Introduction
The Pinnotheridae de Haan, 1833 is a family of brachyuran decapods comprising some 297–312 species (Ng et al., Reference Ng, Guinot and Davie2008; Ahyong et al., Reference Ahyong, Lowry, Alonso, Bamber, Boxshall, Castro, Gerken, Karaman, Goy, Jones, Meland, Rogers, Svavarsson and Zhang2011; Palacios Theil et al., Reference Palacios Theil, Cuesta and Felder2016), with several genera and subfamilies that need to be revised (Palacios Theil et al., Reference Palacios Theil, Cuesta and Felder2016).
Until very recently, four species of the family Pinnotheridae were known to occur in European waters (Becker & Türkay, Reference Becker and Türkay2010; Subida et al., Reference Subida, Arias, Drake, García Raso, Rodríguez and Cuesta2011), namely Afropinnotheres monodi Manning, 1993, Reference Manninga crab originally from Africa, and the native European Nepinnotheres pinnotheres (Linnaeus, 1758), Pinnotheres pectunculi Hesse, Reference Hesse1872 and P. pisum (Linnaeus, Reference Linnaeus1767). For these four species there are data available on their distribution, larval stages and DNA markers (Pérez-Miguel et al., Reference Pérez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019). This information allowed the detection by Marco-Herrero et al. (Reference Marco-Herrero, Drake and Cuesta2018) of some pinnotherid larvae in plankton samples (Project ECOBOGUE) from the coast of Doñana National Park (Gulf of Cádiz, SW Spain) that did not belong, either morphologically or according to DNA analyses, to any of the previously known European pinnotherid species. This was considered as an early detection of a new marine invader in European waters. Since DNA analysis of the larvae indicated that the new species was closer to species of the genus Pinnotheres than to any other Pinnotheridae genus, it was provisionally named Pinnotheres sp. (see Marco-Herrero et al., Reference Marco-Herrero, Drake and Cuesta2018; Pérez-Miguel et al., Reference Pérez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019). Since then, the search was begun for their adult stages, in the context of the AFROBIV project: this is a project to study the pinnotherid species present in the Iberian Peninsula, paying special attention to the effect of A. monodi on their bivalve hosts. Two approaches were adopted in this search: (1) the collection of a large number of individuals of different possible hosts, in the geographic area where larvae appeared; and (2) searching in existing crustacean collections for samples of pinnotherids collected in Iberian waters, for close morphological examination.
During a recent and exhaustive sampling of intertidal bivalves all along the Iberian Peninsula coasts, we did not collect any pinnotherid adults of any unidentifiable species (Pérez-Miguel et al., Reference Pérez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019), and we therefore concluded that adult individuals of Pinnotheres sp. would probably be hosted only by subtidal species. A reexamination of the pea crabs (by morphology and DNA barcodes) deposited in the Biological Reference Collections (CBR) at the Institut de Ciències del Mar (ICM-CSIC), resulted in their classification to three out the four known European species, namely N. pinnotheres, P. pectunculi and P. pisum. However, when reviewing specimens from previous studies by Manjón-Cabeza & García Raso (Reference Manjón-Cabeza and García-Raso1998) one ovigerous female captured in Barbate (Gulf of Cádiz) was identified as Pinnotheres sp. based on DNA barcodes, and it showed a clearly different morphology from that of other European crab species. Also, a soft female living inside the common saddle oyster Anomia ephippium Linnaeus, 1758 (Anomiidae Rafinesque, 1815) had been collected in the same area in 1995 (García Raso & Manjón-Cabeza, Reference García Raso and Manjón-Cabeza2002), and although it was initially identified as P. pisum, it was actually close to the above-mentioned ovigerous female of Pinnotheres sp. Given these findings, we then focused on obtaining samples of Anomia ephippium from the same area to search for more specimens and confirm the habitat and distribution of this species.
In this study, we analyse by morphology and at the molecular level the specimens from Barbate as well as the pea crabs hosted by bivalves collected in two oceanographic surveys conducted in 2017 (ARSA 1117 and MEDITS_ES2017) and another survey in 2018 (ARSA 0318), paying special attention to A. ephippium. All of these specimens clearly belong to an undescribed species of the genus Pinnotheres Bosc, 1801. This new species is described, figured and compared with congeners, and a key for European Pinnotheridae is provided.
Materials and methods
Material examined
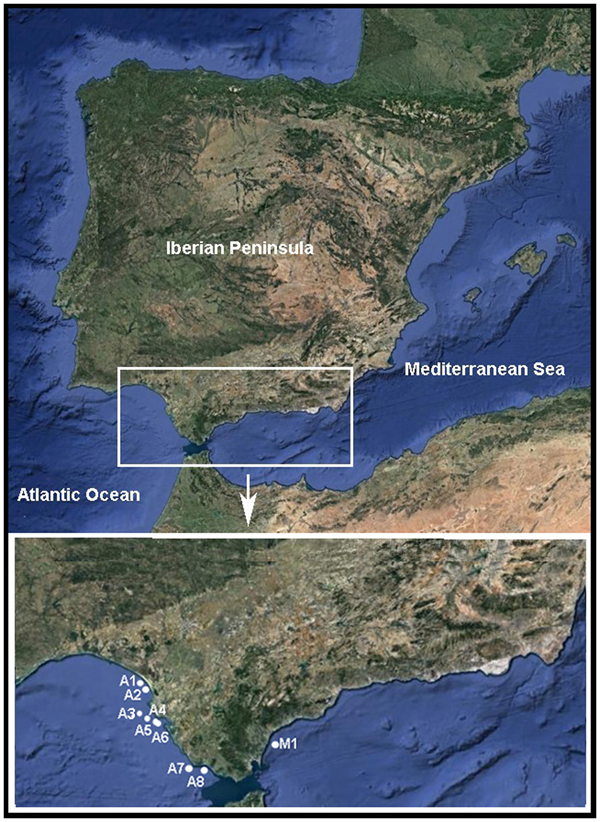
The Pinnotheres bicristatus sp. nov. individuals examined in this study correspond to those hosted by subtidal bivalves collected by the authors during different fishery research cruises along the Atlantic and Mediterranean coasts of Andalusia (South of Iberian Peninsula). Samples of Anomia ephippium were obtained from the ARSA 1117, ARSA 0318 and MEDITS_ES 2017 surveys using bottom trawl gears; an additional pea crab was collected inside A. ephippium, and another was collected free-living in bivalve samples from offshore the Barbate coast (Project PB92-0415) with a dredge (Manjón-Cabeza & García Raso, Reference Manjón-Cabeza and García-Raso1998; García Raso & Manjón Cabeza, Reference García Raso and Manjón-Cabeza2002). The coordinates of all sampling sites in which Pinnotheres bicristatus sp. nov. has been collected are shown in Table 1, and a map showing the position of the sampling stations is displayed in Figure 1. The description of the new species in this paper is only by two of the authors, J.E. García Raso and J.A. Cuesta.
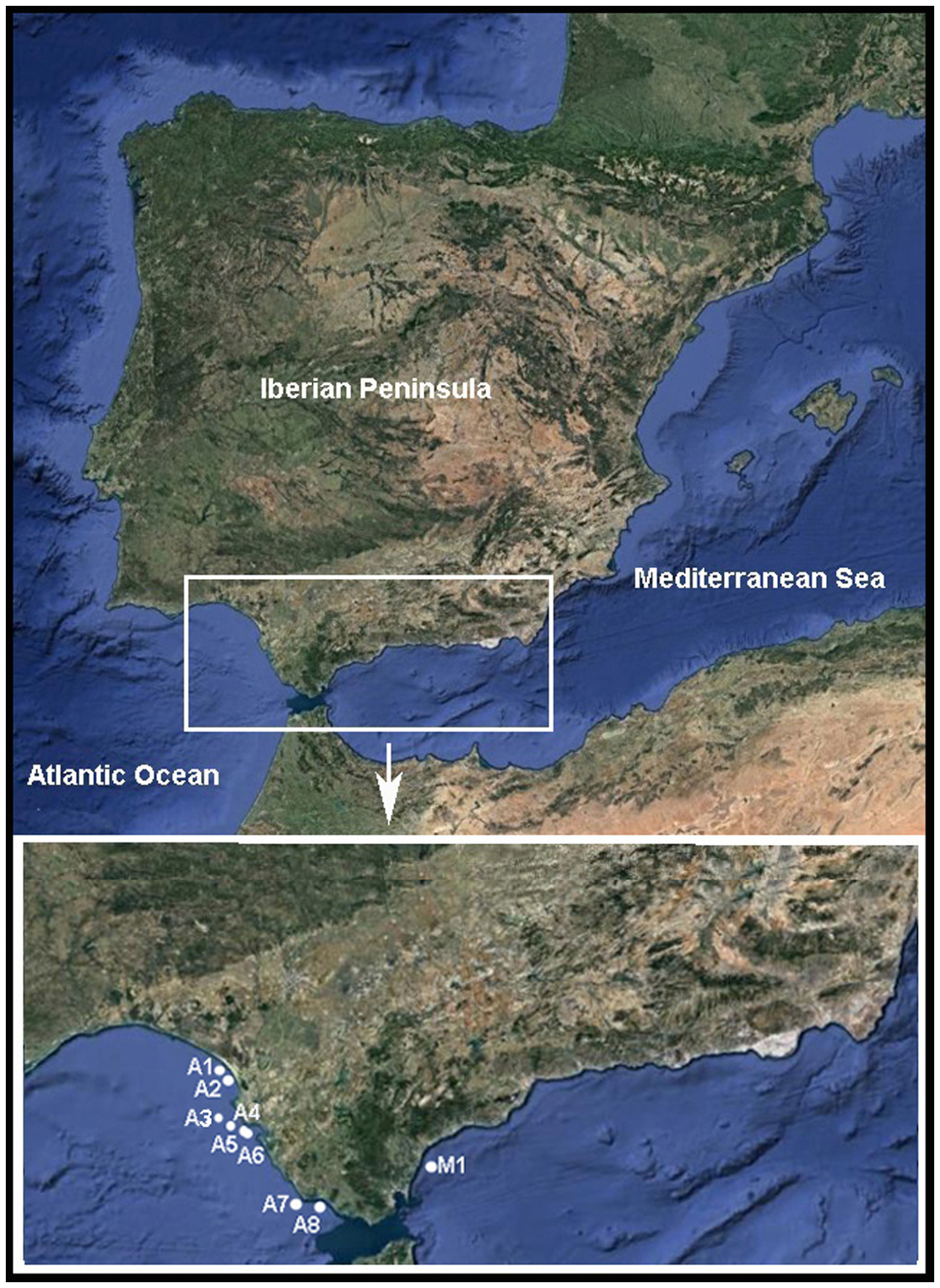
Fig. 1. Map showing sampling sites where Pinnotheres bicristatus sp. nov. was collected. ECOBOGUE 2013: A1, A2, Doñana National Park coast; ARSA 2017: A4, A5, A6 offshore Bay of Cadiz; ARSA 2018: A3, offshore Bay of Cadiz, A7, offshore Barbate coast; Project PB92–0415: A8, Barbate coast; MEDITS_ES 2017: M1, offshore Estepona.
Table 1. Locations, surveys and dates in which the pea crab Pinnotheres bicristatus sp. nov. has been collected
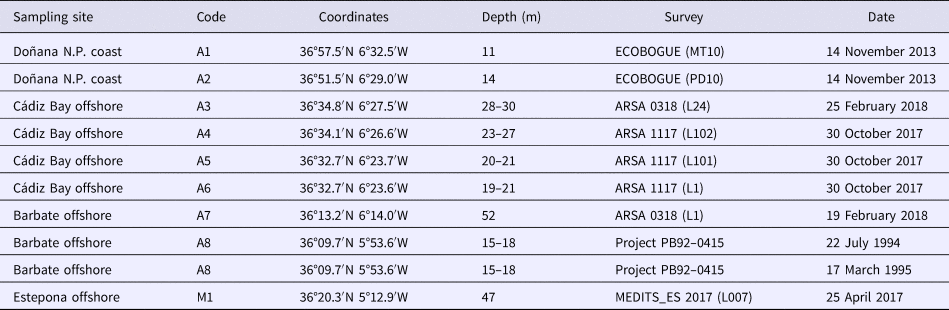
Codes of sampling sites as in Figure 1.
