The rising prevalence of obesity and type 2 diabetes (T2D) has fuelled interest in very low-carbohydrate diets (LC: 20–70 g carbohydrates/d). This is supported by current dietary guidelines advocating personalised management( Reference Evert, Boucher and Cypress 1 ), and increasing evidence demonstrating the long-term efficacy of LC diets for improving glycaemic control and cardiometabolic outcomes compared with traditional high-carbohydrate (HC) diets( Reference Yancy, Westman and McDuffie 2 – Reference Tay, Luscombe-Marsh and Thompson 4 ).
T2D increases the risk for cognitive impairment and dementia( Reference Chung, Pimentel and Jor’dan 5 , Reference Cukierman, Gerstein and Williamson 6 ). Psychomotor efficiency, learning, executive functioning, memory and speed of information processing are often most affected( Reference Chung, Pimentel and Jor’dan 5 , Reference Biessels, Deary and Ryan 7 ). This underscores the importance of considering the effect of diabetes management strategies on cognitive function. A concern with LC diets is that carbohydrate restriction below the recommended daily allowance of 130 g/d, corresponding to the average minimum amount utilised by the brain( 8 ), could impair cognitive function by lowering dietary glucose availability. Consequently, there are concerns that LC diets may adversely affect cognitive function( Reference Crowe 9 ).
A limited number of studies have examined the effects of LC diets on cognitive function beyond a few weeks. To date, only two longer-term (24–52 week) studies have been conducted, and these reported no differences in cognitive performance between participants consuming either an LC or HC diet( Reference Brinkworth, Buckley and Noakes 10 , Reference Makris, Darcey and Rosenbaum 11 ). However, these studies only examined a limited range of cognitive domains in individuals without T2D. It is therefore critical to examine the longer-term effects of LC diets on cognitive performance on a more comprehensive range of cognitive domains in individuals with T2D. This study aimed to compare the effects of a hypoenergetic LC diet with an energy-matched HC diet administered as part of a lifestyle modification programme on cognitive performance in obese individuals with T2D after 52 weeks. It was hypothesised that, compared with a HC diet, an LC diet would reduce cognitive performance.
Methods
Study design and participants
The study was conducted at the CSIRO Clinical Research Unit between May 2012 and September 2013. The participants and study design have been described elsewhere( Reference Tay, Luscombe-Marsh and Thompson 4 ). In brief, 115 overweight/obese adults (forty-nine females/sixty-six males) with T2D (mean age 58 (sd 7) years; HbA1c 7·3 (sd 1·1) % (56 (sd 12) mmol/mol); BMI: 34·6 (sd 4·3) kg/m2; diabetes duration 8 (sd 6) years; highest level of education (15 % high school graduate, 35 % diploma/vocational training, 50 % university/higher degree)) were recruited by public advertisement. In total, twelve participants were taking insulin medication, eighty-seven were taking metformin, thirty-six were taking sulfonylureas, six were taking thiazolidinedione, two were taking glucagon-like peptide-1 agonists and three were taking dipeptidyl peptidase-4 medication. The exclusion criteria included type 1 diabetes; abnormal renal or liver function; any significant endocrinopathy (other than stable treated thyroid disease); history of malignancy or respiratory, gastrointestinal, cerebrovascular, peripheral or CVD; pregnancy or lactation; severe depression and current depression (Beck Depression Inventory Score≥29); history of/or current eating disorder; or smoking. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and the protocol and all procedures were approved by the Human Research Ethics Committees of the Commonwealth Scientific and Industrial Research Organisation (CSIRO), the University of Adelaide and the University of South Australia. Before study commencement, participants provided written informed consent.
