The consumption of a high cholesterol load increases the susceptibility of various organs to oxidative stressReference Konecka and Jezierski1, Reference Homma, Kondo, Kaneko, Kitamura, Nyou, Yanagisawa, Yamamoto and Kakizoe2. Vascular oxidative stress, in particular, may be related to the processes of atherosclerosisReference Parker, Sabrah, Cap and Gill3. One of the possible suggested mechanisms of atherosclerosis is the increased generation of oxidized LDL by superoxide, transition metals, haemoproteins or lipoxygenaseReference Yuan, Brunk and Olsson4–Reference Rusinol, Yang, Thewke, Panini, Kramer and Sinensky6, resulting in the development of foam cells by macrophages in blood vesselsReference Lusis7. Therefore, inhibiting oxidative stress under hypercholesterolaemia is considered to be an important beneficial approach. Many researchers, however, have found that postprandial oxidative stress is restricted when the meal consumed contains foods rich in polyphenolsReference Natella, Ghiselli, Guidi, Ursini and Scaccini8, Reference Natella, Belelli, Gentili, Ursini and Scaccini9. This suggests that polyphenols might reduce hydroperoxideReference Stahl, van den Berg and Arthur10, Reference Sies, Stahl and Sevanian11 or scavenge chelating autoxidation-promoting metal ionsReference Hider, Liu and Khodr12, Reference Murota, Mitsukuni, Ichikawa, Tsushida, Miyamoto and Terao13 in the small intestine.
Anthocyanin, an attractive natural-pigment flavonoid, has been reported to have bioactive properties in vivo Reference Tsuda, Horio and Osawa14–Reference Wang, Cao and Prior16 and inhibits LDL oxidation in vitro Reference Kähkönen and Heinonen17, which suggests that anthocyanin contents of various fruits and vegetables may possibly help to reduce CHDReference Zern, Wood, Greene, West, Liu, Aggarwal, Shachter and Fernandez18. Recently, the anthocyanin of the purple sweet potato has attracted much interest due to its biological functions, including radical scavenging activityReference Philpott, Gould, Lim and Ferguson19, antimutagenicityReference Yoshimoto, Okuno, Yamaguchi and Yamakawa20 and antioxidant activityReference Kano, Takayanagi, Harada, Makino and Ishikawa21. On the other hand, some newly developed potatoes are also considered to be good sources of anthocyaninReference Sorenson22. In a previous study, we have found that purple potato extract prevents the hepatoxicity induced by d-galactosamine in ratsReference Han, Hashimoto, Shimada, Sekikawa, Noda, Yanauchi, Hashimoto, Chiji, Topping and Fukushima23, and that flakes of a medium purple potato (Hokkai no. 92 = Shadow-Queen (SQ)) have antioxidant activity through enhancement of the gene expression of antioxidant enzyme mRNA in rats fed a cholesterol-free dietReference Han, Sekikawa, Shimada, Hashimoto, Hashimoto, Noda, Tanaka and Fukushima24. It was hypothesized that the lower carbohydrate concentration rather than the anthocyanin concentration in the purple potato flake diet contributed to the beneficial health effect because carbohydrate autoxidation products such as glycated proteins in the gastrointestinal tract might affect postprandial oxidative stressReference Stahl, van den Berg and Arthur10, and the absorption and conversion to other metabolites of anthocyanin is limitedReference Prior25. However, which components of purple potato flakes elicit the antioxidant effect is unclear, and there is little information on the effects of purple potato flakes on rats fed a high cholesterol diet. Accordingly, it seems important to examine the effects of purple potato flakes compared to other flakes, and to investigate whether dietary purple potato flakes moderate the metabolic disturbance caused by an exogenous cholesterol load.
In the present study, we investigated the effects of purple potato (Solanum tuberosum cv. Shadow-Queen) flakes on the lipid peroxidation and antioxidant enzyme activities in rats fed a high-cholesterol diet, and the comparative antioxidant efficacy of purple potato flakes with white potato (Solanum tuberosum cv. Toyoshiro (TY)) or dark purple sweet potato (Ipomoea batatas cv. Ayamurasaki (AM)) flakes made under the same conditions.