Collection and measurement of hosts and crabs
The specimens of Anomia ephippium collected during the MEDITS_ES 2017 survey were opened on board the ship and inspected for the presence of pea crabs, which were preserved in 80% ethanol. The A. ephippium collected during the ARSA 1117 and ARSA 0318 (hosting 98.4% of crabs examined) were frozen in the ship, and later examined in the laboratory, where they were measured and inspected for the presence of crabs. For A. ephippium from the ARSA cruises, the maximal shell lengths along the anterior-posterior and dorsal-ventral axes of the shell were measured to the nearest 0.1 mm with a dial caliper (Tesa Cal IP65). Due to the variability in the shell shape, the shell size of A. ephippium was estimated as the mean values of both measures.
All crabs found within each individual host were preserved and stored in 80% ethanol for further demographic observations. The carapace width (CW = maximum cephalothorax width) and carapace length (CL = distance from the rostrum to the posterior margin of the carapace) of each crab was measured to the nearest 0.01 mm under a stereomicroscope provided with a calibrated ocular micrometer. The wet weight (WW) was estimated with a digital balance (precision 0.1 mg). Crabs were sexed based on the presence (male) or absence (female) of gonopods. Furthermore, each female crab was classified either as hard or soft by considering its pleonal morphology, cephalothorax consistency and shape, and pleopods morphology: hard females show a flattened and rounded cephalothorax and swimming legs (similar to those of males), whereas the body of soft females is mostly sub-trapezoidal and lacks swimming legs. Lastly, soft females carrying eggs (embryos) were classified as ovigerous females. Gonadal development of soft females was considered. The drawings were made using a camera lucida and the photographs using a Nikon digital camera DXM1200F, both mounted on stereomicroscopes. Other abbreviations used: MXP3, third maxilliped; P1–P5, pereiopods 1–5 (P1 – cheliped, P2–P5 – walking legs); IEO-CD, Decapod Crustacean Collection from Cadiz Oceanographic Centre; CBR, Biological Reference Collections.
Data treatment of the Gulf of Cádiz population
For each of the two ARSA surveys, the host-size related prevalence of Pinnotheres bicristatus sp. nov. in the host Anomia ephippium was analysed by estimating the number of crabs collected for each 5 mm size group of the host, as well as by representing the cumulative number of A. ephippium in size-increasing order vs the cumulative number of crabs retrieved from them. In addition, for each demographic category of crabs, the relationship between host size and crab size was assessed by regression analysis by fitting data to a linear model. Similarly, the relationship between crab carapace width and crab wet weight was assessed by regression analysis by fitting data to power regression models. Differences between the estimated slopes of regression lines or between the exponents of power models were tested by using Student's t-tests.
To test whether crabs tend to live solitarily, in pairs or aggregated in higher numbers inside the host, the frequency of occurrence of A. ephippium without crabs and with different numbers of crabs was compared with the expected frequencies under a random distribution (binomial distribution) and differences tested by a χ2 test. Similarly, the observed proportion of males to females was tested for deviation from a 1:1 sex ratio by using a χ2 test. Besides, the observed sexual composition of crab pairs sharing a single host individual was compared with the expected frequencies of heterosexual and homosexual pairs in a random distribution by using χ2 test.
A 5% significance level (P = 0.05) was considered for all statistical tests carried out in this study (Zar, Reference Zar2010).
Pinnotheres bicristatus sp. nov. identification by DNA barcodes
Total genomic DNA was extracted from pereiopod muscle tissue, following a modified Chelex 10% protocol by Estoup et al. (Reference Estoup, Largiadèr, Perrot and Chourrout1996). Partial sequences of the mitochondrial 16S and Cox1, and nuclear H3 genes were amplified. Cycling conditions of the polymerase chain reaction (PCR), length of the sequences obtained, and primers used for each gene are the same as in Pérez-Miguel et al. (Reference Pérez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019). PCR products were sent to Stab-Vida laboratories to be purified and then bidirectionally sequenced. Sequences were edited using the software Chromas version 2.0. The obtained final DNA sequences were compared with those from Pinnotheridae available in GenBank database.
An evolutionary distances analysis was carried out in MEGA6 (Tamura et al., Reference Tamura, Stecher, Peterson, Filipski and Kumar2013). A concatenated alignment of the 16S, Cox1 and H3 sequences was built using the sequences obtained in the present study from specimens of Pinnotheres bicristatus sp. nov., as well as the sequences of A. monodi, N. pinnotheres, P. pectunculi, P. pisum, Pinnotheres sp. (larvae), Orthotheres barbatus (Desbonne, 1867) and Zaops ostreus (Say, 1817) downloaded from GenBank (http://www.ncbi.nlm.nih.gov). The phylogeny reconstruction analyses were inferred from neighbour-joining using the p-distance method. The nodal confidence of the obtained topologies was assessed via 2000 bootstrap replicates.
Results
Geographic distribution and host of Pinnotheres bicristatus sp. nov.
The known distribution of P. bicristatus sp. nov. is, so far, the Gulf of Cádiz (NE Atlantic) and western Alboran Sea (SW Mediterranean) coasts (Figure 1). In this study, we have examined one ovigerous female collected free-living in bivalve samples in Barbate offshore (Gulf of Cádiz) and 328 individuals hosted by Anomia ephippium: 325 specimens collected along the coast of the Gulf of Cádiz and 3 specimens collected in the Alboran Sea (Table 2).
Table 2. Characteristics of pea crabs, Pinnotheres bicristatus sp. nov., examined in this study
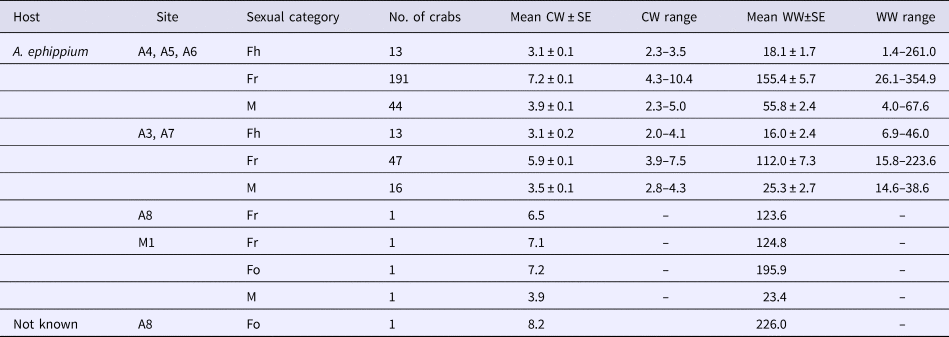
CW, crab carapace width (mm); WW, crab wet weight (mg); Fh, hard females; Fr, reproductive females; M, males.
Codes and locations of sampling sites (A3 to A8, and M1) as in Figure 1.
Gulf of Cádiz population
Crab prevalence and size and sex structure
In the Gulf of Cádiz, the mean prevalence by Pinnotheres bicristatus sp. nov. in its unique known host, Anomia ephippium, was 55.6% (48.15–66.67%) in October 2017 and 77.7% (67.2–88.2%) in February 2018.
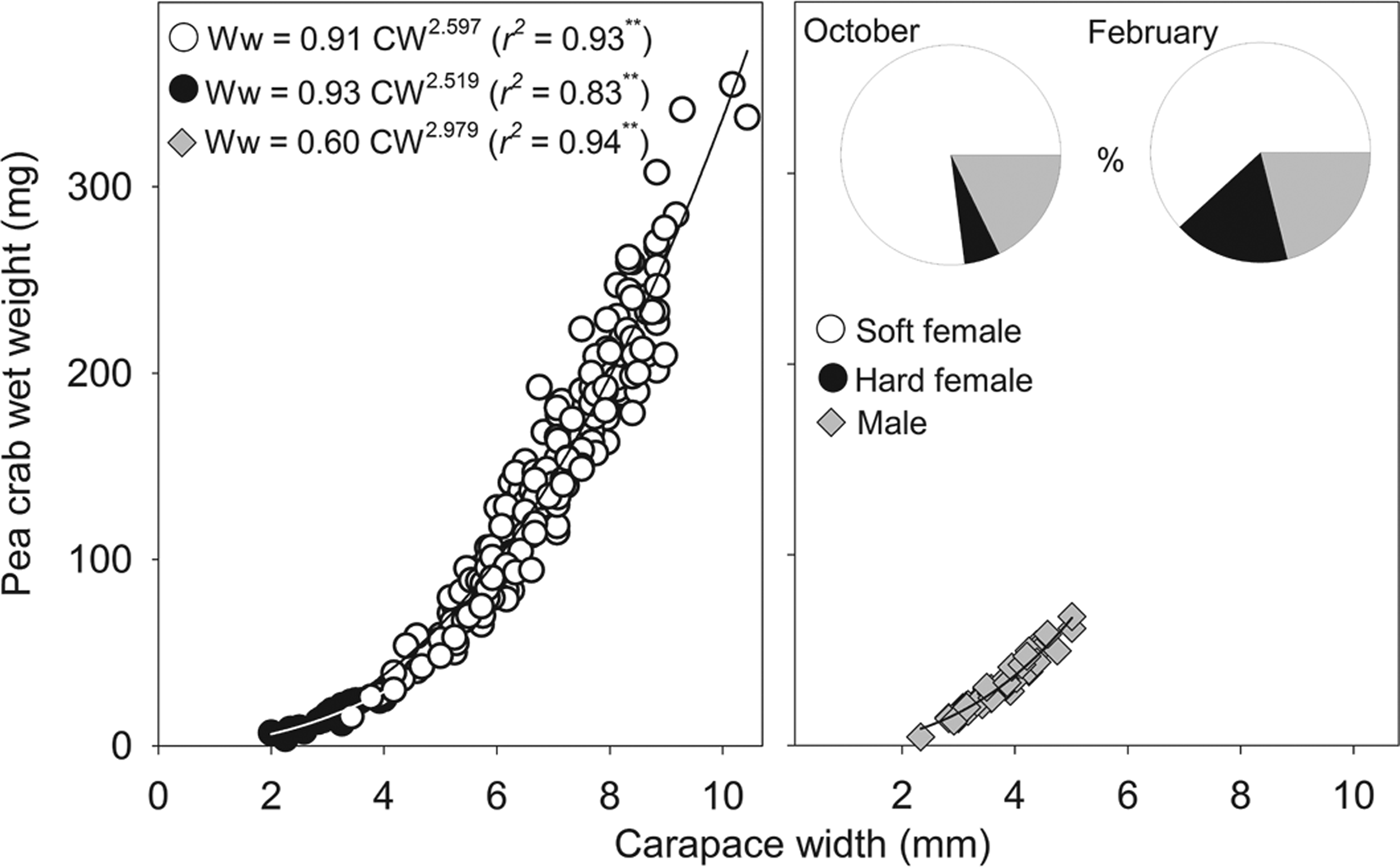
Neither megalopae nor indeterminate (small individuals without sexual characteristics) individuals of P. bicristatus sp. nov. were found inside the host. The three demographic categories observed were males, hard females and soft females (Table 2, Figure 2). In P. bicristatus sp. nov. population from the Gulf of Cádiz (98.4% of the crabs examined), the dominant category was soft females (77.0% in October 2017 and 61.8% in February 2018), followed by males (17.7% in October 2017 and 21.1% in February 2018) and hard females (5.3% in October 2017 and 17.1% in February 2018). The carapace width (and wet weight) of crabs ranged from 2.0 (4.0 mg) to 10.4 mm (354.9 mg), with soft females showing the largest average size of 7.2 ± 0.1 mm (155.4 ± 5.7 mg) in October 2017 and 5.9 ± 0.1 mm (112.0 ± 7.3 mg) in February 2018, followed by males with a mean size of 3.9 ± 0.1 mm (55.8 ± 2.4 mg) in October 2017 and 3.5 ± 0.1 mm (25.3 ± 2.7 mg) in February 2018, and hard females with mean size of 3.1 ± 0.1 mm (18.1 ± 1.7 mg) in October 2017 and 3.1 ± 0.2 mm (16.0 ± 2.4 mg) in February 2018. Most soft females collected in both surveys showed well developed reddish gonads (98.7% in October and 87.2% in February) and the smallest female with this characteristic had a carapace width of 3.5 mm (February 2018).