The study used a 52-week parallel design and participants were block-matched for sex, BMI, age, HbA1c and diabetes medication before they were randomly allocated (by computer generation) to consume either a hypoenergetic (2092–4184 kJ/d deficit (500–1000 kcal/d deficit)) LC, high-unsaturated/low-saturated-fat diet (n 57; 14 % energy as carbohydrate (CHO<50 g/d), 28 % protein (PRO), 58 % fat (<10 % SFA)) or an energy-matched HC diet (n 58; 53 % CHO, 17 % PRO, 30 % fat (<10 % SFA)) that reflected conventional dietary guidelines( Reference Rodbard, Blonde and Braithwaite 12 ), in a 1:1 ratio. Research associates not involved in the data collection or delivery of the intervention performed the randomisation procedures that included sequence generation and allocation concealment. To achieve the targeted macronutrient profile, specific foods and quantities were listed in a food record that was completed daily by participants. Diet plans were personalised for energy requirements and participants received dietetic counselling biweekly for the first 12 weeks, and monthly for the remainder of the study. To facilitate compliance, participants were provided with key foods (approximately 30 % total energy) that reflected the assigned diet profiles for the initial 12 weeks, and for the remainder of study were provided with key foods or a $50AUD voucher on alternating months. All participants also undertook the same supervised moderate-intensity aerobic/resistance exercise sessions (60 min, 3 d/week), consistent with diabetes management guidelines( Reference Colberg, Albright and Blissmer 13 ). These group-based exercise classes were conducted in local community centres, and participants from both diet groups attended classes together.
Detailed description of the diets, exercise programme and the effects of the interventions on weight, glycaemic control and cardiometabolic outcomes have been previously reported( Reference Tay, Luscombe-Marsh and Thompson 4 ). Both groups achieved high dietary compliance and had similar increases in physical activity levels( Reference Tay, Luscombe-Marsh and Thompson 4 ). Exercise-session attendance was similar in both groups (LC diet: 81·2 (sd 18·0) %; HC diet: 77·5 (sd 21·6) %; P=0·47). Compared with HC participants, LC participants reported a lower mean carbohydrate intake (LC 54–74 g/d; HC 202–218 g/d).
Outcome measures
Outcome measures were assessed at baseline (week 0) and at weeks 24 and 52. Body height and weight (assessed monthly) was assessed using a stadiometer (SECA) and electronic scales (Mercury AMZ1), respectively. HbA1c was assessed at a certified pathology laboratory (SA Pathology). The test battery used to assess cognitive performance (Table 1) represented a subset of a previous test battery administered in a clinical study examining the effects of diet on cognitive function( Reference Danthiir, Burns and Nettelbeck 16 ). The present battery comprised several tasks measuring a broad variety of cognitive domains. Tests were selected because they assess cognitive domains most consistently affected by diabetes and have been shown to be sensitive to nutritional interventions( Reference Cukierman, Gerstein and Williamson 6 , Reference Danthiir, Hosking and Burns 17 – Reference van den Berg, Kloppenborg and Kessels 19 ). Specifications of each test have been fully described elsewhere( Reference Danthiir, Burns and Nettelbeck 16 ).
Table 1 Cognitive test batteryFootnote *

* Tasks represent a sub set from Danthiir et al.( Reference Danthiir, Burns and Nettelbeck 16 ).
† Test–retest reliabilities represent the average reliability across sessions and is calculated as Mr=Mean(r 1,2, r 1,3, r 2,3).
Cognitive assessments were conducted at CSIRO’s cognitive laboratory after overnight fasting and water consumed as required. No hypoglycaemic incidents were reported. Tests were administered in a consistent order across test sessions and scored by trained research personnel blinded to participants’ treatment assignment. Each session included computer (Inquisit version 2; Millisecond Software) and paper tasks lasting approximately 1 h. The tasks were administered in the same order as listed in Table 1, with speed and accuracy-based tasks interspersed to reduce the likelihood of fatigue.
Each task was preceded by practice items and queries were addressed before test commencement. No colour blindness was reported. Parallel versions were used for those tasks (word memory, operation span and word endings tests) in which the stimuli might feasibly be remembered between sessions. All other tasks are arguably more process-driven and preclude the ‘learning’ of stimuli, and thus the same version of the test was administered at each assessment( Reference Danthiir, Burns and Nettelbeck 16 ). The average test–retest reliabilities were high and acceptable for most tasks, as shown in Table 1, although reliabilities were lower than anticipated for colour Stroop and word memory tasks.
