently a two-fold aim: an improvement of the crystal chemical knowledge of Te-bearing tetrahedrites and the crystal-chemical study of Se-bearing dantopaite associated with stibiogoldfieldite at the Mohawk mine.
Experimental
Specimen studied
The specimen studied is represented by cotype material of stibiogoldfieldite, kept in the mineralogical collection of the Department of Mineralogy and Petrology of the National Museum in Prague (Czech Republic), under catalogue number P1P 80/2020. Stibiogoldfieldite forms anhedral grains, up to 0.3 mm, associated with pyrite, calaverite, bismuthinite, bohdanowiczite and Se-bearing dantopaite (Fig. 1).

Figure 1. Back-scattered electron image of the cotype specimen of stibiogoldfieldite. Two different degrees of contrast and brightness have been applied in (a) and (b), in order to show all the mineral species. In (a), Te-rich stibiogoldfieldite is grey, whereas all the other phases are white; in (b), Se-bearing dantopaite is dark grey, calaverite is grey, and gold is white. Cotype specimen, National Museum of Prague (Czech Republic), catalogue number P1P 80/2020.
Chemical analysis
Quantitative chemical analysis of stibiogoldfieldite and Se-bearing dantopaite were performed using a Cameca SX 100 electron microprobe (National Museum of Prague, Czech Republic) and the following analytical conditions: wavelength dispersive spectroscopy mode, accelerating voltage of 25 kV, beam current of 20 nA and beam diameter of 0.7 μm. Standards (element, emission line) were: chalcopyrite (CuKα, SKα); Ag metal (AgLα); Sb2S3 (SbLα); PbTe (TeLα); PbS (PbMα); Bi2Se3 (BiMβ); and PbSe (SeLβ). Contents of other elements with Z > 8 are below detection limits. Matrix correction by PAP software (Pouchou and Pichoir, Reference Pouchou, Pichoir and Armstrong1985) was applied to the data. Electron microprobe data of Te-rich stibiogoldfieldite were given in Biagioni et al. (Reference Biagioni, Sejkora, Musetti, Makovicky, Pagano, Pasero and Dolníček2022a), whereas the results on Se-bearing dantopaite are reported in Tables 1 and 2.
Table 1. Chemical data for Se-bearing dantopaite.

e.s.d. = estimated standard deviation; apfu = atoms per formula unit.
Table 2. Chemical data for Se-bearing, Pb-enriched, dantopaite.

e.s.d. = estimated standard deviation; apfu = atoms per formula unit.
Single-crystal X-ray diffraction
Single-crystal X-ray diffraction intensity data of cotype stibiogoldfieldite and Se-bearing dantopaite were collected using a Bruker D8 Venture diffractometer (50 kV and 1.4 mA) equipped with an air-cooled Photon III detector, and microfocus MoKα radiation (C.I.S.U.P., University of Pisa, Italy). The detector-to-crystal distance was set to 38 mm. For both data collections, frames were integrated with the Bruker SAINT software package using a narrow-frame algorithm. Data were then corrected for Lorentz-polarisation, absorption and background. Neutral scattering curves were taken from the International Tables for Crystallography (Wilson, Reference Wilson1992).
Intensity data of stibiogoldfieldite were collected using φ and ω scan modes, in 0.5° slices, with an exposure time of 10 s per frame. A total of 632 frames was collected. Unit-cell parameters, refined on the basis of the XYZ centroids of 4891 reflections above 20 σI with 5.596 < 2θ < 64.959°, are a = 10.2947(3) Å, V = 1091.04(10) Å3 and space group I $\bar{4}$![]() 3m. The crystal structure of cotype stibiogoldfieldite was refined using Shelxl-2018 (Sheldrick, Reference Sheldrick2015) starting from the atomic coordinates of stibiogoldfieldite (Biagioni et al., Reference Biagioni, Sejkora, Musetti, Makovicky, Pagano, Pasero and Dolníček2022a). The following neutral scattering curves were used: Cu vs □ at the M(2) and M(1) sites, Te vs As at X(3), and S at S(1) and S(2) sites. The M(2) site was found to be split and the site occupancy at both sub-positions were refined independently. After several cycles of anisotropic refinement, the agreement factor R 1 converged to 0.0161 for 397 unique reflections with F o > 4σ(F o) and 25 refined parameters. An unsplit model was also refined in order to compare the current results with those given in Biagioni et al. (Reference Biagioni, Sejkora, Musetti, Makovicky, Pagano, Pasero and Dolníček2022a).