Materials and methods
Preparation of flakes and pigmented extracts
Potato tubers, which were harvested in Hokkaido in 2005, were a kind gift from the National Agricultural Research Center for the Hokkaido Region in Japan. TY, SQ and AM flakes were prepared as follows: the tubers were thoroughly washed with water and air dried on filter paper, then they were sliced and mashed. The mashed samples were dried in a drum dryer to minimize enzymatic reactions that degrade anthocyanins. Next, they were ground into flakes. For preparation of pigmented extracts, 5 g of each type of flake were subjected to pigment extraction by exposure to 80 % methanol, boiled at 80°C for 5 min and sonicated for 20 min with a repetitive stream of nitrogen gas to avoid possible oxidation degradation of phenolics. The suspension was centrifuged at 5500 g for 10 min and extraction from the resultant precipitate was repeated under the same conditions. The methanol in the two upper layers was combined, removed using a rotary evaporator at 35°C, and the eluate was first dissolved in 25 ml 99·9 % methanol and diluted to a final volume of 50 ml using distilled water. The mixture was filtered through Whatman no. 2 filter paper and stored at − 4°C until analysis.
Micronutrient contents
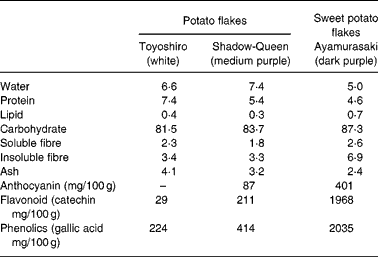
Dietary fibre, protein, lipid, carbohydrate, moisture and ash contents in TY, SQ and AM flakes were measured by the Association of Official Analytical Chemists procedure26. The contents are shown in Table 1.
Table 1 Micronutrient contents (g/100 g powder) of potato and sweet potato flakes
Total polyphenol contents
Total polyphenol contents of the pigmented extracts from TY, SQ and AM flakes were measured by the Folin-Ciocalteu methodReference Singleton, Orthofer and Lamuela-Raventós27. The absorbance was determined at 750 nm using a spectrophotometer (Shimadzu 1600-UV; Shimadzu, Kyoto, Japan). Total phenolic concentrations of TY, SQ and AM flakes were converted into mg gallic acid equivalents per 100 g powder weight.
Flavonoid contents
The absorbance of flavonoids was measured at 510 nm with prepared standards similar to the known (+)-catechin concentrationReference Jia, Tang and Wu28. Flavonoid concentrations of the pigmented extracts from TY, SQ and AM flakes were converted into mg catechin equivalents per 100 g of powder weight.
Anthocyanin contents
The monomeric anthocyanin contents of the pigmented extracts from SQ and AM flakes were measured by the pH differential methodReference Giusti, Wrolstad and Wrolstad29. A Shimadzu 1600-UV spectrophotometer was used to determine the absorbance at 525 nm for SQ (and 524 nm for AM) and 700 nm in buffer at pH 1·0 and 4·5. Anthocyanin contents were calculated using the molar extinction coefficient of cyanidin 3-glucoside (26 900 l/cm per mg) and absorbance
Anthocyanin concentrations of the pigmented extracts from SQ and AM flakes were converted into mg per 100 g powder weight.