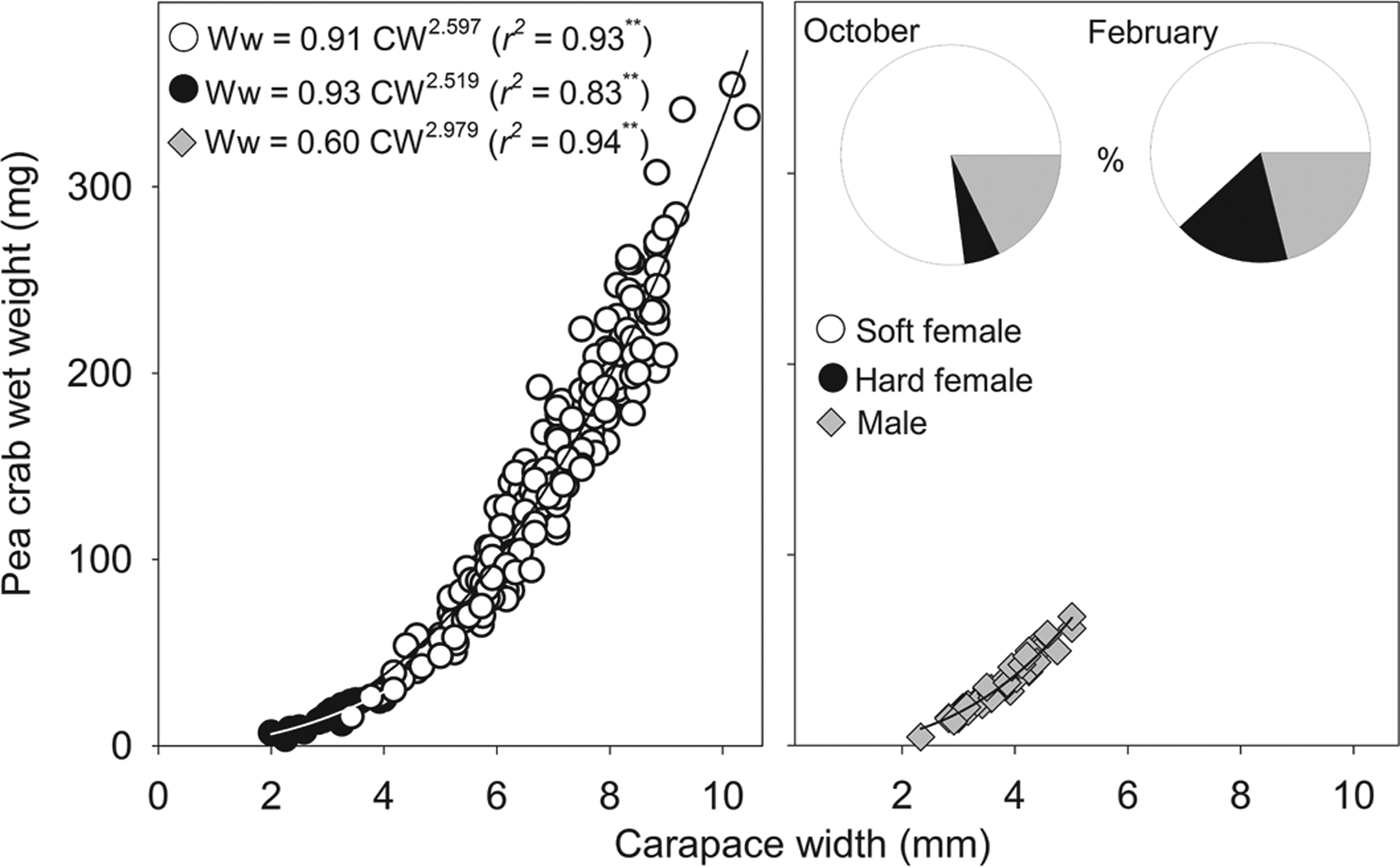
Fig. 2. Relationship between Pinnotheres bicristatus sp. nov. carapace width and wet weight for each demographic category, and percentage represented by each category (pie chart) in October 2017 and February 2018 surveys in Gulf of Cadiz.
The sex-ratio (female/male) was: 4.0 in October 2017, which differed significantly from a 1:1 sex ratio (χ2 = 103.2, P < 0.01), and 3.8 in February 2018, which also differed significantly from a 1:1 sex ratio (χ2 = 13.9, P < 0.01). Irrespective of the sampling date and demographic category, there was a significant (P < 0.01) power relationship between carapace width and wet weight (Figure 2). The exponent of fitted models for males (b = 2.979) was very close to 3 (t-student = 0.1; P > 0.05), whereas those of soft females (b = 2.597) and hard females (b = 2.519) were lower than 3 although this difference was statistically significant for soft females (t = 4.3; P < 0.01) but not for hard females (t = 1.0; P > 0.05).
Interactions between crabs
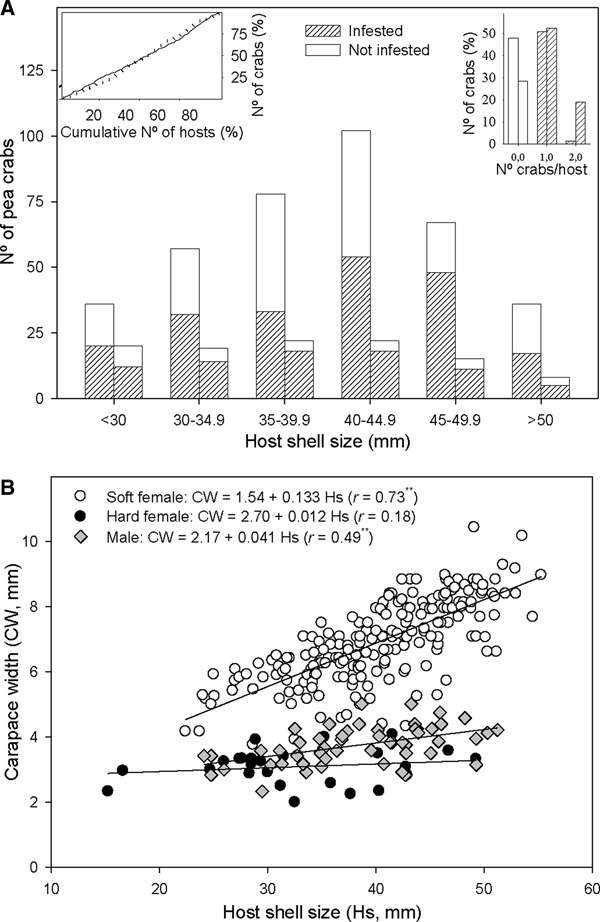
The number of crabs per host varied between 0 and 2, with 96.7% of Anomia ephippium with crabs hosting a single individual in October 2017 and 73.3% in February 2018 (Figure 3A). Thus, crab distribution among hosts did not display a random pattern: there were more bivalves harbouring one crab and fewer bivalves without crabs or harbouring two crabs, than those expected by chance alone. The observed departure from a random pattern was statistically significant in October 2017 (χ2 = 54.3, P < 0.01) and February 2018 (χ2 = 10.7, P < 0.01). A total of six A. ephippium harbouring heterosexual pairs of crabs were collected in October 2017 (3.3% of infested hosts); whereas 12 A. ephippium with crab pairs (26.7%) were collected in February 2018: 10 heterosexual pairs, one 2-males pair and one 2-females pair. Considering the sex ratio observed in each survey (Table 2), there were more heterosexual pairs than expected by chance alone both in October 2017 (χ2 = 9.2; P < 0.01) and in February 2018 (χ2 = 11.3; P < 0.01). One male and one soft female was the combination observed in 55% of heterosexual pairs, whereas one male and one hard female combination was observed in 45% of them.
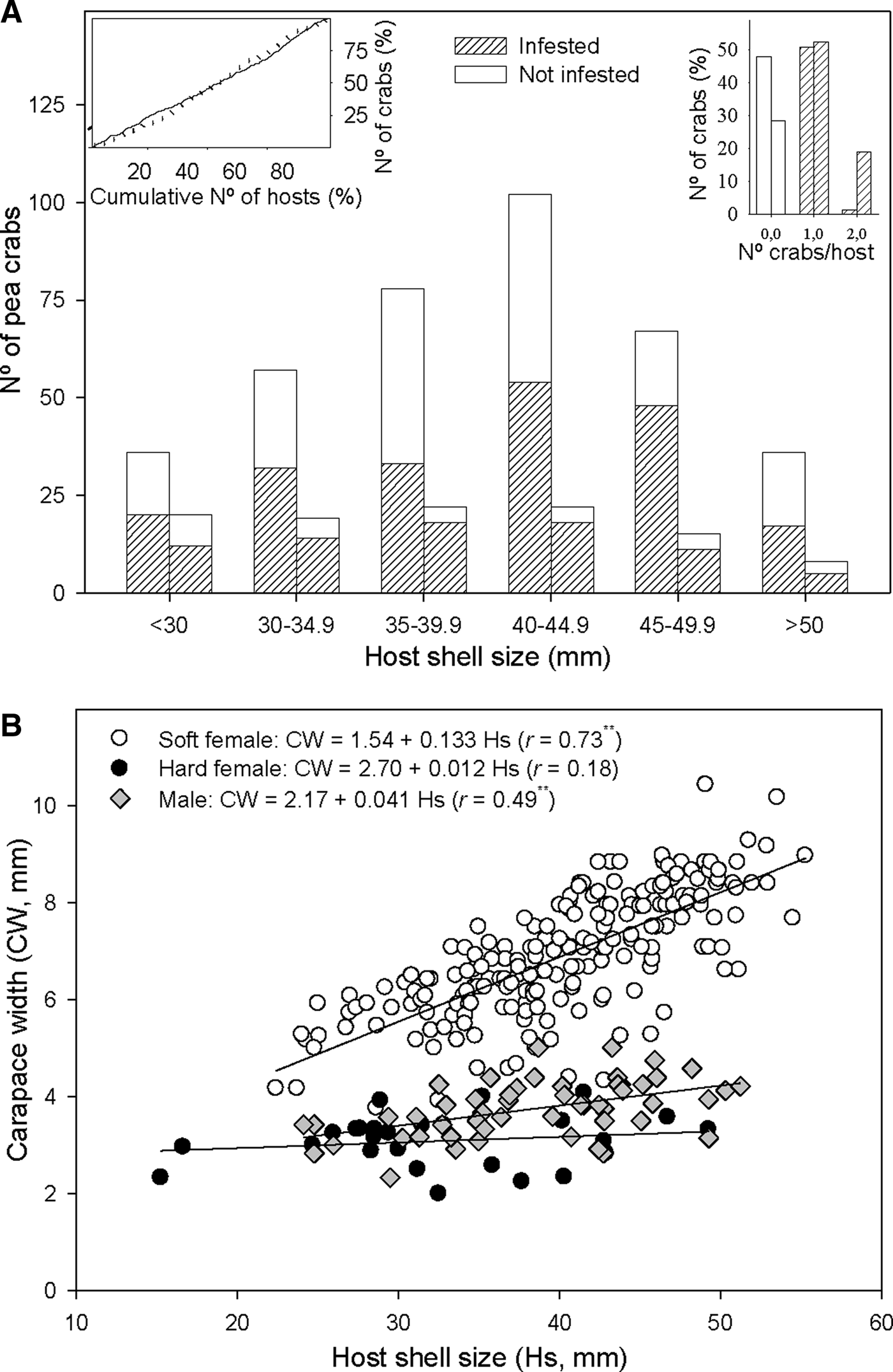
Fig. 3. Relationship between host shell size and Pinnotheres bicristatus sp. nov. population from Gulf of Cadiz. (A) Cumulative number of Anomia ephippium in increasing size order vs cumulative number of pea crabs retrieved from them (plot on left top corner) in October 2017 (solid line) and February 2018 (dotted line) surveys; number of pea crabs by host (bar plot on right top corner) and pea crab prevalence by host size-groups (main bar plot) in October 2017(each left bar) and February 2018 (each right bar) surveys. (B) Host shell size vs carapace width of pea crab for each crab demographic category. ** and *, significance level of correlation coefficient of P < 0.01 and P < 0.05, respectively.
Host size effects
The collected Anomia ephippium in October 2017 survey had a mean size (±SE) of 40.4 (±0.4) mm and ranged between 16.6 mm and 55.3 mm, whereas those of February 2018 had a mean size of 35.6 (±0.8) mm ranging from 15.2 to 52.0; similarly, the mean size of specimens hosting pea crabs was 40.5 (±0.5) mm in October 2017 and of 36.0 (±0.9) mm in February 2018.