3m. The crystal structure of cotype stibiogoldfieldite was refined using Shelxl-2018 (Sheldrick, Reference Sheldrick2015) starting from the atomic coordinates of stibiogoldfieldite (Biagioni et al., Reference Biagioni, Sejkora, Musetti, Makovicky, Pagano, Pasero and Dolníček2022a). The following neutral scattering curves were used: Cu vs □ at the M(2) and M(1) sites, Te vs As at X(3), and S at S(1) and S(2) sites. The M(2) site was found to be split and the site occupancy at both sub-positions were refined independently. After several cycles of anisotropic refinement, the agreement factor R 1 converged to 0.0161 for 397 unique reflections with F o > 4σ(F o) and 25 refined parameters. An unsplit model was also refined in order to compare the current results with those given in Biagioni et al. (Reference Biagioni, Sejkora, Musetti, Makovicky, Pagano, Pasero and Dolníček2022a).
Intensity data of Se-bearing dantopaite were collected in φ and ω scan modes, in 0.5° slices, with an exposure time of 10 s per frame. A total of 1272 frames was collected. Unit-cell parameters, refined on the basis of the XYZ centroids of 3057 reflections above 20 σI with 6.042 < 2θ < 56.50°, are a = 13.518(2), b = 4.0898(6), c = 18.984(3) Å, β = 106.816(6)°, V = 1004.7(3) Å3 and space group C2/m. The crystal structure of this phase was solved initially through direct methods using Shelxt and was refined using Shelxl-2018 (Sheldrick, Reference Sheldrick2015). Once it was clear that this phase corresponded to the N = 6 homologue of the pavonite homologous series, the atomic coordinates were recast into those given by Makovicky et al. (Reference Makovicky, Paar, Putz and Zagler2010) for dantopaite. The following neutral scattering curves were used: Bi vs Ag at the M(1)–M(4) [M(4) was found fully occupied by Bi] and S vs Se at S(1)–S(6) sites. The site occupancies at the Ag, Cu(1), and Cu(2) sites were refined freely using the scattering curves of Ag and Cu. After several cycles of anisotropic refinement, the agreement factor R 1 converged to 0.0504 for 1230 unique reflections with F o > 4σ(F o) and 82 refined parameters.
Data collection details and refinements for both stibiogoldfieldite and dantopaite are in Table 3, whereas fractional atomic coordinates and displacement parameters, as well as selected bond distances, are given in Tables 4 and 5. Bond-valence sums (BVS), calculated using the bond parameters of Brese and O'Keeffe (Reference Brese and O'Keeffe1991) are in Tables 6 and 7. The crystallographic information files (cifs) have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 3. Summary of crystal data and parameters describing data collections and refinements for Te-rich stibiogoldfieldite and Se-bearing dantopaite.

1 w = 1/[σ2(F o2) + (0.0271P)2 + 1.6352P] for Te-rich stibiogoldfieldite; w = 1/[σ2(F o2) + (0.0001P)2 + 265.7769 P] for Se-bearing dantopaite.
2 Flack (Reference Flack1983)
Table 4. Sites, Wyckoff positions, site occupancy, fractional atomic coordinates and isotropic (*) or equivalent isotropic displacement parameters (Å2) for Te-rich stibiogoldfieldite and Se-bearing dantopaite.

Table 5. Selected bond distances (in Å) for Te-rich stibiogoldfieldite and Se-bearing dantopaite.

Table 6. Weighted bond-valence sums (in valence units) for Te-rich stibiogoldfieldite.

Note: left and right superscripts indicate the number of equivalent bonds involving cations and anions, respectively. The following site occupancies were used: M(2a) = Cu0.73; M(2b) = Cu0.08; M(1) = Cu1.00; X(3) = Te0.64Sb0.20As0.14Bi0.02.
Table 7. Weighted bond-valence sums (in valence units) for Se-bearing dantopaite.

Note: left and right superscripts indicate the number of equivalent bonds involving cations and anions, respectively. The site occupancies given in Table 4 for both cations and anions were used.