Quality control included regular audit of test sessions, independent dual-scoring and regular review of session notes. Depression, a potential confounder and frequent comorbidity in T2D, was assessed by the Beck Depression Inventory-II (BDI-II)( Reference Beck, Steer and Brown 20 ) to control for possible effects of mood disturbances or affective disorders. Participants’ BDI-II scores were in the subclinical range (mean LC 6·3 (sd 6·2); HC 5·2 (sd 4·7), P=0·29, Table 2). Eight LC participants reported taking antidepressant medications (ADM) and two HC participants began taking ADM during the study. None of the participants were on dementia medications or taking any cognitive enhancing drugs or nootropics.
Table 2 Baseline characteristics of participants by diet assignmentFootnote * (Mean values and standard deviations; numbers and percentages)
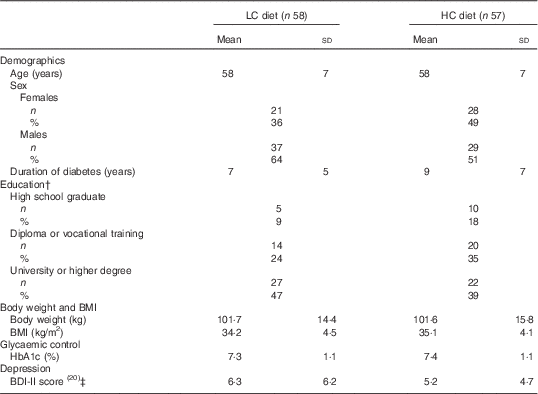
LC diet, very low-carbohydrate, high-unsaturated/low-saturated-fat diet; HC diet, high-carbohydrate, low-fat diet; BDI-II, Beck Depression Inventory-II.
* Total analysed n 115 (LC: 58, HC: 57) for all data unless otherwise stated. All baseline characteristics were not significantly different between diet groups (P>0·05) by independent sample t test (continuous variables) or χ 2 test (categorical variables).
† Data on highest education level unavailable for seventeen participants (LC: twelve, HC: five).
‡ BDI-II score: possible range 0–63. Higher scores indicate greater symptom burden.
Statistical analysis
The initial sample size calculation for this study was based on the primary study outcome of HbA1c, as previously reported( Reference Tay, Luscombe-Marsh and Thompson 4 ). However, a priori power analyses were also conducted to determine the power to detect clinically relevant differences between groups for cognitive function measures using G*Power software( Reference Faul, Erdfelder and Buchner 21 ). For a univariate repeated measures model, we determined that a minimum combined sample size of n 82 was necessary to detect a small (f=0·15, equivalent to Cohen’s d=approximately 0·30) time×diet interaction effect with 95 % power, assuming α=0·05 for the cognitive function tests and correlation among measures of r 0·66. The minimum number required to retain 80 % power to detect the same time×diet small interaction effect (f=0·15, α=0·05, r 0·66) is n 64. Therefore, the sample size that completed the present study (n 78) was suitable to assess the cognitive outcome measures.
Baseline demographic and clinical characteristics between the groups were compared using independent t tests for continuous variables and χ 2 tests for categorical variables. Mean response latencies for speed tasks were converted to work rates (1000/mean latency) in order to normalise the distributions. Work rates represent the average number of items completed per second of measured response time, and higher scores indicate better performance. The effect of the diets on changes in cognitive function over time was analysed by random-coefficient analysis, restricted maximum likelihood and mixed effects models using an unstructured covariance that assumed data to be missing at random. Participants with a cognitive assessment at baseline and at least 24 or 52 weeks of follow-up were included in the final analyses (n 114 for word memory, two-choice reaction time and odd-man-out letter (OMO); n 113 for digit symbol substitution (DSST), word endings, number memory scanning (MScan) and colour Stroop; n 112 for letter sets and operation span tasks). Four (LC: three, HC: one) participants were excluded from various cognitive function analyses because of non-compliance to the test protocol, accuracy score <50 %, language difficulty and time constraints precluding completion of tests. Mean test–retest reliability representing the average reliability across sessions was calculated as Mr=Mean(r 1,2, r 1,3, r 2,3). The following fixed effects were included in the model: main effect for each time point, diet group assignment and diet group by time point interaction. Estimated marginal means (95 % CI) and change from week 0 to 52 are reported. Analyses with adjustment for sex, age, depression and education status were conducted for sensitivity analyses. The magnitudes of effect size (d) for time effects were calculated as the mean difference in performance from week 0 to week 52 divided by the pooled sd of the difference( Reference Cohen 22 ). Simple linear regression analysis was used to determine relationships between the change in anthropometric markers (weight and BMI) and changes in cognitive function. For cognitive variables, absolute change scores were calculated and used as the difference score (week 52−week 0), and weight loss was expressed as percentage of total weight loss ((difference score/weight at week 0)×100). Where significant univariate effects were found, multivariate models were used to adjust for the impact of sex and age. SPSS 20.0 for Windows (SPSS Inc.) was used to perform the analyses and statistical tests were two-tailed with P<0·05 considered statistically significant.