A higher number of crabs was collected in the most frequent size classes of A. ephippium in both surveys. This homogeneous prevalence of crabs throughout the host size range was evidenced by an increase of the cumulative number of crabs collected proportional to that of the cumulative number of hosts when the latter was estimated by ordering hosts by increasing size (Figure 3A).
For crabs collected in the 2017 and 2018 surveys, there was no significant correlation between hard female sizes and those of their hosts (r = 0.07; P > 0.05), whereas a significant positive correlation between host and crab sizes was observed for both soft females (r = 0.73; P < 0.01) and, to lesser extent, males (r = 0.49; P < 0.01). Nevertheless, soft females hosted by A. ephippium of a specific size were larger than males hosted by A. ephippium of the same size (Figure 3B).
Pinnotheres bicristatus sp. nov. identification by molecular analysis
All the sequences obtained for the three genes (mithocondrial 16S and Cox1, and nuclear H3) fit 99–100% with those of Pinnotheres sp. larvae (zoeae and megalopa) deposited in GenBank under the accession codes MF069149 and MF069150 (16S), MF134396 (Cox1) and MF138913 (H3) obtained by Marco-Herrero et al. (Reference Marco-Herrero, Drake and Cuesta2018) and Pérez-Miguel et al. (Reference Pérez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019). In the final tree, all sequences of Pinnotheres bicristatus sp. nov. (adults and larvae) clustered together within a clade, separate from the other European Pinnotheres species (P. pisum and P. pectunculi) (Figure 4).
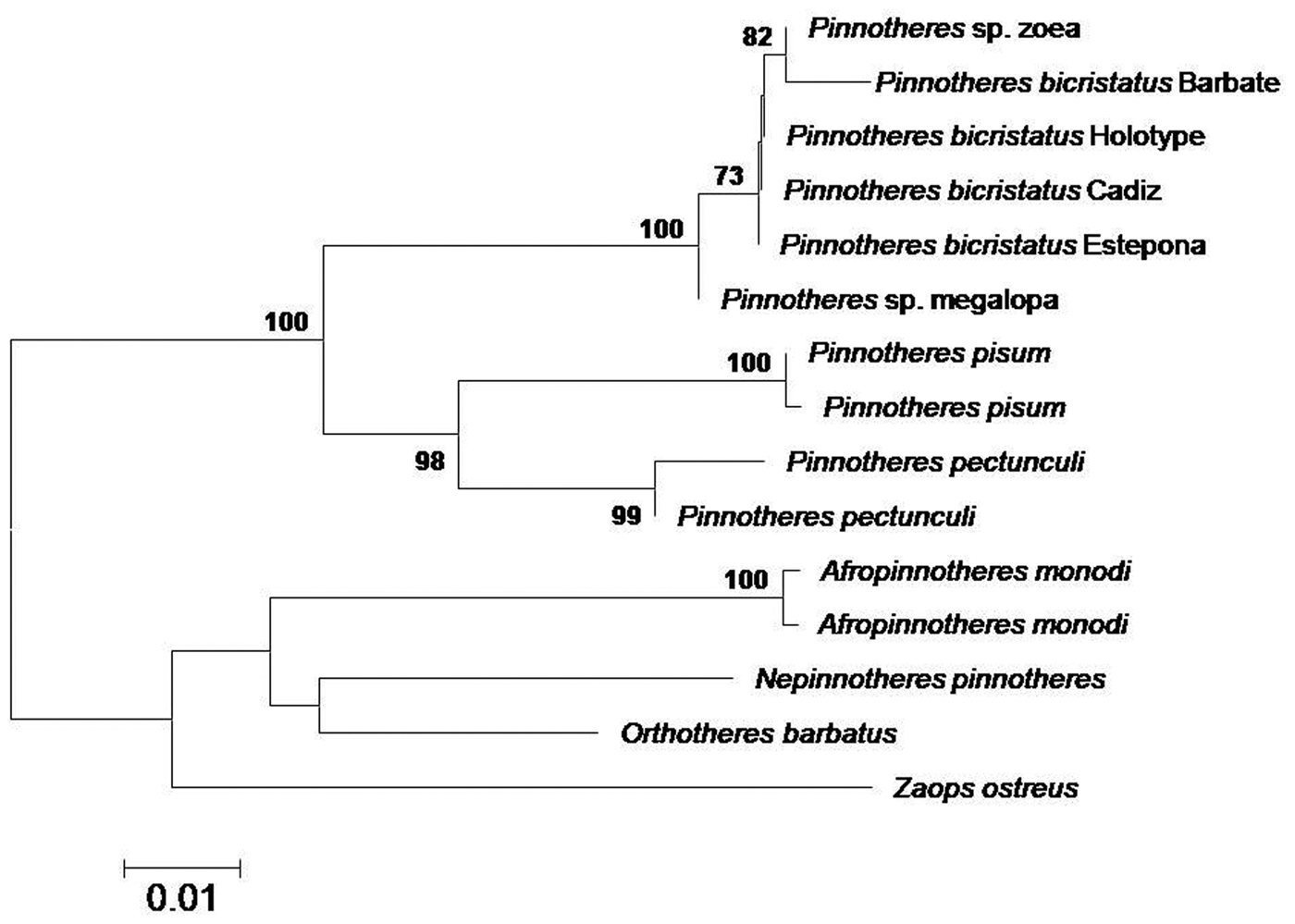
Fig. 4. Topology of neighbour-joining tree based on concatenated 16S and Cox1 mtDNA and H3 nuclear gene sequences, showing inferred phylogenetic relationships within European Pinnotheridae, with American Zaops ostreus and Orthotheres barbatus as outgroups. Numbers close to nodes indicate bootstrap support (only values above 80% shown).
Species description
Systematic
Order DECAPODA Latreille, 1802
Infraorder BRACHYURA Latreille, 1802
Family PINNOTHERIDAE De Haan, 1833
Genus Pinnotheres Bosc, 1801
Pinnotheres bicristatus sp. nov. García Raso & Cuesta
(Figures 5–8 & 9G)
Pinnotheres sp.: Manjón-Cabeza & García Raso (Reference Manjón-Cabeza and García-Raso1998), ovigerous female; Pinnotheres pisum: García Raso & Manjón-Cabeza (Reference García Raso and Manjón-Cabeza2002), soft female; Pinnotheres sp.: Marco-Herrero et al. (Reference Marco-Herrero, Drake and Cuesta2018), zoea II, zoea III, zoea IV and megalopa.
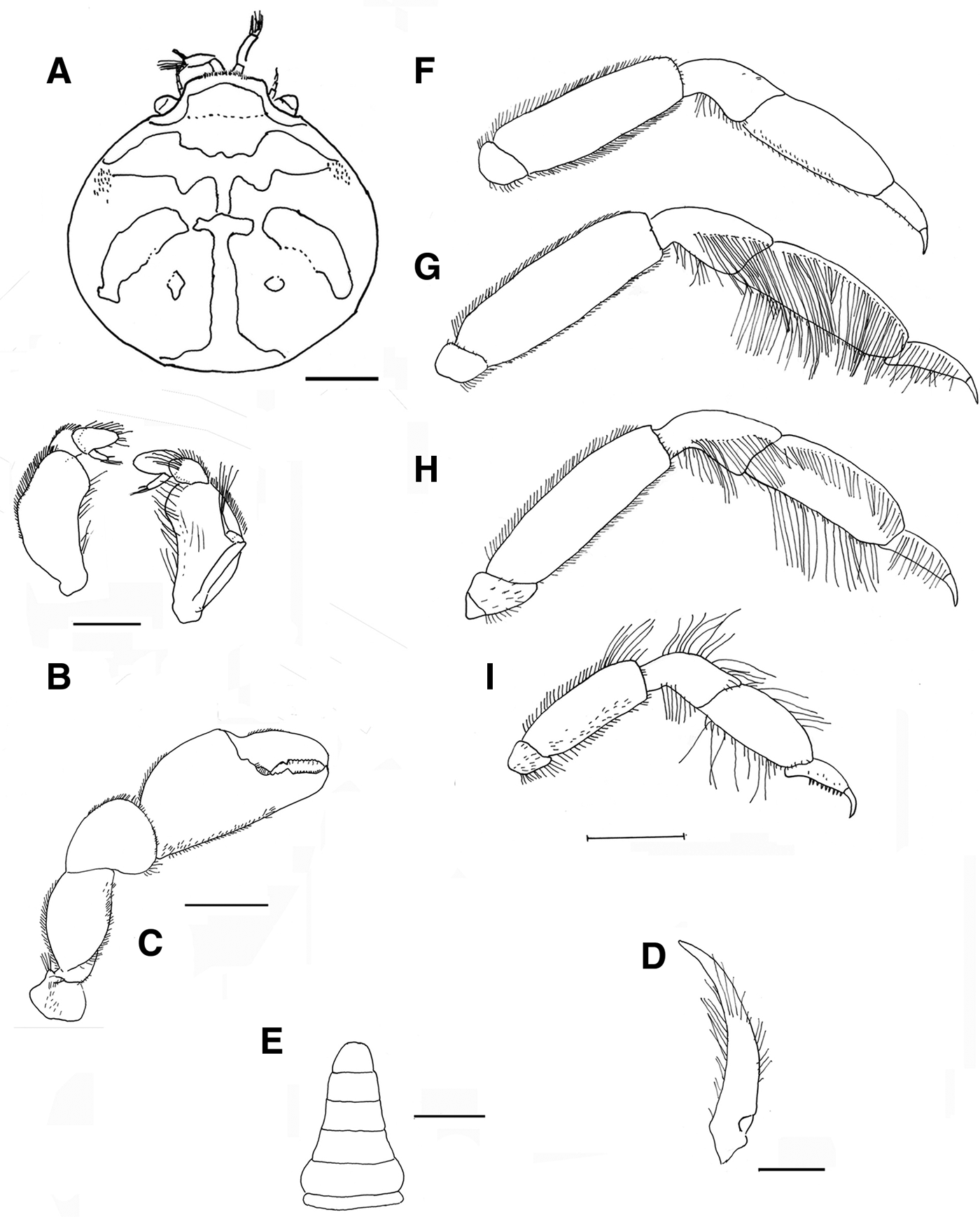
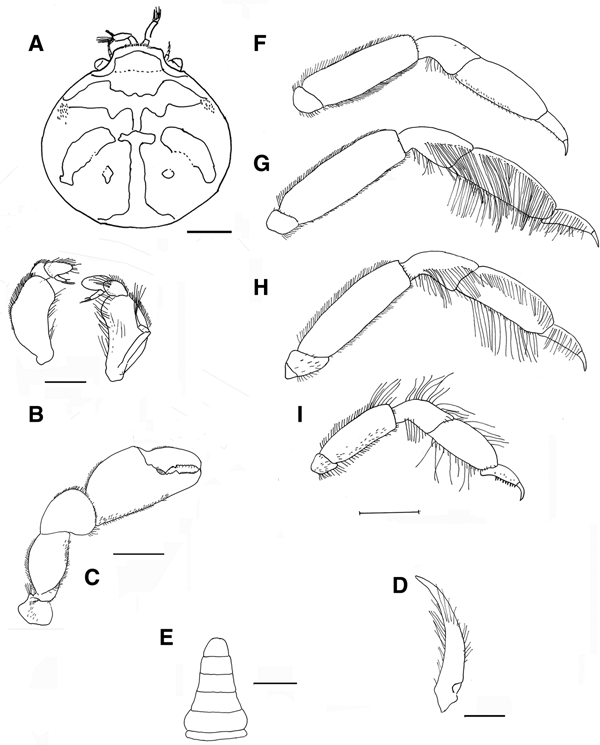
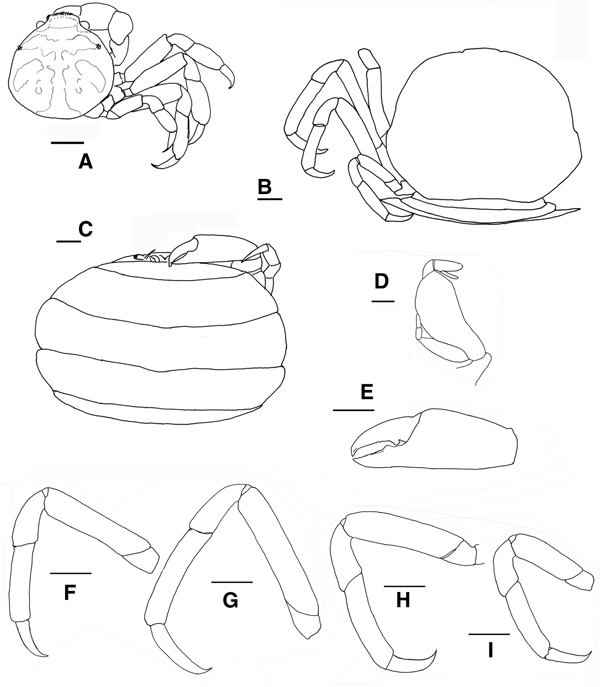
Fig. 5. Pinnotheres bicristatus sp. nov. Holotype male IEO-CD-ICMAN/2419 (4.4 mm carapace width). (A) Carapace, dorsal view and figure pattern; (B) third maxilliped, outer and inner views; (C) right cheliped, outer view; (D) male first gonopod; (E) pleon, outer view; (F–I) outer views of the second to fifth right pereiopods. Scale bars: (A, C), (E–I), 1.0 mm; (B, D), 0.5 mm.