Results and discussion
Tellurium-rich stibiogoldfieldite
The crystal structure of cotype stibiogolfieldite agrees with the general features of the members of the tetrahedrite group. As reported in the Introduction, previous chemical data given by Biagioni et al. (Reference Biagioni, Sejkora, Musetti, Makovicky, Pagano, Pasero and Dolníček2022a) indicated that cotype stibiogoldfieldite is Te-rich, with 2.57 Te atoms per formula unit (apfu). Such a Te content should be coupled with the occurrence of vacancy (□) at the M(2) site and the composition of cotype material should be idealised as (Cu11.4□0.6)[(Sb0.8As0.6)Σ1.4Te2.6]S13 =M (2)(Cu5.4□0.6)M (1)Cu6X (3)[Sb0.8As0.6Te2.6]S(1)S12S(2)S.
In the material studied, the M(2) site is split into two sub-positions M(2a) and M(2b), with M(2a)–M(2b) = 0.52(4) Å. The first one shows a planar triangular coordination, whereas the second one has a flat trigonal pyramidal coordination, in agreement with previous studies (e.g. Welch et al., Reference Welch, Stanley, Spratt and Mills2018; Sejkora et al., Reference Sejkora, Biagioni, Vrtiška and Moëlo2021; Biagioni et al., Reference Biagioni, Sejkora, Moëlo, Marcoux, Mauro and Dolníček2022b). Average Me–S bond distances are 2.219 and 2.288 Å for M(2a) and M(2b), respectively. Both sub-positions are partially occupied by Cu, whereas the Ag content is negligible (≈0.03 apfu, according to electron microprobe analysis). In holotype stibiogoldfieldite, the M(2) site was not found split. In order to compare the Me–S bond distances observed in holotype and cotype specimens, an unsplit model of cotype material was refined (R 1 = 0.0194). The average Me–S bond distance at M(2) is 2.230 Å, to be compared with 2.251 Å observed by Biagioni et al. (Reference Biagioni, Sejkora, Musetti, Makovicky, Pagano, Pasero and Dolníček2022a) in the holotype sample. The observed mean atomic number (MAN) at M(2a) + M(2b) corresponds to 25.64 electrons, comparable to those calculated from electron microprobe analysis, i.e. 25.84 electrons assuming the site occupancy M (2)(Cu0.883Ag0.005□0.112), thus supporting the occurrence of a vacancy at the M(2) site. In holotype material, M(2) was found fully occupied by Cu. The bond-valence sum for M(2a) + M(2b) is 0.97 valence units (vu), in accord with the presence of monovalent Cu.
The tetrahedrally coordinated M(1) site is a pure Cu site. The average Me–S bond distance is 2.323 Å, close to the <M(1)–S(1)> distance of 2.329 Å observed in holotype material (Biagioni et al., Reference Biagioni, Sejkora, Musetti, Makovicky, Pagano, Pasero and Dolníček2022a). The observed MAN, 28.56 electrons, agrees with the theoretical value of 29.00 electrons. The BVS at M(1) is 1.16 vu.
The X(3) site, on the basis of electron microprobe data, should have the site occupancy (Te0.64Sb0.20As0.14Bi0.02) which corresponds to a calculated MAN of 49.76 electrons, in accord with the value obtained during the crystal structure refinement, i.e. 48.96 electrons. In holotype stibiogoldfieldite, the site occupancy at the X(3) site is (Te0.50Sb0.28As0.16Bi0.06) (Biagioni et al., Reference Biagioni, Sejkora, Musetti, Makovicky, Pagano, Pasero and Dolníček2022a). The higher amount of Te and the lower content of Sb in cotype material results in a slight contraction of the average <X(3)–S(1)> distance. Indeed, this value is 2.387(4) Å in the holotype specimen, and 2.3775(12) Å in cotype material. This is in keeping with the shorter Te–S distances with respect to Sb–S ones. The bond-valence sum at the X(3) site is 3.48 vu, to be compared with the theoretical value of 3.64 vu based on the X(3) site occupancy.
The S(1) site is four-fold coordinated and is bonded to two M(1), one M(2) and one X(3). Its BVS is 2.06 vu. S(2) is octahedrally coordinated by atoms hosted at M(2) sites, with BVS of 1.98 vu. Both sites are fully occupied by S. Minor Se (0.20 apfu, as detected by electron microprobe analysis) was located at neither of the S(1) or S(2) sites; it could possibly be disordered among the two positions.