Results
Baseline characteristics were not significantly different between diet groups (P>0·05, Table 2). As previously reported( Reference Tay, Luscombe-Marsh and Thompson 4 ), the two groups had similar rates of study completion (LC 71 %, HC 65 %, P=0·51) (Fig. 1), reductions in weight (mean LC −9·8; 95 % CI −11·7, −7·9; HC −10·1; 95 % CI −12·0, −8·2 kg, P=0·18) and HbA1c (LC −1·0; 95 % CI −1·2, −0·7; HC −1·0; 95 % CI −1·3, −0·8 %, P=0·65).

Fig. 1 The study flow diagram.
Cognitive outcomes based on estimated marginal means from primary mixed-models analyses are shown in Table 3. No statistically significant differences in cognitive test performance scores between the diet groups over time for any of the cognitive outcomes assessed were observed (P≥0·24 time×diet). Some main effects of time were observed. Specifically, word endings (Cohen’s d=1·01, P<0·001) and word memory scores (Cohen’s d=0·31, P=0·005) decreased over time, whereas performance on DSST (Cohen’s d=−0·28, P=0·03), MScan (Cohen’s d=−0·34, P<0·001) and OMO performance (Cohen’s d=−0·49, P<0·001) improved over time. Fully adjusted models controlling for covariates associated with cognitive function (age, sex, education level and depression) did not alter the results of the responses between the diet groups. Removal of the participants taking ADM did not change the outcomes.
Table 3 Estimated marginal means derived using linear mixed-effects model analysis for cognitive performance across 52 weeks for low-carbohydrate (LC) diet and high-carbohydrate (HC) diet groupsFootnote * (Mean values and 95 % confidence intervals)

LC diet, very low-carbohydrate, high-unsaturated/low-saturated-fat diet; HC diet, high-carbohydrate, low-fat diet; DSST, digit symbol substitution test; OMO, odd-man-out letter test; MScan, number memory scanning test.
* Outcome measures for cognitive tasks described in Table 1.
Linear regressions showed a higher percentage change in body weight (corresponding to a greater reduction) significantly correlated to the change (increase) in DSST performance (β=0·25, P=0·03). This effect remained significant after controlling for age and sex (β=0·23, P=0·03).
Discussion
This study showed after participation in a 52-week lifestyle intervention that both an LC diet and a HC diet had comparable effects on cognitive performance in overweight and obese individuals with T2D, as assessed by a comprehensive neuropsychological test battery. This is consistent with previous longer-term studies (24–52 weeks) conducted in populations without T2D that showed no difference in cognitive effects following consumption of LC and HC weight-loss diets on tasks that assessed working memory and speed of processing( Reference Brinkworth, Buckley and Noakes 10 , Reference Makris, Darcey and Rosenbaum 11 ), reaction time and attention, problem-solving and short-term memory( Reference Makris, Darcey and Rosenbaum 11 ). Results from smaller, shorter-term studies (3–8 weeks), however, do suggest some differences between HC and LC diets for speed of processing, attention and memory performance( Reference D’Anci, Watts and Kanarek 23 , Reference Halyburton, Brinkworth and Wilson 24 ). While these studies suggest that differences in cognitive performance may occur between HC and LC diets over the short term, these effects are unlikely to be sustained over a longer period, as demonstrated by the present results.