Animals and diets
Male F344/DuCrj rats (8 weeks old) were purchased from Charles River Japan (Yokohama, Japan). The animal facility was maintained on a 12 h light–dark cycle at a temperature of 23 ± 1°C and relative humidity of 60 ± 5 %. Animals were randomly assigned into four groups (n 5). This experimental animal design was approved by the Animal Experiment Committee of Obihiro University of Agriculture and Veterinary Medicine. All animal procedures conformed to National Institutes of Health guidelines30. There was no significant difference in body weight at the start of the experiment. Body weight and food consumption were recorded weekly and daily, respectively. The diet compositions, based on the AIN-93G semi-purified rodent dietReference Reeves, Nielsen and Fahey31, are shown in Table 2. Control rats were fed a high-cholesterol diet (0·5 % cholesterol plus 0·125 % sodium cholate) containing 543 g α-maize starch/kg for 4 weeks. Flake-treated rats were fed a high-cholesterol diet supplemented with one of the following diets containing a mixture of 243 g α-maize starch/kg plus 300 g TY, SQ or AM flakes/kg, resulting in final flake concentrations of 30 %. At the end of the experimental period of 4 weeks, blood samples (1 ml) were collected to analyse the serum lipids from fasted rats. The samples were taken into tubes without an anticoagulant. After the samples were allowed to stand at room temperature for 2 h, the sera were separated by centrifugation at 1500 g for 20 min. Soon after, the rats were fed the diets again. Rats were anaesthetized with Nembutal (sodium pentobarbital, 40 mg/kg body weight; Abbott Laboratories, Abbott Park, IL, USA) 24 h after blood was collected, and then were killed. Blood samples were collected and taken into tubes without an anticoagulant. After the samples were allowed to stand at room temperature for 2 h, the sera were separated by centrifugation at 1500 g for 20 min. Then the livers were quickly removed, washed with cold saline (9 g NaCl/l), blotted dry on filter paper and weighed before freezing for storage at − 80°C.
Table 2 Composition of the experimental diets (g/kg of diet)
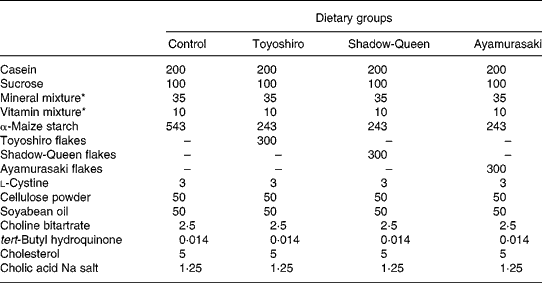
*These diets were based on the AIN-93G diet composition.
For details of procedures, see p 919.
Serum lipid assay
At the end of the experimental period of 4 weeks, serum cholesterol, TAG, phospholipid and NEFA concentrations were measured enzymatically using commercially available reagent kits (Abbott Laboratories).
Serum and hepatic lipid peroxidation
In the serum collected after killing the rats, the degree of serum oxidation was determined using a commercial assay kit (Lipid Hydroperoxide Assay Kit; Wako, Tokyo, Japan). Liver samples (0·5 g) were homogenized in 10 volumes of PBS (pH 7·4). The degree of oxidation was immediately measured by the thiobarbituric acid reactive substances (TBARS) assayReference Ohkawa, Ohishi and Yagi32. Protein concentrations were determined by Lowry assay (Bio-Rad, Hercules, CA, USA)Reference Lowry, Rosebrough, Farr and Randall33.
Serum urate level and Trolox equivalent antioxidant coefficient value
The serum uric acid level was determined using a commercial assay kit (Uric Acid C-Test; Wako, Tokyo, Japan), and the total antioxidant capacity expressed as the Trolox equivalent antioxidant coefficient value was determined using a commercial kit (Randox Laboratories, Antrim, UK), based on scavenging of 2,2′-azinobis(3-ethylbenzothiazoline-6-sulphonate) radical cationsReference Re, Pellegrini, Proteggente, Pannala, Yang and Rice-Evans34.
Hepatic glutathione level and antioxidant enzyme activity
The hepatic glutathione (GSH) concentration was determined by the method of Cohn & LyeReference Cohn and Lyle35. For the assays of glutathione reductase (GSH-R), glutathione S-transferase (GST), glutathione peroxidase (GSH-Px) and catalase, livers were weighed and homogenized in 10 volumes of 0·25 m-sucrose. The supernatant fractions were prepared by centrifugation at 105 000 g for 1 h. For superoxide dismutase (SOD) assay, livers were homogenized in 10 volumes of 1 mm-Tris-HCl buffer (pH 7·4) in 0·25 m-sucrose and centrifuged at 78 000 g for 1 h. Then each supernatant was stored at − 80°C until use. The assay procedure used for the determination of GSH-R activity was based on the method of Worthington & RosemeyerReference Worthington and Rosemeyer36. GST activity was measured by the method of conjugation of 1-chloro-2,4-dinitrobenzene with GSH developed by Habig et al. Reference Habig, Pabst and Jakoby37. GSH-Px activity was measured by the method of Lawrence & BurkReference Lawrence and Burk38. Catalase activity was determined by measuring the rate of H2O2 depletion using a spectrophotometer at 240 nmReference Aebi39. Total SOD activity was determined using a commercial kit (Dojindo Laboratories, Kumamoto, Japan).