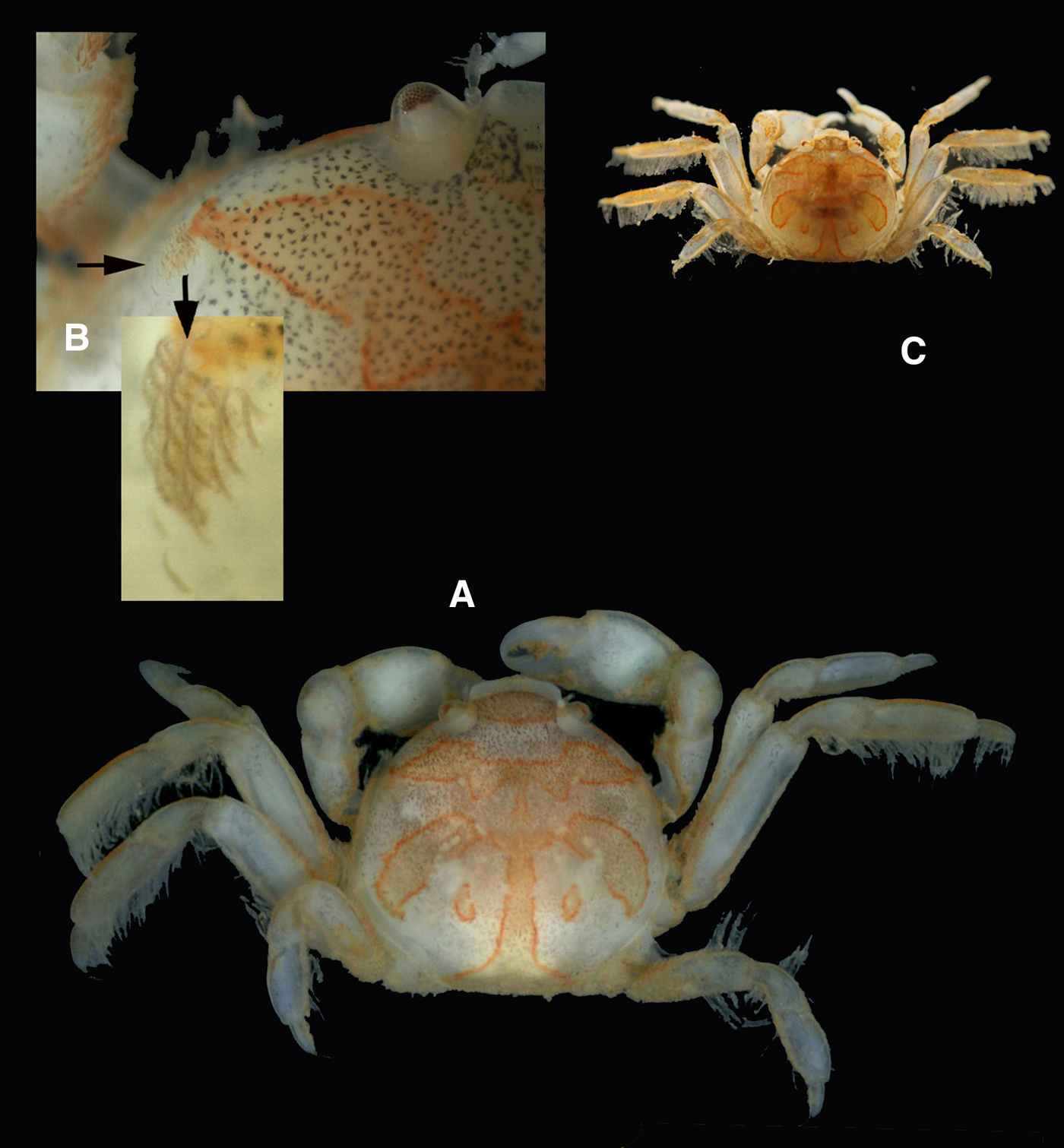
Fig. 6. Pinnotheres bicristatus sp. nov. Holotype male IEO-CD-ICMAN/2419 (A, C) dorsal view, showing colour pattern; (B) detail of dorsoanterolateral carapace region with tufts of short curved setae.
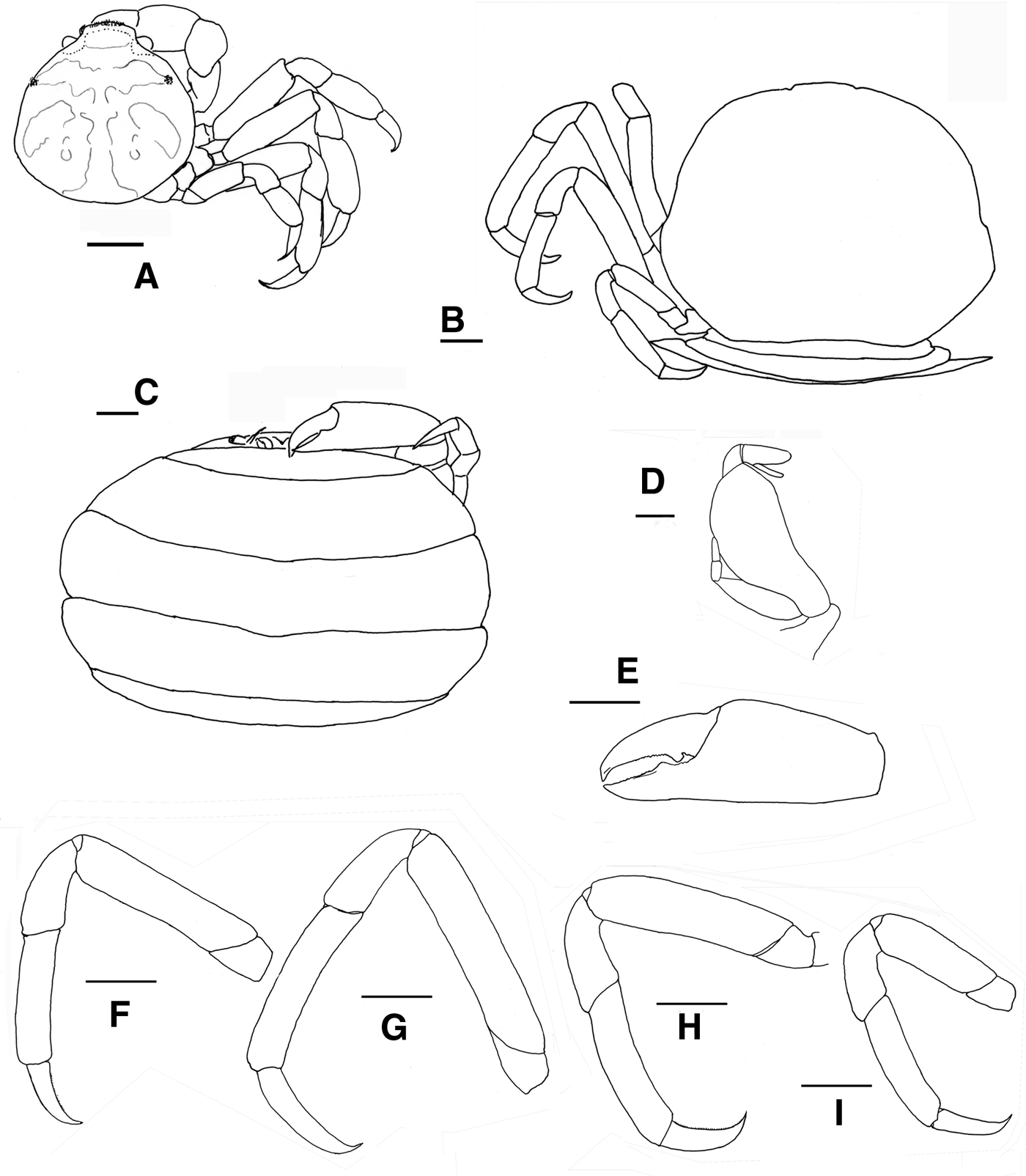
Fig. 7. Pinnotheres bicristatus sp. nov. (A) Paratype hard female (3.27 mm carapace width) IEO-CD-ICMAN/2420, dorsal view; (B–I) Paratype ovigerous female (8.2 mm carapace width), IEO-CD-ICMAN/2440, from Barbate (setae not represented): (B) carapace in dorsal view; (C) ventral view; (D) third right maxilliped (outer view); (E) chela of first left pereiopod (outer view); (F–I) outer view of second to fifth left pereiopod. Scale bars: (A–C), (E–I), 1.0 mm; (D), 0.5 mm.

Fig. 8. Pinnotheres bicristatus sp. nov. (A, B) Paratype hard female IEO-CD-ICMAN/2420, habitus: (A) dorsal view; (B) ventral view. (C, D) Paratype ovigerous female IEO-CD-ICMAN/2440, habitus, dorsal and ventral views (left and right respectively); (E) Paratype soft female (7.88 mm carapace width), IEO-CD-ICMAN/2428, from Gulf of Cadiz, in dorsal view (right) and detail of walking left legs in ventral view (left).