Se-bearing dantopaite
Dantopaite, ideally Ag5Bi13S22, is the 6P homologue of the pavonite series (Makovicky et al., Reference Makovicky, Paar, Putz and Zagler2010). In addition to its type locality, Erzwies, Austria, it is currently reported only from the Rozália mine, Slovakia and the Clara mine, Germany (Jeleň et al., Reference Jeleň, Pršek, Kovalenker, Topa, Sejkora, Števko and Ozdín2012; Kolitsch et al., Reference Kolitsch, Bayerl and Topa2019). The identification of dantopaite at the Mohawk mine, Goldfield, USA, is thus the fourth world-occurrence.
Chemical composition
The chemical formula of dantopaite from the Mohawk mine (Table 1), calculated on the basis of 22 (S+Se+Te) apfu, is Cu1.36Ag4.39Pb0.12Bi12.62Sb0.06(S14.01Se7.91Te0.08). The empirical formula shows an excess of cations (~0.55 apfu), probably as a result of the accumulation of Cu in the column of octahedra in the thinner slab, in agreement with the structural model of Makovicky et al. (Reference Makovicky, Paar, Putz and Zagler2010). In addition to this predominant mineral, which was used for the crystal structure study, rare grains with higher Pb and Se contents (Fig. 2a), up to 30 μm in size, were also found in the polished sections (Table 2). Their average chemical formula, calculated on the same basis, is Cu0.89Ag4.50Pb0.49Bi12.53Sb0.07(S11.26Se10.74) and shows an excess of cations corresponding to ~0.48 apfu. These grains are usually intergrown with S-bearing bohdanowiczite (Ag1.01Bi1.01Se1.37S0.61).

Figure 2. Relationship between (a) S/(S+Se+Te) and Pb; (b) Cu and Ag; and (c) (Cu+Ag) and (Bi+Sb), in atoms per formula unit (apfu), in Se-bearing dantopaite. Lines indicate the theoretical trend of the corresponding substitutions. For comparison, literature data are shown from Makovicky et al. (Reference Makovicky, Paar, Putz and Zagler2010) and Jeleň et al. (Reference Jeleň, Pršek, Kovalenker, Topa, Sejkora, Števko and Ozdín2012).
Karup-Møller and Makovicky (Reference Karup-Møller and Makovicky1979) distinguished two different kinds of Cu, on the basis of their structural role. They identified some Cu fitting the ideal formula of pavonite homologues which they indicated as “substitutional Cu”, sCu, and some Cu occurring in the interstices of the crystal structure, namely “interstitial Cu”, iCu. On the basis of this distinction, they proposed three possible N numbers (N = order number of the homologue within the pavonite series) assuming that all Cu is interstitial (N P1), all Cu is substitutional (N P2), and that Cu is equally distributed between sCu and iCu (N P3). These three different approaches led to the following values (minor Sb was added to Bi): N P1 = 5.29; N P2 = 5.95; and N P3 = 5.62 for the variety studied through single-crystal X-ray diffraction and N P1 = 5.69; N P2 = 6.13; and N P3 = 5.91 for the Se- and Pb-enriched grains. These values may suggest that Cu acts both as a substitutional and interstitial cation in the material studied.
Dantopaite from the Mohawk mine has some chemical peculiarities with respect to previous known occurrences.
(1) The Cu content is similar with that measured in dantopaite from the type locality, with a Cu/(Cu+Ag) atomic ratio of 0.24 (0.16 in the case of Pb-enriched variety), to be compared with 0.20 measured by Makovicky et al. (Reference Makovicky, Paar, Putz and Zagler2010). In dantopaite from the Rozália mine, Cu/(Cu+Ag) = 0.38 (Jeleň et al., Reference Jeleň, Pršek, Kovalenker, Topa, Sejkora, Števko and Ozdín2012), indicating a larger replacement of Ag by Cu.
(2) The Pb content in dantopaite from the Mohawk mine is the lowest so far reported for the species. Indeed, only 0.12 Pb apfu were measured (0.49 for Pb-enriched variety), to be compared with 0.90 and 1.32 apfu in dantopaite from Erzwies and the Rozália mine, respectively (Makovicky et al., Reference Makovicky, Paar, Putz and Zagler2010; Jeleň et al., Reference Jeleň, Pršek, Kovalenker, Topa, Sejkora, Števko and Ozdín2012).