Statistical analysis
Data are presented as means and standard deviations. The significance of differences among treatment groups was determined by ANOVA with Duncan's multiple range test (SAS Institute, Cary, NC, USA). Differences were considered significant at P < 0·05.
Results
Table 1 shows the micronutrient contents in TY, SQ and AM flakes. The total phenol contents in TY, SQ and AM flakes were 224, 414 and 2035 mg/100 g powder, respectively. The flavonoid contents in TY, SQ and AM flakes were 29, 211 and 1968 mg/100 g powder, respectively. Moreover, total monomeric anthocyanin contents in SQ and AM flakes were 87 and 401 mg/100 g powder, respectively. Total phenol, flavonoid and anthocyanin contents were higher in the order of AM, SQ and TY flakes.
Table 3 shows body weight, food intake, feed efficiency and liver weight in rats fed maize starch or maize starch plus TY, SQ or AM flakes. There was no difference in the body weight among the groups. Food intake in the TY and AM groups tended to decrease compared to that in the control and SQ groups. However, there was no significant difference in feed efficiency among any groups. Liver weights were similar in all groups.
Table 3 Body weight, food intake, feed efficiency and liver weight in rats fed Toyoshiro, Shadow-Queen and Ayamurasaki flakes for 4 weeks (Mean values and standard deviations for five rats per group)
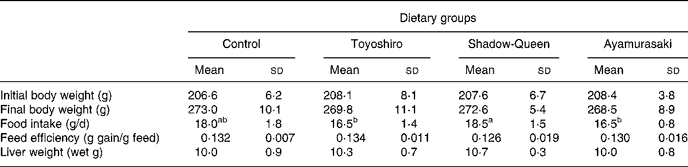
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Table 4 shows serum total antioxidant capacity, and urate, TBARS and GSH concentrations in rats fed maize starch or maize starch plus TY, SQ or AM flakes. There were no significant differences in antioxidant potential capacity among the groups. When the denominator was changed into the cholesterol concentrationReference Bocanegra, Benedí and Sánchez-Muniz40, however, antioxidant potential capacity (IU/mmol cholesterol) in the SQ group was significantly higher (P < 0·05) than in the control group. Urate levels in the TY, SQ and AM groups were significantly lower (P < 0·05) than in the control group. TBARS levels in the SQ and AM groups were significantly lower (P < 0·05) than those in the control and TY groups. There was no significant difference in the GSH level among the groups. Table 4 also shows serum cholesterol, TAG, phospholipid and NEFA concentrations in rats fed maize starch or maize starch plus TY, SQ or AM flakes. The total cholesterol concentration in the SQ group was significantly lower (P < 0·05) than in the control and AM groups. However, there were no significant differences in TAG, phospholipid and NEFA concentrations among the groups.
Table 4 Serum total antioxidant capacity, and urate, thiobarbituric acid reactive substances (TBARS), glutathione and lipid concentrations in rats fed Toyoshiro, Shadow-Queen and Ayamurasaki flakes for 4 weeks (Mean values and standard deviations for five rats per group)

TEAC, Trolox equivalent antioxidant coefficient.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0.05).
Table 5 shows hepatic lipid, TBARS and GSH concentrations, and enzyme activities in rats fed maize starch or maize starch plus TY, SQ or AM flakes. There was no significant difference in the total lipid concentration among the groups. The TBARS levels in the SQ and AM groups were significantly lower (P < 0·05) than in the control and TY groups. The GSH levels in the SQ and AM groups were higher (P < 0·05) than in the control and TY groups. GSH-R activity in the SQ and AM groups was significantly greater (P < 0·05) than in the control group. Furthermore, GST activity in the TY, SQ and AM groups was significantly higher (P < 0·05) than in the control group. Total SOD activity in the SQ and AM groups tended to increase more than in the control group.