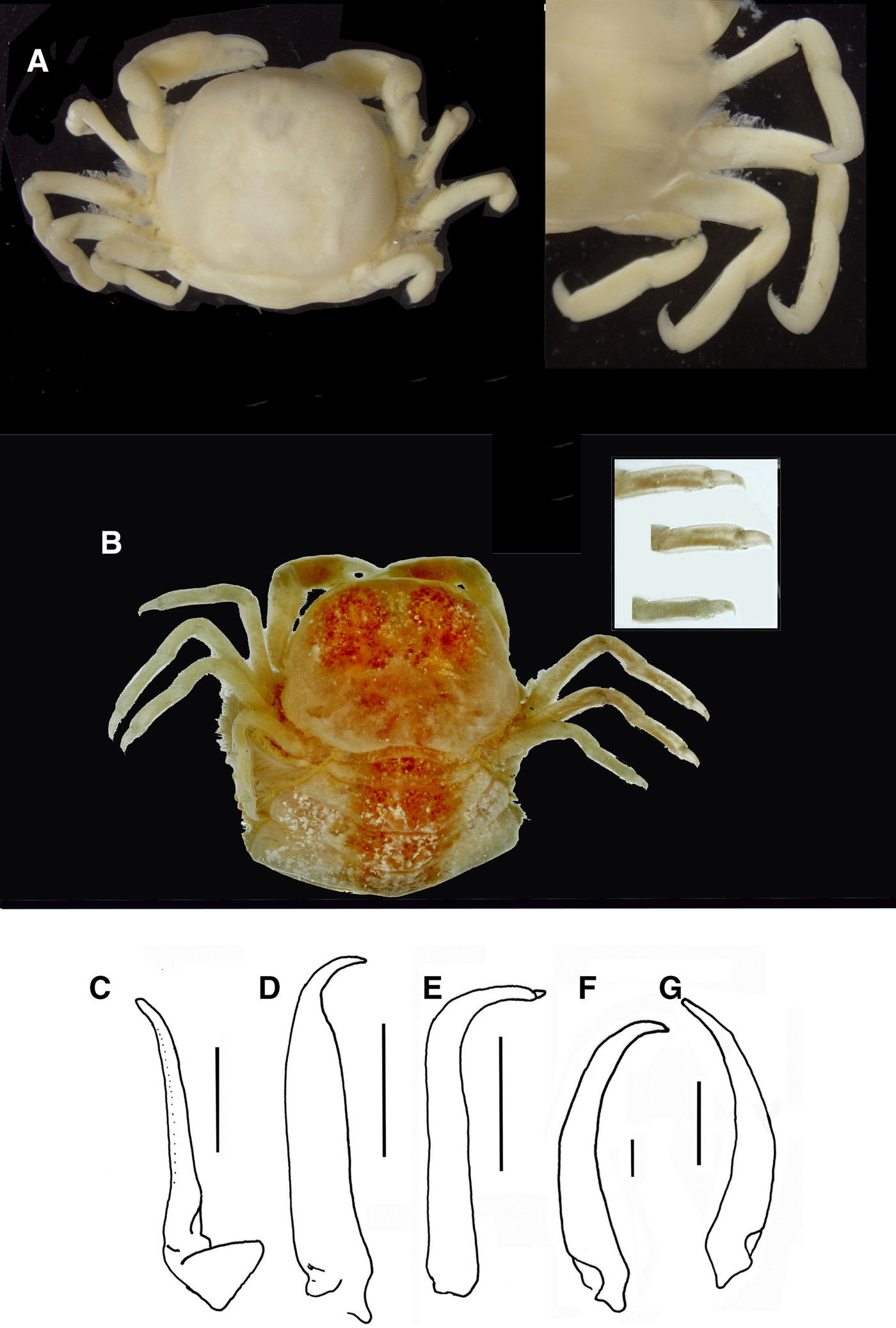
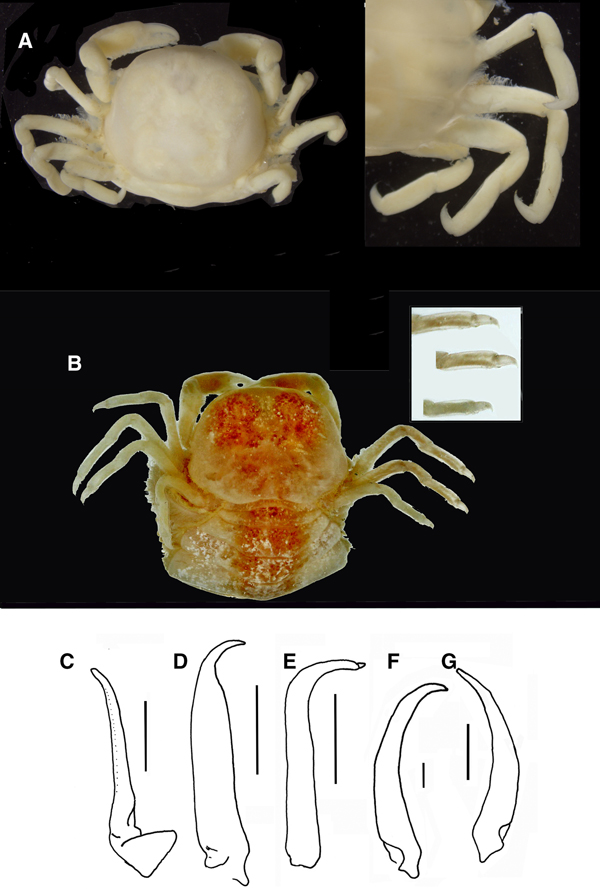
Fig. 9. Pinnotheres pectunculi: (A) soft female of San Roque, Cádiz, habitus in dorsal view (left) and details of left walking legs (right); (B) soft female from Marbella, Málaga, habitus in dorsal view and details of propodus and dactylus of right walking legs P3–P5. Schematic drawings of first gonopod of European pinnotherids (setae removed): (C) Afropinnotheres monodi (IEO-CD-ICMAN/2442, Gulf of Cadiz); (D) Pinnotheres pisum (from Barbate Cadiz, June1994, Manjón-Cabeza & García Raso, Reference Manjón-Cabeza and García-Raso1988); (E) Nepinnotheres pinnotheres (from Malaga, 1978 within Atrina pectinata); (F) Pinnotheres pectunculi (figure modified from Becker & Türkay, Reference Becker and Türkay2010); (G) Pinnotheres bicristatus sp. nov. (holotype IEO-CD-ICMAN/2419). Scale bars: (C–E), 1.0 mm; F (from Becker & Türkay, Reference Becker and Türkay2010), 200 µm; G, 0.5 mm.
Material examined
Holotype: ♂ (4.4 mm CW) (IEO-CD-ICMAN/2419, GenBank accession numbers MK415384, MK426944 and MK468907) in Anomia ephippium Linnaeus, 1758 (Bivalvia, Anomiidae), 36°34.1′N 6°26.6′W, Gulf of Cádiz, 23–27 m depth, detritic bottom, coll. Luis Silva (ARSA cruise 1117, 30 October 2017).
Paratypes: in Anomia ephippium Linnaeus, 1758 (Bivalvia, Anomiidae), 36°34.1′N 6°26.6′W, Gulf of Cádiz, 23–27 m depth, detritic bottom, coll. Luis Silva (ARSA cruise 1117, 30 October 2017): 1 ♀ (hard) (3.27 mm CW) (IEO-CD-ICMAN/2420), 1♂ and 1♀ (soft) (3.8 and 7.5 mm CW) (IEO-CD-ICMAN/2421–2422), 1 ♀ (soft) (7.35 mm CW) (IEO-CD-ICMAN/2423); 1 ♀ (soft) (7.8 mm CW) (IEO-CD-ICMAN/2424), 1 ♀ (soft) (7.50 mm CW) (IEO-CD-ICMAN/2425), 1 ♂ and 1 ♀ (soft) (3.8 and 5.08 mm CW) (IEO-CD-ICMAN/2426–2427), 1 ♀ (7.88 mm CW) (IEO-CD-ICMAN/2428), 1 ♀ (soft) (8.03 mm CW) (IEO-CD-ICMAN/2429), 1 ♀ (soft) (6.89 mm CW) (IEO-CD-ICMAN/2430), 1 ♀(soft) (8.26 mm CW) (IEO-CD-ICMAN/2431), 1 ♀ (soft) (5.68 mm CW) (IEO-CD-ICMAN/2432), 1 ♀ (soft) (7.42 mm CW) (IEO-CD-ICMAN/2433), 1 ♂ (3.6 mm CW) (IEO-CD-ICMAN/2434), 1 ♀ (soft) (5.76 mm CW) (IEO-CD-ICMAN/2435), 1 ♂ (3.8 mm CW) (IEO-CD-ICMAN/2436). In Anomia ephippium, 36°34.8′N 6°27.5′W, Gulf of Cádiz, Spain, 28–30 m depth, detritic bottom, coll. Luis Silva (ARSA expedition 0318, 25 February 2018): 1♀ (hard) (2.59 mm CW) (IEO-CD-ICMAN/2437), 1 ♂ and 1 ♀ (hard) (3.6 and 2.9 mm CW) (IEO-CD-ICMAN/2438–2439). In Barbate offshore, 36°09.7′N 5°53.6′W, free in bivalve samples of detritic bottom, 15–18 m depth, 22 July 1994, coll. Manjón-Cabeza and García Raso (1988, as Pinnotheres sp.): 1 ovigerous ♀ (8.2 mm CW) (IEO-CD-ICMAN/2440, GenBank accession numbers MK426940 and MK468903). In Anomia ephippium, Barbate offshore, 36°09.7′N 5°53.6′W, detritic bottom, 15–18 m depth, 17 March 1995, coll. García Raso and Manjón Cabeza (2002, as Pinnotheres pisum): 1 ♀ (soft) (6.5 mm CW) (IEO-CD-ICMAN/2441). In Anomia ephippium, Estepona offshore, 36°20.3′N 5°12.9′W, 47 m depth, coll. Pere Abelló (MEDITS_ES 2017, 25 April 2017): 1 ♂ (3.9 mm CW) (ICMD002583, GenBank accession numbers MK415382, MK426942 and MK468905), 1 ♀ (soft) (7.1 mm CW) (ICMD002584, GenBank accession numbers MK415381, MK426941 and MK468904), 1 ovigerous ♀ (7.2 mm CW) (ICMD002585, GenBank accession numbers MK415383, MK426943 and MK468906).
Description of male
Carapace (Figures 5A & 6A–C) circular (CL/CW = 0.99). Minimum and maximum width: 3.64 and 4.4 mm, respectively. Frontal margin projected anteriorly with dense brush of short setae medially. Dorsoanterolaterally, on both sides of carapace, characteristic dense group of short and thick setae (more than 30) present, superficially resembling tubercles (Figure 6B). Eyes clearly visible in dorsal view. Antenna short, with large basal segment followed by 4 segments, and with 2 or 3 terminal setae on distal segment.
Third maxilliped (Figure 5B) with ischium and merus indistinguishably fused, inner, outer margins with distal obtuse angle; palp 3-segmented with tuft of long simple setae from tip; propodus longer than carpus, dactylus inserted underneath propodus, on proximal half of ventral margin of propodus, apex not reaching end of propodus. First thoracic sternite with anterior border concave.
Chelipeds (Figures 5C & 6A) similar. Palm (pal) slightly longer than dactylus (d) (pal d−1 = 1.05), relationship length/width of palm = 1.1. Dactylus with well-developed tooth basally, smaller tooth present anteriorly; cutting edge of fixed finger of propodus with large tooth (in anterior position to that of mobile finger), with about 15 short thick setae between apex of tooth. Tips of fingers curved.
Walking legs (Figure 5F–I, pereiopods 2–5) slender. P3, P4 longer than P2, P5. P3 slightly longer than P4. Relative length P3 > P4 > P2 > P5. Merus of P2–P5 with short setae on dorsal and ventral sides. P3 and P4 with long swimming setae, in carpus (on lateral outer side), propodus and dactylus (on dorsolateral outer, and ventral sides), and some on merus of P5 (dorsodistal and ventral parts), carpus and propodus (dorsal and ventral parts). Without long swimming setae on P2 (Figure 5F). Relative lengths of merus are: P3 > P4 > P2 > P5; relationships length/width of merus leg P2 to P5 are 3.1, 3.1, 3.6 and 2.6 respectively. Proportion of length of merus/length of dactylus of P2 to P5 are 2.7, 2.4, 2.4 and 2.0 respectively. Propodus relative lengths are: P3 > P4 > P2 > P5. Dactylus of P2–P5 almost straight, with long curved distal part (claw), with small spiniform setae on ventral concave faces, longer in P5. Dactylus of P3 and P4 longer than others, subequal in length, P3 ≥ P4 > P2 > P5 (Figure 5F–I).
Male pleopod curving regularly, with apex narrowing progressively from base to tip (Figure 5D), with abundant setae from base to apex, especially on concave face.
Pleon (Figure 5E) triangular, gradually narrowing, with 6 somites and telson all free with rectilinear distal margin.
Description of hard female
These small females have long swimming setae on their pereiopods, similar to those of males. The general morphology is also very similar to that of males; the carapace is circular, with the same characteristic dorsoanterolateral tufts of short setae (Figures 7A & 8A). Also, the morphology of chelipeds is similar to that of males. Relative length of walking legs is: P3 > P4 > P2 > P5. The dactyli of P3 and P4 are the longest and both are of similar lengths. The length/width relationships of the propodus (P2–P5) are: 2.18, 2.42, 2.33 and 1.73. Propodus/dactylus length relationships (P2–P5) are: 1.60, 1.45, 1.56 and 1.33. Pleon only slightly wider than that of adult males (Figure 8B).
Description of soft females
Carapace (Figures 7B & 8C, E) subtrapezoidal, wider than long (CL/CW = 0.76 to 0.81). Minimum and maximum width of carapace: 5.08 and 8.2 mm, respectively. Dorsal surface smooth, without dorsoanterolateral tufts of short setae. Front almost rectilinear, not projected, without setae (slightly convex in smallest soft females, AR-156: 5.08 mm CW). Small eyes, hardly visible in dorsal view. Antenna short, with large basal segment followed by 4 segments, and with 2 or 3 terminal setae on distal segment.