Despite the lack of any differential effect of diet composition on cognitive outcomes, significant improvements in performance on the DSST, MScan and OMO tasks were observed over 52 weeks in both groups. Because of the absence of a non-intervention control group, it is likely that the improvements over time reflect practice effects( Reference Machulda, Pankratz and Christianson 25 , Reference van den Berg, Reijmer and de Bresser 26 ), which are relatively common for cognitive testing( Reference Lowe and Rabbitt 27 ), and are probably not due to the effects of the lifestyle interventions tested. However, it cannot be dismissed that the improvements observed may be attributed to increases in physical activity following participation in the planned exercise sessions, combined with the sustained weight loss and metabolic improvements resulting from the intervention. Previous studies have shown that exercise and particularly resistance training improves cognitive outcomes including information-processing speed, attention, memory formation and other forms of executive function( Reference Chang, Pan and Chen 28 – Reference Blondell, Hammersley-Mather and Veerman 30 ). In the present study, only improvements in speed-related cognitive domains were observed. These differences may be attributed to differences in study populations (healthy elderly with intact cognition v. those with mild cognitive impairment), and the intensity and frequency of the exercise prescriptions. However, as the study design was intended to compare differences in cognitive function between diet groups and the exercise intervention involved combined aerobic and resistance training that was implemented in both groups, the independent effect of resistance training on cognition cannot be elucidated.
Further, a significant relationship between the change in percentage body weight and DSST was also observed. Witte et al.( Reference Witte, Fobker and Gellner 31 ) showed that cognitive performance improved in overweight individuals following 3 months of energy intake restriction, and weight loss was associated with improved metabolic control compared with no improvement in a non-dieting control group. Given that any practice effects would have been similar in both groups, the experimental design of the present study would be appropriate to test the hypothesis of comparing the effects of consuming either an LC or HC diet on cognitive function. Further studies that specifically examine the effect of weight loss and lifestyle modification should be conducted to determine the potential clinical benefits of this intervention strategy in populations with T2D who are at an increased risk of cognitive impairment.
An interesting finding in the present study was the significant decline in performance on the word endings test. Parallel forms of this test were used and they were designed to be of equivalent difficulty. However, these scores may have decreased at follow-up assessments because of increased test complexity. Evidence of this is apparent in the magnitude of this effect; performance decreased by 1 sd, which is a very large effect. While other prospective studies have shown a decline in verbal fluency test performance in adults with T2D, the effect sizes (for time) were comparatively smaller (Cohen’s d=0·2)( Reference van den Berg, Reijmer and de Bresser 26 ), mean annual decline of 0·4 points or 0·1±1 sd units( Reference Christman, Matsushita and Gottesman 32 ), and these effects emerged after 4–6 years. Therefore, given the 12-month time frame of the present study and magnitude of the effect observed, it is possible that the decline was due to increasing test difficulty and poor test equivalence that could be considered a limitation of the test battery design. Future studies should be cautious of these methodological issues.