Table 5 Liver lipids, thiobarbituric acid reactive substances (TBARS) and glutathione concentrations, and antioxidant enzyme activities of rats fed Toyoshiro, Shadow-Queen and Ayamurasaki flakes for 4 weeks (Mean values and standard deviations for five rats per group)

GSH-Px, glutathione peroxidase; GSH-R, glutathione reductase; GST, glutathione S-transferase; SOD, superoxide dismutase.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0.05).
Discussion
Excess cholesterol consumption promotes oxidative stressReference Tsai41, as demonstrated through increased serum levels of oxidized cholesterol in ratsReference Homma, Kondo, Kaneko, Kitamura, Nyou, Yanagisawa, Yamamoto and Kakizoe2. That process of cholesterol oxidation is highly related to the early process of atherosclerosis developmentReference Steinbrecher, Zhang and Lougheed42. Thus, it might be useful to inhibit oxidative stress or to lower cholesterol concentrations in serum under hypercholesterolaemia. Recently, anthocyanins from edible fruits and vegetables have been shown to have free radical-scavenging activityReference Wang, Cao and Prior16, Reference Lapidot, Harel, Akiri, Granit and Kanner43 and inhibit LDL oxidation in vitro Reference Kähkönen and Heinonen17. In the present study, we administered 30 % TY, SQ or AM flakes to rats fed 0·5 % cholesterol together with 0·125 % sodium cholate, with the result that serum TBARS levels were lowered in the SQ and AM groups compared to the control group. The present results were similar to the result of Tsuda et al. Reference Tsuda, Horio and Osawa14, who reported that feeding 0·2 % cyanidin-3-glucoside increased the ex vivo oxidation resistance of serum in rats. However, there was no significant difference between the control and TY groups for the serum TBARS level. The variation in serum TBARS levels in the flake groups might be due to the different phenolic concentrations in the flakes because TY flakes contain a lower polyphenol concentration, not including anthocyanin, than SQ and AM flakes. Furthermore, the lowered serum TBARS level in the SQ group was likely related to the serum cholesterol concentration because it was lower in the SQ group than in the control group, but those in the TY and AM groups were not significantly different from the control group.
The present results also showed that SQ and AM flakes effectively reduced the hepatic TBARS level in rats fed a cholesterol diet. Ramirez-Tortosa et al. Reference Ramirez-Tortosa, Andersen, Gardner, Morrice, Wood, Duthie, Collins and Duthie44 reported that an anthocyanin-rich extract decreased hepatic lipid peroxidation in vitamin E-depleted rats. Tsuda et al. Reference Tsuda, Horio and Osawa15, Reference Tsuda, Horio, Kitoh and Osawa45 also reported that the consumption of cyanidin-3-glucoside suppressed ischaemia/reperfusion-induced hepatic oxidative stress in rats. Furthermore, there is abundant evidence that anthocyanins from edible plants have free radical-scavenging activityReference Wang, Cao and Prior16, Reference Lapidot, Harel, Akiri, Granit and Kanner43. Natella et al. Reference Natella, Belelli, Gentili, Ursini and Scaccini9 have also suggested the postprandial oxidative stress induced by high consumption of lipids is restricted when a meal is consumed together with foods rich in polyphenols. Other researchers have suggested that the bioavailability of anthocyanin is not necessarily highReference Lotito and Frei46, because a meaningful amount of anthocyanins is poorly absorbed from the intestinesReference Prior25 which may be due to such compounds in the food matrix interacting with other components of the luminal contents and become unabsorbableReference Stahl, van den Berg and Arthur10. During gastrointestinal passage, however, anthocyanins are capable of reducing hydroperoxidesReference Stahl, van den Berg and Arthur10, Reference Prior25 and of chelating autoxidation-promoting metal ionsReference Hider, Liu and Khodr12, Reference Murota, Mitsukuni, Ichikawa, Tsushida, Miyamoto and Terao13. Therefore, the present findings suggest that polyphenol/anthocyanin in SQ and AM flakes might limit the absorption of hydroperoxide in the gastrointestinal tract, furthermore an absorbed polyphenol/anthocyanin or their metabolites might scavenge free radicals generated in the serum. Such scavenging action in the intestinal tract might result in the reduction of oxidative damage in the liverReference Ramirez-Tortosa, Andersen, Gardner, Morrice, Wood, Duthie, Collins and Duthie44, Reference Yeh and Yen47.