(3) Bismuth is replaced by minor Sb (0.06–0.07 apfu).
(4) The metal excess is 0.55 apfu (0.48 apfu for the Pb-enriched variety), to be compared with 0.43 apfu in dantopaite from the Austrian type locality (Makovicky et al., Reference Makovicky, Paar, Putz and Zagler2010); this excess is definitely lower than that found in the sample from Slovakia, i.e. 1.43 apfu (Jeleň et al., Reference Jeleň, Pršek, Kovalenker, Topa, Sejkora, Števko and Ozdín2012).
(5) The Se content is exceptional, with a Se/(S+Se+Te) ratio of 0.360 for sample used for single crystal study and even 0.488 in the case of Pb-enriched variety. In type dantopaite, S was replaced by minor Te (0.11 apfu, similar to that detected in the sample from the Mohawk mine), but no Se was reported. On the contrary, Jeleň et al. (Reference Jeleň, Pršek, Kovalenker, Topa, Sejkora, Števko and Ozdín2012) gave 0.15 Se apfu, i.e. Se/(S+Se+Te) = 0.007.
In the empirical formula of dantopaite from the Mohawk mine, several homo- and heterovalent substitutions take place. A ‘substitution-free’ formula of dantopaite used for the structural study can be obtained applying the following substitutions. Selenium and minor Te can be accounted for through the substitution (Se,Te)2– = S2– (Fig. 2a) and minor Sb3+ is replaced by Bi3+ according to the homovalent substitution Sb3+ = Bi3+. The occurrence of Pb could be related to the substitution Cu+ + Pb2+ = □ + Bi3+; after subtracting minor Pb, the formula is Cu1.24Ag4.39Bi12.80S22. The presence of Cu can be partially due to the Ag+ = Cu+ substitution, however, Fig. 2b does not show a clear negative relationship between these two elements and their mutual replacement does not explain the remaining metal excess, i.e. 0.43 apfu. A possible mechanism could involve the replacement of Bi3+ by Cu+ (or Ag+) and the occupancy of interstitial positions by Cu+, according to the substitution rule 3(Cu, Ag)+ = Bi3+ + 2□ (Fig. 2c). In this equation, one could consider the amount of Bi3+ as the difference between its ideal content (13 apfu) and 12.80 apfu, i.e. 0.20. Using this calculation the formula can be recast as Cu0.64Ag4.39Bi13.00S22. However, alternatively, one could assume that 2□ = 0.43 apfu, and then the formula becomes Cu0.595Ag4.39Bi13.015S22. The homovalent substitution Cu+ = Ag+ leads to Ag5.03Bi13.00S22 and Ag4.985Bi13.015S22, respectively, close to the ideal formula Ag5Bi13S22.
Crystal structure
The crystal structure of Se-bearing dantopaite is isotypic with that of dantopaite (Makovicky et al., Reference Makovicky, Paar, Putz and Zagler2010) and it is composed of two kinds of slabs parallel to (001), i.e. a PbS-like thick slab and a thin slab (Fig. 3).

Figure 3. Crystal structure of Se-bearing dantopaite as seen down b. Violet, grey and blue circles represent Bi, Ag and Cu-hosting sites, whereas yellow and green circles show S- and Se-dominant positions. Drawn using CrystalMaker® software.
The thicker slab is formed by Bi- and Ag-centred octahedra, namely the M(1)–M(3) sites. In agreement with the order number N = 6, there are six octahedra in a diagonal row across the thicker slab, according to the sequence M(3)–M(1)–M(2)–M(2)–M(1)–M(3).
The M(1) site is a mixed (Bi,Ag) position, with a Bi/(Bi+Ag) ratio of 0.66. In type dantopaite, this site has a pure Bi occupancy. The site is characterised by a short M(1)–S(3) bond of 2.637 Å, with S(3) located on interslab surfaces. This distance is shorter than that observed in dantopaite from Erzwies (2.664 Å), notwithstanding the significant replacement of S by Se [site occupancy at S(3) = Se0.57S0.43]. The coordination of M(1) is completed by five additional bonds ranging between 2.901 and 2.968 Å. The average <M(1)–S> distance is 2.889 Å, longer than that observed in dantopaite (2.832 Å) and agreeing with the replacement of S by the larger anion Se2–. The bond-valence sum at this site is 2.57 vu, slightly higher than the theoretical value of 2.33 vu based on the proposed site occupancy.