A strength of the study was the use of a cognitive test battery that was more comprehensive and assessed multiple cognitive domains compared with previous studies examining LC and HC diets, particularly in individuals with T2D. However, the study has several limitations. The absence of a non-intervention, control group makes it difficult to decipher the effects observed overtime between practice effects relating to repeated testing or the direct effect of weight loss and the lifestyle intervention. The study also examined a relatively modest sample size. However, previous short-term studies with smaller sample sizes have been sufficient to demonstrate differences in cognitive performance between HC and LC diets( Reference D’Anci, Watts and Kanarek 23 , Reference Halyburton, Brinkworth and Wilson 24 ), and the total number of participants recruited exceeded those determined by a priori power analyses. This study was also conducted in a Caucasian, middle-aged, T2D population with relatively good metabolic control and minimal cognitive deficits based on age-specific normative scores( Reference Hoyer, Stawski and Wasylyshyn 33 ). However, clinically relevant cognitive decrements in T2D occur mainly in those with diabetes-related vascular complications and older adults >65 years of age( Reference Biessels, Deary and Ryan 7 ). Therefore, although our participants represent an age group at a critical window of opportunity for targeted interventions that may slow diabetes complication progression, further studies that examine the effects of these dietary patterns in older individuals with diabetes, pre-existing diabetes complications, poorer cognitive function and/or metabolic control and ethnically diverse populations are required to better understand the wider generalisability of the current findings. In addition, it is possible that the tasks used to assess changes in cognitive performance lacked sufficient sensitivity to detect any differences between the diets examined. However, the tests comprising the large battery of cognitive tasks used in this study were selected based on their known sensitivity to dietary manipulation( Reference Hoyland, Lawton and Dye 18 ), and assessed different cognitive domains sensitive to T2D( Reference Cukierman, Gerstein and Williamson 6 ). This suggests that the current battery would have been suitable to identify any diet differences. Nevertheless, the test battery did not assess all possible cognitive domains, such as long-term/delayed verbal memory, the impairment of which is well known in diabetes( Reference Awad, Gagnon and Messier 34 ). Future studies could further extend the cognitive domains tested. The study also examined the cognitive effects of the LC and HC diets when administered as part of prescriptive energy reduced diet plan that achieved substantial weight loss and metabolic improvement. Whether similar responses between the dietary patterns would be observed over a longer follow-up period and with the dietary patterns administered ad libitum requires further investigation.
In summary, in overweight and obese adults with T2D, both LC and HC weight-loss diets combined with exercise training had similar effects on cognitive performance. This suggests that both dietary patterns can be used as a strategy for diabetes and weight management without concerns of negative impacts on cognitive function. In fact, the cognitive improvements observed over time in both diet groups and the modest relationship between weight loss and improvements in cognitive performance demonstrate the potential benefits of combined diet and lifestyle interventions in T2D. Further studies should be undertaken to evaluate the cognitive effects of these diets in older adults with T2D, pre-existing diabetes complications, poorer cognitive function and glycaemic control, who are at greater risk of cognitive decline.
Acknowledgements
The authors would like to thank the participants for their invaluable help with the study. The authors also gratefully acknowledge the Clinical Research Team at the CSIRO – Food and Nutrition, Adelaide, South Australia: Julia Weaver, Anne McGuffin and Vanessa Courage for trial coordination; Lindy Lawson and Theresa Mckinnon for nursing expertise; Pennie Taylor, Janna Lutze, Paul Foster, Gemma Williams, Hannah Gilbert and Fiona Barr for assisting in the design and implementing the dietary intervention; Heidi Long and Laura Edney for administering the cognitive tests; Julie Syrette for data management expertise; Kathryn Bastiaans for assisting with cognitive data processing; Kelly French, Jason Delfos, Kristi Lacey-Powell, Marilyn Woods, John Perrin, Simon Pane, Annette Beckette (South Australian Aquatic Centre & Leisure Centre, South Australia), Angie Mondello and Josh Gniadek (Boot Camp Plus, South Australia), and Luke Johnston and Annie Hastwell (Fit for Success, South Australia) for implementing the exercise intervention.
Funding was provided by a project grant received from National Health and Medical Research Council of Australia (103415). J. T. was supported by a postgraduate research scholarship from the Agency for Science, Technology and Research, Singapore. The funding sponsors had no role in the design, data collection and analysis, the preparation of the manuscript or decision to publish.
G. B. had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: all authors. Data analysis and interpretation: all authors. Manuscript drafting: J. T., I. T. Z. and G. D. B. Critical revision the manuscript for intellectual content: N. D. L.-M., C. H. T., V. D., M. N., J. D. B. and G. A. W. Approval of final manuscript: all authors. Obtained funding: G. D. B., M. N., J. D. B., N. D. L.-M. and C. H. T. Study supervision: G. D. B., N. D. L.-M., C. H. T., M. N. and J. D. B.
The authors declare that there are no conflicts of interest.