Recently, Yeh & YenReference Yeh and Yen47 reported that various polyphenol supplements increased the activities and expression of SOD, GSH-Px and catalase in the liver and small intestine in rats fed a cholesterol-free diet. Previously, we similarly reported that a 25 % purple potato flake diet up-regulated the hepatic mRNA expression related to antioxidant enzymes in rats fed a cholesterol-free dietReference Han, Sekikawa, Shimada, Hashimoto, Hashimoto, Noda, Tanaka and Fukushima24. On the other hand, Lee et al. Reference Lee, Park, Bok, Jung, Kim, Park, Huh, Kwon and Choi48 reported that cinnamic acids decreased hepatic GSH-Px and catalase activities without any change in SOD activity in rats fed a cholesterol diet. In fact, the present results showed that hepatic GSH-Px and catalase activities in the TY, SQ and AM groups were decreased more than those in the control group, and that hepatic SOD activity in the TY, SQ and AM groups was not different from that in the control group. This might be explained by the findings that exogenous cholesterol loads increase hepatic GSH-Px and/or catalase activities in ratsReference Bocanegra, Benedí and Sánchez-Muniz40, Reference Mahfouz and Kummerow49. Thus, it is possible that polyphenols in flakes might reduce the increase in hepatic GSH-Px and/or catalase activities to maintain the homeostasis in rats fed a cholesterol diet. Contrary to hepatic GSH-Px and catalase activities in the TY, SQ and AM groups, however, hepatic GST activity in the TY, SQ and AM groups was increased more than in the control group. Although it is uncertain whether a cholesterol-loaded diet can affect hepatic GST activity, Bradfield et al. Reference Bradfield, Chang and Bjedances50 reported that hepatic GST activity in male C57BL/6 mice fed a 40 % sweet potato diet was increased 1·3-fold compared to the control group. Furthermore, Kawabata et al. Reference Kawabata, Yamamoto, Hara, Shimizu, Yamada, Matsunaga, Tanaka and Mori51 reported that ferulic acid, which is one of the main polyphenols in TYReference Nara, Miyoshi, Honma and Koga52, increased hepatic GST activity in rats fed a CE-2 diet (CLEA Japan, Tokyo, Japan). Thus, the polyphenols/anthocyanins in TY, SQ or AM flakes might increase hepatic GST activity, and that action is likely to contribute to the antioxidant potential in rats fed a cholesterol diet.
GSH (reduced form) is an essential intercellular substrate of GST or GSH-Px, and plays an important role in the maintenance of thiol groups on intracellular proteins and in protection of cells against oxidative stressReference Reed53. It has been reported that a higher concentration of intercellular GSH improves cellular functionality upon exposure to oxidized lipidsReference Dickinson, Moellering, Iles, Patel, Levonen, Wigley, Darley-Usmar and Forman54. Recently Tsuda et al. Reference Tsuda, Horio, Kitoh and Osawa45 reported that the decrease in the hepatic GSH level in rats subjected to hepatic ischaemia/reperfusion was significantly suppressed by feeding them with 0·2 % cyanidin-3-glucoside for 14 d. In the present study, the prevention of SQ and AM flakes from lowering the hepatic GSH level, which suggested that the polyphenol/anthocyanin in SQ and AM flakes might act as antioxidants to protect against oxidative damage induced by a cholesterol load. Furthermore, since GSH-R is involved in the maintenance of a suitably high GSH level, we think that the increase in GSH-R activity in the SQ and AM groups had a modulatory effect on the GSH level. Therefore, it is assumed that factors up-regulating GST and GSH-R activities of rats following the intake of SQ and AM flakes may lead to inhibition of hepatic lipid peroxidation, which may be involved in the postprandial oxidative stress induced by a high-cholesterol diet.