The M(2) site is located at the centre of the thick PbS-like slab. In agreement with Makovicky et al. (Reference Makovicky, Paar, Putz and Zagler2010), this position is actually split into two sub-positions, occupied by Bi [M(2a)] and Ag [M(2b)], respectively, with a Bi/(Bi+Ag) ratio of 0.70. In the sample studied by Makovicky et al. (Reference Makovicky, Paar, Putz and Zagler2010), the M(2) site hosted Pb and was split into two sub-positions, with a ratio 82:18. Both sub-positions have the same average distance (~2.88 Å, slightly longer than those observed in dantopaite from Erzwies, i.e. ~2.85 Å), with M(2a) more regular than M(2b). A more pronounced distortion was observed in the Austrian sample, with bond distances at the Ag2 position ranging between 2.59 and 3.03 Å; in dantopaite from the Mohawk mine such distances range between 2.74 and 3.03 Å. According to Makovicky et al. (Reference Makovicky, Paar, Putz and Zagler2010), the M(2) site could host Pb. In type material, there are 0.9 Pb apfu, whereas the specimen studied in this work is Pb-poor, with only 0.12 Pb apfu. Lead could be hosted at M(2), however no evidence was found during the structure analysis. Indeed, bond-valence sums for M(2a) and M(2b) sub-sites are in reasonable agreement with expected values, i.e. 2.28 vs 2.10 vu and 0.32 vs 0.30 vu, respectively.
The M(3) site is located on the margins of the PbS-like slab and it is a pure Bi position. As for M(1), it shows a short M(3)–S(6) distance of 2.643 Å. Also in this case, the anion is located on the interslab surface. The observed distance can be compared with 2.628 Å given by Makovicky et al. (Reference Makovicky, Paar, Putz and Zagler2010) for dantopaite. The M(3) has two additional relatively short bonds (2.829 Å, longer than those reported by Makovicky et al., Reference Makovicky, Paar, Putz and Zagler2010, i.e. 2.779 Å), two bonds at 2.983 Å and a long bond at 3.135 Å. The average <M(3)–S> distance is 2.900 Å, longer than that observed in the Austrian sample (2.857 Å – Makovicky et al., Reference Makovicky, Paar, Putz and Zagler2010). As for the M(1) site, M(3) is also slightly overbonded, with a bond-valence sum of 2.81 vu, compared with a calculated value of 2.66 vu based on the Bi/Ag ratio.
The thinner slab contains single columns of edge-sharing Ag-centred octahedra (Ag site), flanked by double columns of Bi-centred square pyramids [M(4) site].
The Ag site has a 2+4 distorted octahedral coordination, with two linearly arranged Ag–S(6) bonds, with distance of 2.376 Å and a S(6)–Ag–S(6) angle of 180.0(2)°. Four additional bonds at 2.940 Å complete the bonding environment of this position. The site occupancy points to a partially occupied Ag position (Ag0.66). Actually, the smaller Ag–S(6) distance observed with respect to that reported by Makovicky et al. (Reference Makovicky, Paar, Putz and Zagler2010) (~2.50 Å) may suggest the partial replacement of Ag by Cu, in agreement with the overbonding of the bond-valence sum at the Ag site (Table 7). However, the average bond distance, 2.752 Å, is only slightly smaller than that in the corresponding Ag1 site of the sample from Erzwies, 2.764 Å. The shortest distances at the Ag site can be compared with those observed in linearly-coordinated Ag atoms in other minerals, e.g. ~2.45 Å in pyrargyrite (Laufek et al., Reference Laufek, Sejkora and Dušek2010), 2.432 Å in pyrostilpnite (Biagioni et al., Reference Biagioni, Zaccarini, Roth and Bindi2020) and 2.416 Å in neyite (Makovicky et al., Reference Makovicky, Balić-Žunić and Topa2001).
