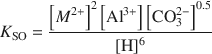Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Radha, A V
and
Kamath, P Vishnu
2003.
Aging of trivalent metal hydroxide/oxide gels in divalent metal salt solutions: Mechanism of formation of layered double hydroxides (LDHs).
Bulletin of Materials Science,
Vol. 26,
Issue. 7,
p.
661.
Johnson, C. Annette
and
Abbaspour, Karim C.
2004.
Hydrological and geochemical factors controlling leachate composition in incinerator ash landfills.
Geological Society, London, Special Publications,
Vol. 236,
Issue. 1,
p.
607.
Johnson, C. Annette
2004.
Cement stabilization of heavy-metal-containing wastes.
Geological Society, London, Special Publications,
Vol. 236,
Issue. 1,
p.
595.
Funke, H.
Scheinost, A. C.
and
Chukalina, M.
2005.
Wavelet analysis of extended x-ray absorption fine structure data.
Physical Review B,
Vol. 71,
Issue. 9,
Evans, David G.
and
Slade, Robert C. T.
2005.
Layered Double Hydroxides.
Vol. 119,
Issue. ,
p.
1.
Panfili, Frédéric
Manceau, Alain
Sarret, Géraldine
Spadini, Lorenzo
Kirpichtchikova, Tatiana
Bert, Valérie
Laboudigue, Agnès
Marcus, Matthew A.
Ahamdach, Noureddine
and
Libert, Marie-Françoise
2005.
The effect of phytostabilization on Zn speciation in a dredged contaminated sediment using scanning electron microscopy, X-ray fluorescence, EXAFS spectroscopy, and principal components analysis.
Geochimica et Cosmochimica Acta,
Vol. 69,
Issue. 9,
p.
2265.
Voegelin, Andreas
and
Kretzschmar, Ruben
2005.
Formation and Dissolution of Single and Mixed Zn and Ni Precipitates in Soil: Evidence from Column Experiments and Extended X-ray Absorption Fine Structure Spectroscopy.
Environmental Science & Technology,
Vol. 39,
Issue. 14,
p.
5311.
Catalano, Jeffrey G.
Warner, Jeffrey A.
and
Brown, Gordon E.
2005.
Sorption and precipitation of Co(II) in Hanford sediments and alkaline aluminate solutions.
Applied Geochemistry,
Vol. 20,
Issue. 1,
p.
193.
Voegelin, Andreas
Pfister, Sabina
Scheinost, Andreas C.
Marcus, Matthew A.
and
Kretzschmar, Ruben
2005.
Changes in Zinc Speciation in Field Soil after Contamination with Zinc Oxide.
Environmental Science & Technology,
Vol. 39,
Issue. 17,
p.
6616.
Vespa, M.
Dähn, R.
Wieland, E.
Grolimund, D.
and
Scheidegger, A. M.
2006.
The influence of hydration time on the Ni uptake by cement.
Czechoslovak Journal of Physics,
Vol. 56,
Issue. 1,
p.
D599.
Peltier, Edward
Allada, Ramakumar
Navrotsky, Alexandra
and
Sparks, Donald L.
2006.
Nickel Solubility and Precipitation in Soils: A Thermodynamic Study.
Clays and Clay Minerals,
Vol. 54,
Issue. 2,
p.
153.
Lothenbach, Barbara
and
Winnefeld, Frank
2006.
Thermodynamic modelling of the hydration of Portland cement.
Cement and Concrete Research,
Vol. 36,
Issue. 2,
p.
209.
Vespa, M.
Dähn, R.
Wieland, E.
Grolimund, D.
and
Scheidegger, A. M.
2006.
The influence of hydration time on the Ni uptake by cement.
Czechoslovak Journal of Physics,
Vol. 56,
Issue. S4,
p.
D599.
Vespa, M.
Dähn, R.
Gallucci, E.
Grolimund, D.
Wieland, E.
and
Scheidegger, A. M.
2006.
Microscale Investigations of Ni Uptake by Cement Using a Combination of Scanning Electron Microscopy and Synchrotron-Based Techniques.
Environmental Science & Technology,
Vol. 40,
Issue. 24,
p.
7702.
Allada, Rama kumar
Peltier, Edward
Navrotsky, Alexandra
Casey, William H.
Johnson, C. Annette
Berbeco, Hillary Thompson
and
Sparks, Donald L.
2006.
Calorimetric Determination of the Enthalpies of Formation of Hydrotalcite-Like Solids and Their Use in the Geochemical Modeling of Metals in Natural Waters.
Clays and Clay Minerals,
Vol. 54,
Issue. 4,
p.
409.
Dijkstra, Joris J.
van der Sloot, Hans A.
and
Comans, Rob N.J.
2006.
The leaching of major and trace elements from MSWI bottom ash as a function of pH and time.
Applied Geochemistry,
Vol. 21,
Issue. 2,
p.
335.
Vespa, M.
Dähn, R.
Grolimund, D.
Wieland, E.
and
Scheidegger, A. M.
2006.
Spectroscopic Investigation of Ni Speciation in Hardened Cement Paste.
Environmental Science & Technology,
Vol. 40,
Issue. 7,
p.
2275.
Grünewald, Gritta
Kaiser, Klaus
and
Jahn, Reinhold
2007.
Alteration of secondary minerals along a time series in young alkaline soils derived from carbonatic wastes of soda production.
CATENA,
Vol. 71,
Issue. 3,
p.
487.
Liu, Zhaoping
Ma, Renzhi
Ebina, Yasuo
Iyi, Nobuo
Takada, Kazunori
and
Sasaki, Takayoshi
2007.
General Synthesis and Delamination of Highly Crystalline Transition-Metal-Bearing Layered Double Hydroxides.
Langmuir,
Vol. 23,
Issue. 2,
p.
861.
Vespa, M.
Wieland, E.
Dähn, R.
Grolimund, D.
and
Scheidegger, A.M.
2007.
Determination of the elemental distribution and chemical speciation in highly heterogeneous cementitious materials using synchrotron-based micro-spectroscopic techniques.
Cement and Concrete Research,
Vol. 37,
Issue. 11,
p.
1473.
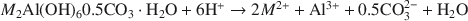 where
where