Several researchers have reported that anthocyanin leads to increased serum antioxidant potential in experimental animalsReference Auger, Laurent, Laurent, Besancon, Caporiccio, Teissedre and Rouanet55 and human subjectsReference Cao, Russell, Lischner and Prior56, Reference Mazza, Kay, Cottrell and Holub57. We also previously reported that a 25 % purple potato flake diet increases the serum Trolox equivalent antioxidant coefficient value in rats fed a cholesterol-free dietReference Han, Sekikawa, Shimada, Hashimoto, Hashimoto, Noda, Tanaka and Fukushima24. In the present study, however, there was no change in the serum Trolox equivalent antioxidant coefficient value in any rats fed a cholesterol diet. This might be explained by the decrease in the serum uric acid level induced by the flake diets, because serum urate could act like an antioxidant and contribute to the serum antioxidant potential to a sizeable extentReference Benzie and Strain58. Furthermore, Jacob et al. Reference Jacob, Spinozzl, Simon, Kelley, Prior, Hess-Pierce and Kader59 reported that the anthocyanin-rich cherry lowers the plasma urate level in healthy women. However, it is uncertain how the flake diets could modulate purine metabolism leading to a decrease in serum urate in rats fed a cholesterol diet. Therefore, it would be interesting to investigate further in a future study the mechanism of the decreasing serum urate level following intake of the flakes, because such an effect would be beneficial for healthReference Weir, Muir, Walters and Lees60.
In a previous study, antioxidant activity of a 25 % purple potato flake diet in rats fed a cholesterol-free diet was hypothesized to lower the carbohydrate concentration in the dietReference Han, Sekikawa, Shimada, Hashimoto, Hashimoto, Noda, Tanaka and Fukushima24 because the possible inhibitory action against postprandial oxidative stress is considerable due to the reduced primary or secondary carbohydrate autoxidation products such as glycated proteins in the gastrointestinal tractReference Stahl, van den Berg and Arthur10, Reference Sies, Stahl and Sevanian11. In the present study, however, the TBARS levels in liver and serum of the TY group (white potato) did not show any significant difference from those in the control group despite the comparable carbohydrate concentrations in all flake diets (TY, 637·7 g/kg diet; SQ, 644·2 g/kg diet; AM, 655·1 g/kg diet) and the lower carbohydrate concentration compared to the control diet (693·2 g/kg diet). Furthermore, TY flakes had a lower polyphenol concentration not including anthocyanin. Therefore, the present findings suggest that the antioxidant activity of SQ and AM flakes might be highly related to the polyphenol/anthocyanin concentration, not to the carbohydrate concentration, and that SQ and AM flakes have the capacity to prevent postprandial oxidative stress in rats fed a high-cholesterol diet. On the other hand, the antioxidant efficiency of SQ flakes was similar to that of AM flakes, although the polyphenol/anthocyanin concentration was approximately fourfold lower than in AM flakes. It might be that the polyphenol/anthocyanin concentration of SQ flakes has the optimal effect on the antioxidant potential in rats fed a cholesterol diet.
In conclusion, the present study suggests that anthocyanin-containing SQ flakes improve the antioxidant status against oxidative damage in rats fed high-cholesterol diets. Such antioxidant effects might result from increments of GST and GSH-R activities, and GSH in the liver. However, a further study is necessary to investigate the mechanism decreasing the serum urate level following intake of TY, SQ and AM in rats fed a cholesterol diet.
Acknowledgements
We are grateful to Dr Takahiro Noda (The National Agricultural Research Center for the Hokkaido Region) and Mr Hisashi Tanaka (Somatech Center, House Foods Corporation) who kindly provided the potato and sweet potato samples for this study. This work was supported in part by a grant from the Research and Development Program for New Bio-industry Initiatives of the Bio-oriented Technology Research Advancement Institution, by a grant from Cooperation of Innovative Technology and Advanced Research in Evolutional Area (CITY AREA), and by a grant from the 21st Century COE Program (A-1), Ministry of Education, Culture, Sports, Science and Technology of Japan.