In agreement with the structural model of dantopaite proposed by Makovicky et al. (Reference Makovicky, Paar, Putz and Zagler2010), the Ag-centred octahedron is flanked by partially occupied, tetrahedrally coordinated, Cu-centred sites, namely Cu(1) and Cu(2). The Cu(1) site is situated along the column of Ag octahedra, between two adjacent polyhedra. Its average bond distance is 2.44 Å, to be compared with 2.41 Å observed by Makovicky et al. (Reference Makovicky, Paar, Putz and Zagler2010). However, in Se-bearing dantopaite, the coordination tetrahedron is very distorted, with a very short distance of 2.09 Å and two long distances at 2.62 Å; on the contrary, the tetrahedron described by Makovicky et al. (Reference Makovicky, Paar, Putz and Zagler2010) was more regular, with distances ranging between 2.35 and 2.50 Å. The Cu(2) site is located above and below the Ag site. Its average bond distance, 2.47 Å, can be compared with the value given by Makovicky et al. (Reference Makovicky, Paar, Putz and Zagler2010), 2.54 Å. The Cu(1) and Cu(2) sites have occupancies Cu0.10 and Cu0.21, respectively; with respect to dantopaite from Erzwies, Cu(1) has a lower occupancy (0.10 vs 0.20), whereas Cu(2) is more occupied (0.21 vs 0.08). Considering site multiplicity, an excess of 0.57 apfu occurs, to be compared with the excess found during electron microprobe analysis (0.55 apfu). Such accumulation of Ag and Cu requires an ordering of these elements to avoid too short Me–Me contacts. Indeed, several short distances can be observed (in Å): Cu(1)–Cu(2) = 1.19(4), Ag–Cu(2) = 1.20(3), Cu(1)–Cu(1) = 1.69(11), Cu(2)–Cu(2) = 1.69(7), and Ag–Cu(1) = 2.21(2). An acceptable distance is represented by Ag–Cu(2) = 2.89(3) Å, whereas the distance Cu(2)–Cu(2) = 2.40(7) Å is shorter than the Cu–Cu distance in metallic Cu (e.g. 2.56 Å – Otte, Reference Otte1961). However, as these sites show high values of their displacement parameters, it is possible that they may deviate from their average positions giving rise to tolerable bond lengths, favouring the occurrence of the metal excess observed in dantopaite.
The M(4) site has a square pyramidal coordination (average distance 2.825 Å), with two additional longer bonds at 3.479 Å. This site was refined as a pure Bi position; the occurrence of minor Pb cannot be excluded, as the bond-valence sum shows a small underbonding (2.87 vu), possibly agreeing with the presence of minor Pb. Usually, Pb replaces Bi in the thick slab (e.g. Makovicky et al., Reference Makovicky, Mumme and Watts1977; Makovicky and Mumme, Reference Makovicky and Mumme1979), but Topa et al. (Reference Topa, Makovicky and Balić-Žunić2008) described the occurrence of Pb in the thin slab of cupromakovickyite. However, this deviation from the expected value is probably within the experimental error and consequently the actual location of Pb in Se-bearing dantopaite cannot be found.
Among the six independent anion sites, three are dominated by S, i.e. S(1), S(2) and S(6), whereas the remaining sites have Se > S. The bond-valence sums at these sites range between 2.02 and 2.16 vu, close to the expected value of 2 vu; only S(4) has a high value of 2.32 vu, indicating too short Me–anion bonds. As this position is bonded to the split site M(2) and shows the highest U eq value among anion sites, it could be possible that the actual position of the atoms could be slightly displaced from the average position refined during this study.
The chemical formula obtained through single-crystal X-ray diffraction is t[Cu1.47Ag1.32Bi4(S6.92Se1.08)Σ8.00] T[Ag3.20Bi8.79(Se7.64S6.36)Σ14.00], where superscript t and T indicate thin and thick slabs, respectively. It is worth noting that the t and T slabs have Se/(S+Se) ratios of 0.135 and 0.546, respectively, indicating the preferential partitioning of Se in the PbS-like thick slab, whereas the thin slab preferentially hosts S. The sum of these two t and T structural modules gives the formula Cu1.24Ag4.52Bi12.79(S13.28Se8.72)Σ22.00 (Z = 1) that can be compared with that obtained through quantitative chemical analysis, i.e. Cu1.36Ag4.39Pb0.12Bi12.62Sb0.06(S14.01Se7.91Te0.08). The number of electrons e – refined at the cation positions is 1309.97, to be compared with 1306.13 e – calculated from the chemical data. As regards the anions, 497.26 electrons expected on the basis of chemical data can be compared with 508.96 e – obtained during the crystal structure refinement.