Third maxilliped (Figure 7D) obliquely positioned on buccal cavity, not different from P. pisum (see Figure 5C in Becker & Türkay, Reference Becker and Türkay2010), ischium and merus indistinguishably fused, inner, outer margins with distal obtuse angle; palp 3-segmented with tuft of long simple setae at tip; propodus longer than carpus; dactylus inserted underneath propodus, almost subterminal, at or about half length of ventral margin of propodus, apex reaches to or does not reach end of propodus.
Chelipeds (Figure 7E) similar. Palm longer than dactylus (pal d−1 = 1.2–1.3), relationship length/width of palm = 1.2–1.3 (narrower than that of males and hard females). Dactylus with well-developed tooth basally, another on cutting edge of fixed finger of propodus.
Walking legs (Figures 7F–I) slender. P3 longer than rest. Total relative length is: P3 > P4 > P2 > P5. Merus relative length: P3 > P4 ≈ P2 > P5. Length/width relationships of merus (P2–P5) are 4.8, 5.1, 4.1 and 3.3 (P3 > P2 > P4 > P5), respectively. Propodus relative lengths are: P3 > P4 > P2 > P5. Dactylus of P2–P5 almost straight with curved distal part, with continuous row of tiny spines on ventral face of P2 and P4 (not in P3 and P5). Dactylus of P3 longer than rest (P3 > P4 > P2 > P5) (Figures 7B, G & 8C–E); more clearly in large specimens (Figures 7B, G & 8C, D). Dactylus of P5 narrower than on others. Relationships between merus (m), propodus (p) and dactylus (d) lengths of each P2 to P5 are as follow: m d−1 = 2.5, 2.0, 2.3 and 1.6; p d−1 = 1.5, 1.4, 1.7 and 1.5.
Pleon, with 6 somites, very broad (Figures 7C & 8D); telson well separated with rectilinear distal margin, covering almost completely ventral side, even covering almost completely the third maxillipeds (in large females), with setose margin and smooth outer surface.
Etymology: The specific name bicristatus is an adjective (gender masculine as Pinnotheres) referring to the two ‘crests’ or dense tufts of setae located dorsoanterolaterally on the carapace of males and hard females.
Habitat, host: All specimens collected were found inside Anomia ephippium collected between 11 and 47 m depth, on detritic bottoms.
Colouration in life: Males and hard females carapace with yellow-reddish colour with dark spots and with a characteristic pattern; it resembles a palm tree (Figures 6A & 8A), with a well-defined central ‘trunk’ and two pairs of lateral projections as ‘leaves’, the posterior ones separated from this ‘trunk’. Behind, there are two well-defined rounded areas. The whole outline of this figure is reddish. Soft females with a pale-yellow colour: when females present developed gonads, they are reddish.
Discussion
Morphology
Taxonomically, within the genus Pinnotheres the species richness is high, with a total of 52 species (WoRMS 2018) or 61 species (Palacios Theil et al., Reference Palacios Theil, Cuesta and Felder2016), but only two species are known in the area studied: P. pisum and P. pectunculi. In the past this genus was the most specious of the Pinnotheridae, but after several studies, especially those of Manning (Reference Manning1993a, Reference Manning1993b) and Campos (Reference Campos1989, Reference Campos1993, Reference Campos2002), new genera have been recognised for many former Pinnotheres species. In contrast, no new species of Pinnotheres s. str. have been described since Pinnotheres taichungae Sakai, Reference Sakai2000.
Morphological differences with other European pinnotherids
In European waters pinnotherid species have been known since Greek and Roman times (see Becker, Reference Becker2010), although no descriptions of species were made until the 1700s. According to Ng et al. (Reference Ng, Guinot and Davie2008) there are several old studies that were overlooked in the first description of Pinnotheres pisum (as Cancer pisum) by Linnaeus (Reference Linnaeus1767) and subsequent studies. Scopoli (Reference Scopoli1763) described Cancer nutrix as a crab living inside Ostrea edulis from the Adriatic Sea. As Ng et al. (Reference Ng, Guinot and Davie2008) pointed out, de Wulfen (Reference de Wulfen1791) also described, from the Adriatic coasts, Cancer minutus, a species very similar to Cancer nutrix and living inside O. edulis, but also in other bivalves. According to the very brief descriptions given by these authors and considering that there are no type specimens extant available for checking, we believe that both taxa belong to P. pisum, mainly because this species is present in that part of the Mediterranean Sea and is known to inhabit several different species of hosts, including O. edulis. At present, the complete distribution of Pinnotheres bicristatus sp. nov. is unknown and it cannot be excluded that this new species could be one of those described by Scopoli (Reference Scopoli1763) and de Wulfen (Reference de Wulfen1791). We have, however, found only P. bicristatus sp. nov. inside Anomia ephippium, and not in any other bivalve species.
Assuming that C. nutrix can be assigned to P. pisum, and that Scopoli's description in 1763 has priority with respect to that of Linnaeus (Reference Linnaeus1767), the correct name for P. pisum would be Pinnotheres nutrix (Scopoli, Reference Scopoli1763). Prior to being more conclusive, as suggested by Ng et al. (Reference Ng, Guinot and Davie2008), it could be necessary to assign neotypes to these species by collecting fresh specimens hosted by O. edulis from the Adriatic Sea and also take into account that Ŝtevčić (Reference Ŝtevčić1990) also mentioned N. pinnotheres inside Ostrea edulis in this region. This is, however, beyond the scope of the present study.
Two other species have been described from European waters, Pinnotheres ascidicola Hesse, Reference Hesse1872 and P. marioni Gourret, Reference Gourret1888. Recently, Becker & Türkay (Reference Becker and Türkay2010) made a detailed study of European pinnotherids and concluded that both species are junior synonyms of N. pinnotheres, based on the original descriptions and figures (because there is no type material extant) and together with the fact that the hosts of both species are ascidians. Based on the same evidence and the previous paper by Gourret (Reference Gourret1887), we have also concluded that they are junior synonyms of N. pinnotheres, and we are confident neither can be P. bicristatus sp. nov.
When comparing P. bicristatus sp. nov. with the recognized species of European pinnotherids, males and hard females from all those species can be easily differentiated by two peculiar characteristics on the cephalothorax: (a) a pair of dorsoanterolateral tufts of curved setae (short and very close to each other) that resemble two tubercles (Figures 5A, 6B & 7A), not described in the previous known European species or in other species for which we have evidence; and (b) the orange-reddish ‘palm tree’ marking covering the dorsal surface (Figures 5A, 6A, C, 7A & 8A). Concerning this latter feature, males of P. pisum show a somewhat similar, although different, marking in which the ‘trunk of the palm tree’ is missing and the colour is yellow-orangish (see pictures in the Crustikon – Crustacean photographic website – Tromsø Museum, University of Tromsø, http://archive.li/rHrj6, by Cédric d'Udekem d'Acoz; and Figure 1B in Becker & Türkay, Reference Becker and Türkay2010).
Pinnotheres bicristatus sp. nov. can be differentiated from the African species Afropinnotheres monodi by the morphology of the third maxilliped: in A. monodi the propodus is shorter than the carpus (this is not the case in the genus Pinnotheres and Nepinnotheres), and the dactylus extends well beyond the end of the propodus (see A. monodi Figure 1A, in Manning, Reference Manning1993a), while these features are not present in the rest of European species (at most, the dactylus extends only very slightly in N. pinnotheres; see Figure 3C in Becker & Türkay, Reference Becker and Türkay2010). Also, in A. monodi, the dorsal surface of the carapace in males and hard females is finely pubescent.
Soft females of the new species and those of Nepinnotheres pinnotheres can be differentiated easily by the apex of the dactylus of the third maxilliped, which reaches or exceeds the distal part of the propodus in N. pinnotheres (see Figure 24B in Manning, Reference Manning1993a; Figure 3C in Becker & Türkay, Reference Becker and Türkay2010), but not in P. bicristatus sp. nov. (Figure 7D). The carapace morphology is subglobular, with width greater than length, in N. pinnotheres, whereas it is subtrapezoidal in P. bicristatus. However, both species possess a broad triangular tooth at about mid-length of the fixed finger of the chelipeds (which does not exist, or else is very small, in P. pisum), and the dactylus lengths of P5 are more than half the length of their propodus. However, in the new species, the dactylus of P3 is clearly longer than those of the rest of its walking legs, a difference especially pronounced in large females (Figures 7B, G & 8C, D).
Soft females of Pinnotheres pectunculi have a subtrapezoidal carapace with an approximately rectilinear front that is not projected (see Figure 25 in d'Udekem d'Acoz, 1989 and Figure 9A, B in the present work) as in P. bicristatus sp. nov. (Figures 7B & 8C, E) (in N. pinnotheres the frontal area is a little projected, bilobed by a median incision, whereas in P. pisum it is slightly rounded). They can also be easily distinguished by the dactyli of the walking legs, which are very different. Thus, in P. pectunculi they are very short (d'Udekem d'Acoz, 1989) (Figure 9A, B) (even shorter than those of P. pisum), while they are long in the new species, especially in large soft females, whose legs are longer, and thinner (stylized) than those of hard individuals.
With regards to morphology, Pinnotheres pisum is the species closest to P. bicristatus sp. nov. They can be separated by the relative length of the dactyli of the walking legs; these are shorter (clearly less than half as long as the propodus) and more curved in P. pisum (see Figure 4A in Becker & Türkay, Reference Becker and Türkay2010 and Figures 7B, F–I & 8C–E in present study). In addition, in soft females of P. pisum, the dactyli are of almost equal length on all walking legs (see Figure 5 in Becker & Türkay, Reference Becker and Türkay2010), while in the new species the dactyli is clearly longer on the third pereiopod (Figure 7G). In addition, the carapace in P. pisum is subglobular (see Figure 5 in Becker & Türkay, Reference Becker and Türkay2010), not subtrapezoidal as in the new species (Figures 7B & 8C, E).
Identification key for European Pinnotheridae
1. Mxp3 with propodus conical, tapering distally, shorter than carpus. Dactylus inserted at base of ventral margin of propodus, overreaching end of propodus Afropinnotheres monodi
Mxp3 with propodus oval or spatulate, longer than carpus. Dactylus inserted at mid-length or at base of ventral margin of propodus but apex not extending to end of propodus2
2. Carapace of hard specimens (males and females) with dorsoanterolateral tuft of setae. Soft females with subtrapezoidal carapace, dactylus of P3 longer than others, dactylus of P5 narrower, with a relation propodus/dactylus length of 1.5Pinnotheres bicristatus sp. nov.
Carapace of hard specimens (males and females) without dorsoanterolateral tuft of setae. Soft females with subtrapezoidal or subglobular-rounded carapace, dactylus of P2-P5 more or less similar in length3
3. Carapace and pereiopods covering with tomentum. P2–P5 with long dactylus, that of P5 slightly longer than others, approximately as long as three-quarters of propodus. Dactylus of Mxp3 styliform or spatulate, inserted at or near midlength of ventral margin of propodus, apex extending to or overreaching end of propodus Nepinnotheres pinnotheres
Carapace and pereiopods smooth. P2–P5 with short dactylus. Dactylus of Mxp3 styliform, inserted at base of ventral margin of propodus, apex not extending to end of propodus 4
4. Carapace of soft females subglobular-rounded. Eyes visible on dorsal view. Dactylus of walking legs (P2–P5) with short, curved tips, less than half as long as propodus. Fixed finger of chela (propodus of P1) with single tooth Pinnotheres pisum
Carapace of soft females subtrapezoidal. Small eyes. Walking legs (P2–P5) slender with short, pointed, curved dactylus, considerably less than half as long as propodus. Fixed finger of chela (propodus of P1) with a well-developed basal tooth followed by additional small onesPinnotheres pectunculi
All males of the European pinnotherid species can be distinguished by the morphology of the first gonopod (see Figure 9C–G). Each first gonopod has a different degree of curvature, from that of A. monodi, which is practically straight and only lightly curved at the apex (Figure 9C) to that of N. pinnotheres, which is almost L-shaped (Figure 9E); in Pinnotheres spp., it has an intermediate shape. The first gonopod of P. pisum is straight and wide over almost its total length, but its distal part is narrowed and curved (Figure 9D), while in P. pectunculi and P. bicristatus sp. nov. the gonopod is curved along its full length; however, the curvature of gonopod is more pronounced in P. pectunculi (Figures 6C, D in Becker & Türkay, Reference Becker and Türkay2010, Figure 9F in present study) than in P. bicristatus (Figure 9G).