Nomenclature issues
Following the results of the crystal structure analysis, the ‘substitution-free’ formula of the sample studied in this work is Ag5Bi13S12Se10, being an intermediate member between dantopaite, Ag5Bi13S22 (Makovicky et al., Reference Makovicky, Paar, Putz and Zagler2010), and a hypothetical Se-end-member with formula Ag5Bi13Se22. Following Nickel and Grice (Reference Nickel and Grice1998), the sample of Se-bearing dantopaite could be theoretically considered as a different mineral species, because “at least one structural site […]” is “predominantly occupied by a different chemical component than that which occurs in the equivalent site in an existing mineral species”.
The occurrence of Se in pavonite homologues was previously known. Topa and Paar (Reference Topa and Paar2008), for instance, reported 0.25 Se apfu in cupromakovickyite, and up to 0.10 Se apfu were found by Žák et al. (Reference Žák, Frýda, Mumme and Paar1994) in makovickyite. Finally, Kolitsch et al. (Reference Kolitsch, Bayerl and Topa2019) described the possible existence of the Se-analogue of dantopaite from the Clara mine, Germany. However, to the best of our knowledge, no structural data about the roles of Se in pavonite homologous series have been available so far. The study of Se-bearing dantopaite from the Mohawk mine shows a preferential partitioning of Se in the PbS-like slab, in agreement with the existence of the Se-isotype of galena, clausthalite. However, it is not clear what the maximum Se content in dantopaite isotypes could be; the rare grains showing higher Pb and Se contents than those measured in the structurally characterised grain suggest that further Se may be hosted in the PbS-like slab, probably increasing the Se/(Se+S) atomic ratio at the S(2)–S(5) sites. A full replacement of S by Se in this slab would lead to the chemical formula Ag5Bi13Se14S8. However if the Se-to-S replacement is assumed to also occur in the thin slab, the existence of Ag5Bi13Se22 would be possible.
For the sake of simplicity, we think that S(1)–S(6) sites should be considered as an aggregate site for nomenclature purposes, avoiding the creation of different isotypes of dantopaite based on different S/Se ratios. On the contrary, species with S > Se should be classified as dantopaite, and those with Se > S could be described as a new mineral species.
Conclusion
The crystal chemical study of Te-rich stibiogoldfieldite and Se-bearing dantopaite allows the addition of new data, improving the knowledge of these very rare sulfosalt species.
The occurrence of vacancies at the M(2) site of stibiogoldfieldite related to the high Te content was confirmed, supporting the results of previous studies (e.g. Dmitrieva et al., Reference Dmitrieva, Yefremov and Kovalenker1987; Pohl et al., Reference Pohl, Ließmann and Okrugin1996). This new crystal structure refinement completes the first description of stibiogoldfieldite provided by Biagioni et al. (Reference Biagioni, Sejkora, Musetti, Makovicky, Pagano, Pasero and Dolníček2022a), adding structural data for cotype material.
More interesting results have been achieved in the crystal chemical study of Se-bearing dantopaite. Indeed, this is the first investigation of a Se-rich natural member of the pavonite homologous series and its structural characterisation allows a description of the different S and Se partitioning between the two different structural slabs typical of this series. In particular, Se is preferentially hosted in the PbS-like slab, whereas S is located in the thin slab. This finding supports the possible natural occurrence of Se-dominant homologues of the pavonite series, confirming the results of Kolitsch et al. (Reference Kolitsch, Bayerl and Topa2019) who suggested the occurrence of the Se-isotype of dantopaite at the Clara mine, Germany. Further research on the crystal chemistry of dantopaite and related phases will improve our understanding of the crystal chemistry of this complex homologous series of sulfosalts.
Acknowledgements
The paper was improved through the comments of the Associate Editor Owen Missen and two anonymous reviewers. CB acknowledges financial support from the Ministero dell'Istruzione, dell'Università e della Ricerca through the project PRIN 2017 “TEOREM – deciphering geological processes using Terrestrial and Extraterrestrial ORE Minerals”, prot. 2017AK8C32. The Centro per l'Integrazione della Strumentazione scientifica dell'Università di Pisa (C.I.S.U.P.) is acknowledged for the access to the C.I.S.U.P. X-ray Laboratory. The study was also financially supported by the Ministry of Culture of the Czech Republic (long-term project DKRVO 2024–2028/1.II.a; National Museum, 00023272) for JS and ZD.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1180/mgm.2023.77.
Competing interests
The authors declare none.