The first gonopod of A. monodi was not described when the species was described by Manning (Reference Manning1993a), because the only male specimen lacked these appendages; therefore, this is the first time that the gonopod of A. monodi has been illustrated (Figure 9C).
About the specific host and associated pinnotherid species
Until now, only four species of pinnotherid crabs have been reported in association with bivalves of the genus Anomia (see Schmitt et al., Reference Schmitt, McCain, Davidson, Gruner and Holthuis1973). Pinnotheres gordoni Shen, Reference Shen1932, with distribution in China and Japan (Silas & Alagarswami, Reference Silas and Alagarswami1967), has been reported as living inside Anomia cytaeum Gray, 1850 (syn. Anomia lischkei Dautzenberg & Fischer, 1907), which has a general distribution in the waters of China, Japan and India (Higo et al., Reference Higo, Callomon and Goto1999; Souji & Radhakrishna, Reference Souji and Radhakrishna2015). According to the description by Shen (Reference Shen1932), P. gordoni has pubescent chelipeds and walking legs, P4 being the longest pereiopod, while the dactylus of P5 is subequal to the propodus of the same leg. In these characters, it is clearly different from P. bicristatus.
Two pinnotherid crabs from West Atlantic waters, namely Tumidotheres maculatus (Say, 1818), known to occur from Massachusetts to Argentina (Kruczynski, Reference Kruczynski1974), and Zaops ostreus (Say, 1817), present from Massachusetts to Cuba, Guadalupe and Brazil (Palacios et al., Reference Palacios Theil, Cuesta and Felder2016), have been found inside Anomia simplex d'Orbigny 1853. This bivalve has a general distribution ranging from Nova Scotia to Mexico and the Caribbean (Rosenberg, Reference Rosenberg2009). Given that T. maculatus and Z. ostreus belong to different genera from that of Pinnotheres bicristatus sp. nov., the differences are major (see Campos, Reference Campos1989; Manning, Reference Manning1993a, Reference Manning1993b). Among these differences to be noted are the size of the dactylus of P5, which is longer than the dactyli of P2–P4 in T. maculatus and Z. ostreus; in P. bicristatus sp. nov., P3 has the longest dactylus.
Finally, the fourth species reported as being hosted by Anomia is the European Pinnotheres pisum, which has a general distribution from Norway to the Mediterranean Sea and Canary Islands (Triay-Portella et al., Reference Triay-Portella, Pérez-Miguel, González and Cuesta2018), and has been recorded twice in Anomia ephippium (Miranda y Rivera, Reference Miranda y Rivera1921; Delongueville & Scaillet, Reference Delongueville and Scaillet2002). This bivalve is distributed from Norway to Morocco, and Angola, including also the Mediterranean Sea (MolluscaBase 2018, accessed through WoRMS). The oldest record in A. ephippium is one by Miranda y Rivera (Reference Miranda y Rivera1921), from Malaga (Alboran Sea) at 30 m depth, which mentioned the presence of two females of P. pisum hosted in A. ephippium, and a second (and the most recent known to the authors) is one female reported in Rota (Gulf of Cádiz) by Delongueville & Scaillet (Reference Delongueville and Scaillet2002). Both locations are within the area sampled in the present study. Unfortunately, we cannot confirm these identifications because the two specimens from Malaga (Miranda y Rivera, Reference Miranda y Rivera1921) no longer exist in the collection where they were deposited (IEO), and the photograph of the female from Rota in Delongueville & Scaillet (Reference Delongueville and Scaillet2002) does not allow a correct identification. However, we have analysed a large amount of material of A. ephippium from the Gulf of Cádiz and Málaga and the only pea crab found living in association with this bivalve is Pinnotheres bicristatus sp. nov. On this evidence, it is very likely that the records of P. pisum in A. ephippium by Miranda y Rivera (Reference Miranda y Rivera1921) and Delongueville & Scaillet (Reference Delongueville and Scaillet2002) are actually P. bicristatus sp. nov. The present new crab species probably has a wider distribution throughout European and African waters (North-east Atlantic and Mediterranean Sea), occurring wherever A. ephippium is found.
With these two possible exceptions of Miranda y Rivera (Reference Miranda y Rivera1921) and Delongueville & Scaillet (Reference Delongueville and Scaillet2002), Pinnotheres bicristatus sp. nov. had not been previously recorded in European waters. This oversight of its presence could be explained if P. bicristatus sp. nov. is not native to the region of study and its introduction into European A. ephippium had occurred recently. However, the relative high abundance of P. bicristatus in A. ephippium from the Gulf of Cadiz and the distribution range presently known support instead the hypothesis that this new pinnotherid is a native species, whose presence has been omitted because its host A. ephippium, which is not a commercial species, has been rarely collected and, consequently, has not been well studied. Besides, morphological and molecular results of this study suggest that P. bicristatus sp. nov. is a native species since it is closely related to P. pisum and P. pectunculi: it has been shown that the geographic distribution of species plays an important role in their phylogenetic relationships (Cuesta et al., Reference Cuesta, Drake, Martínez-Rodríguez, Rodríguez and Schubart2012), and it would be more difficult to find this molecular proximity between this new pinnotherid and the other two native species if the former was an introduced exotic species. For this reason, molecular data would support an Atlantic-Mediterranean origin of P. bicristatus sp. nov.
Pinnotheres bicristatus sp. nov. population hosted by Anomia ephippium
The high prevalence of Pinnotheres bicristatus sp. nov. in the common saddle oyster Anomia ephippium populations of the Gulf of Cádiz suggests that this crab forms a well-established population in the area. Although the smallest sex-indeterminate individuals of P. bicristatus were not found inside A. ephippium, the presence of at least 20% of males, together with hard and reproductive females, in this host makes it possible that a stable population of this crab exists even without any additional host. For other pinnotherid species, sex ratios biased towards a lower proportion of males have been explained by possible predation when males leave their hosts to find mates (Trottier & Jeff, Reference Trottier and Jeffs2012). However, it is also plausible that other bivalve species were facultative hosts of males and of smaller specimens that were not found inside A. ephippium, as is the case for Afropinnotheres monodi found in the same area (Drake et al., Reference Drake, Marco-Herrero, Subida, Arias and Cuesta2014; Pérez-Miguel et al., Reference Pérez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019). The mussel Mytilus galloprovincialis is the primary host of the soft (reproductive) females of A. monodi, whereas other bivalves of small size (such as Cerastoderma spp. and Scrobicularia plana) are the primary hosts of hard females and males. Notwithstanding, about 15.8% of A. monodi crabs hosted by mussels were males (Drake et al., Reference Drake, Marco-Herrero, Subida, Arias and Cuesta2014; Pérez-Miguel et al., Reference Pérez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019). If males and hard females of P. bicristatus were using another bivalve species as primary host, it would probably be the case that this unknown host species is also subtidal, since we did not collect any specimens of this crab (Pérez-Miguel et al., Reference Pérez-Miguel, Cuesta, Navas, García Raso and Drake2018) during an exhaustive sampling of intertidal bivalves along the coasts of the Iberian Peninsula in 2016 and 2017.
Since the timing and duration of reproductive periods is influenced by water temperature and/or the light-dark cycle (Sastry, Reference Sastry and Vernberg1983), it is reasonable to assume that Pinnotheres bicristatus sp. nov. has one single reproductive period throughout the known distribution area (Figure 1). Currently, the available information on this topic is the presence of larval stages (zoeae and megalopae) found in November 2013 (Marco-Herrero et al., Reference Marco-Herrero, Drake and Cuesta2018), one ovigerous female found in July 1995 in the Gulf of Cádiz, and one ovigerous female found in April 2017 in the Alboran Sea (this study). According to this information, P. bicristatus sp. nov. would have a long reproductive period extending from spring to autumn. Furthermore, there was a clear predominance of reproductive females with well-developed gonads both in October 2017 and in February 2018, whereas ovigerous females were absent, suggesting that a slowdown of the final gonadal development occurred during the cold period and that spawning does not take place until the water temperature starts to rise. Concerning crab pairs, there was a remarkable increase between October 2017 (3.3% of hosts infested) and February 2018 (26.7% infested). A positive relationship between crab prevalence and the proportion of multiple infested hosts has been found in other pinnotherid crabs (Asama & Yamaoka, Reference Asama and Yamaoka2009; Mena et al., Reference Mena, Salas-Moya and Wehrtmann2014; Pérez-Miguel et al., Reference Pérez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019). Thus, it is not possible to reject the view that the increased multiple infestations may be partially associated with the increase in the proportion of hosts infested by P. bicristatus sp. nov. observed between October 2017 and February 2018: on average, 55.6 vs 77.7% of host specimens infested, respectively. However, since heterosexual pairs were collected more frequently than expected by chance, a more probable explanation of the increase of male-female pairs inside a single host in February was the increase of encounters for mating just before starting the spring spawning period. Mating that takes place inside the hosts, where soft female crabs remain stationary, and with males visiting various bivalves hosting soft females, has been suggested for other pinnotherid species (Soong, Reference Soong1997; Ocampo et al., Reference Ocampo, Nuñez, Cledón and Baeza2012).
Females of Pinnotheres bicristatus reached larger sizes than males, a sexual dimorphism that is very common among pea crabs (e.g. see Soong, Reference Soong1997; Ocampo et al., Reference Ocampo, Nuñez, Cledón and Baeza2012; Drake et al., Reference Drake, Marco-Herrero, Subida, Arias and Cuesta2014). Concerning the relationship between host size and crab size, a strong correlation was found between Anomia ephippium size and ovigerous female size of P. bicristatus sp. nov.; this suggests that availability of space inside a host is a relevant factor in determining the final size of the crab in its sedentary phase (i.e. soft females). Although a significant correlation between host size and male crab size was also found in the population studied, the correlation was weaker than for females. Furthermore, for each specific host size, male crabs were smaller than females hosted by it (Figure 3B). A similar sex-dependent pattern in the relationship between host size and the symbiotic/parasitic crab size has been found previously for other pinnotherid species (Soong, Reference Soong1997; Kane & Farley, Reference Kane and Farley2006; Ocampo et al., Reference Ocampo, Nuñez, Cledón and Baeza2012; Drake et al., Reference Drake, Marco-Herrero, Subida, Arias and Cuesta2014). As stated by Soong (Reference Soong1997), there is a closer correlation in females than in males, between crab size and host size; this may be due to the longer association between a female crab and its host (females stay longer in the same host than males do). Considering all the information available, we hypothesize that, initially, P. bicristatus sp. nov. hard females do not make their selection of host by size (there is no correlation between the size of a hard female and that of its host). However, after their metamorphosis to soft females, they cannot leave the host and their growth might be limited by the host size, which explains the observed strong correlation in size between soft females of P. bicristatus sp. nov. and the specimens of A. ephippium hosting them. In the case of the smaller and more motile males, they might not be limited by host size but, as they may change hosts frequently, larger males may avoid relatively small (growth-limiting) hosts and this behaviour could explain the significant but low correlation in size found between hosts and male crabs.
Final remark
As previously commented, Pinnotheres bicristatus sp. nov. probably has a wider distribution throughout European and African waters linked to the occurrence of its host Anomia ephippium. However, due to the scarce studies on this bivalve species, the existence of P. bicristatus sp. nov. could have been overlooked until now. Therefore, we would like to underline the importance of studying the plankton-collected larvae in combination with DNA analyses, to discover, as in the present case, hidden cryptic new species or non-indigenous species introduced in new areas.
Author ORCIDs
Jose A. Cuesta, 0000-0001-9482-2336; J. E. García Raso, 0000-0003-3092-9518
Acknowledgements
We are grateful to all participants in the ARSA and MEDITS surveys run by the Spanish Institute of Oceanography (IEO), from which many of the samples come, particularly to their chief scientists (Ignacio Sobrino and Cristina García). We want to thank Carlos Sánchez (ICMAN) for laboratory work. Thank are also due to Maria Eugenia Manjón-Cabeza for sharing specimens collected in Barbate samples. We want to thank two anonymous referees, and especially to Peter Ng for his detailed corrections, comments and suggestions that clearly improved the manuscript, and Royston Snart for the linguistic revision of the English text.
Financial support
This study was partially funded by the Spanish ‘Ministerio de Economía y Competitividad (MINECO), Plan Nacional I + D’ and the European FEDER funds through project AFROBIV (CGL2014-53557-P), and the Project PB92-0415, supported by the Spanish M.E.C, DGICYT funds.