Introduction
Despite a decrease in the incidence rates and number of tuberculosis (TB) cases in the past 5 years, London still has the biggest burden of TB in England, accounting for 39% of all cases in England in 2016; an incidence rate of 25 per 100 000 population. In the same year there were significant variations among London boroughs, with rates ranging between 5 and 58 per 100 000 population. Four out of 33 London boroughs had TB rates higher than 40/100 000 and another four boroughs had TB rates above 30/100 000 [1,2].
TB control is a public health priority [3] for London and TB contact investigation and management are important elements of TB control. Household and close contacts of pulmonary cases are routinely screened in accordance with National Institute for Health and Care Excellence (NICE) guidelines [4]. Contacts of cases of infectious TB outside of household settings, such as those in schools or workplaces, are generally considered to be at lower risk compared to household contacts. However, if following a risk assessment, they are considered to have had significant exposure to the case or are particularly vulnerable then TB screening will be offered.
The ‘Control and prevention of tuberculosis in the United Kingdom: Code of Practice 2000’, published by the British Thoracic Society (BTS) suggests an index case should be considered highly infectious if either transmission to more than 10% of close contacts is demonstrated, or in the circumstances of an outbreak [5]. This document and other national guidelines recommend the principle of ‘stone in the pond’ for ‘prioritising contacts in order of intensity of exposure and risk of being infected’ [6]. It is recommended that contact investigations should be expanded beyond the first circle of contacts (those with highest degree of exposure) if the proportion of contacts tested positive for latent tuberculosis infection (LTBI) is greater than expected suggesting recent transmission from the index case. TB services and public health teams in England commonly use a threshold of 10% as the ‘expected’ levels of LTBI and consider higher yields as an indication of recent transmission from an index case.
A proportion of contacts might have been exposed to TB at any point during the course of their lives and when a group of individuals are screened such latent infections due to previous exposures may be detected, alongside infection as a result of recent transmission. The risk factors for latent TB infection include links with communities or groups with high TB incidence rates, such as being born, living or working in a high incidence country, frequent travelling to or having visitors from such countries or having a family member with history of TB.
In this paper, we present our findings following a TB incident in a school where screening was offered to a large number of adolescents judged to have had significant exposure to the index case, and where screening was subsequently extended to a second circle of contacts with less exposure. We describe the yield, and factors associated with latent or active TB infection among contacts.
The incident
The index case
A 15-year-old child was diagnosed with pulmonary tuberculosis in 2014. The case was sputum smear and culture positive with a strain fully sensitive to first-line anti-tuberculosis drugs. Chest X-ray was reported as abnormal, but no cavities were seen in the lungs. Symptoms included a productive cough for five weeks. Of the five household contacts identified and screened three were children. One of the children was treated for active non-infectious tuberculosis and another diagnosed with latent tuberculosis infection. Adult contacts were screened with a chest X-ray but showed no evidence of active TB. The case attended a secondary school for four weeks during their infectious period. Case was considered infectious since onset of respiratory symptoms, i.e. productive cough, five weeks prior to the diagnosis date, but attended school for four weeks during this period.
The school
The school was located in one of the 20% most deprived boroughs/districts/unitary authorities in England, with 1 in every 3 child living in a low-income family. More than 60% of the population was from ethnic and minority groups, and it was one of the seven London boroughs with TB rates of more than 40 per 100 000 population in 2014.
TB screening and outcomes
There were 291 pupils in the same year as the index case, aged between 14 and 15 years. A risk assessment was carried out and 100 pupils who spent at least 8 h per week in the same room with the case during their infectious period were identified for screening. In June 2014 on-site screening was carried out by TB specialist nurses from the London Tuberculosis Extended contact tracing (LTBEx) team and the local TB Clinic. A consent form and a questionnaire were sent to parents of the students invited for screening. The questionnaire included questions on BCG history, ethnicity, country of birth, history of living or travelling overseas or having long-term visitor from overseas and any previous contact with someone with TB. Questionnaires were returned to TB screening nurses prior to screening.
Screening of students was carried out using a tuberculin skin test (TST or Mantoux test). If the test resulted in an induration of 15 mm or more with a BCG history or scar, or more than 5 mm in the absence of a BCG scar or reliable history, the contacts were offered an Interferon Gamma Release Assay (IGRA) blood test, in line with the NICE guidelines at the time.
There was a very high screening uptake rate and only four children did not attend the screening. Of the remaining 96 children, three were diagnosed with active TB and 22 with latent TB infection (Table 1). Molecular genotyping (24-loci Mycobacterial Interspersed Repetitive Units -Variable Number Tandem Repeat - MIRU -VNTR) was available for one child which identified the same strain as the index case. There were no specimens available for culture or strain typing for the other two children who were diagnosed with non-infectious TB, based on positive TST and IGRA tests, abnormal chest X-ray findings and corresponding TB symptoms. The outcomes suggested a high transmission rate with a yield of 26.0%.
Table 1. Outcomes of TB screening in secondary school students
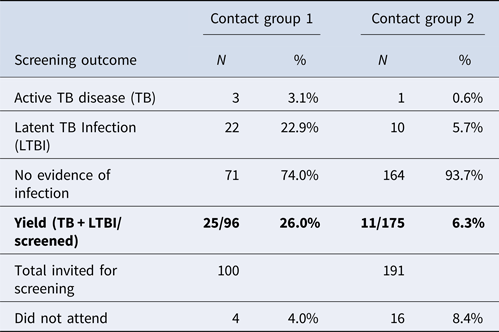
Based on the principle of ‘stone in the pond’ and considering the screening results of the first group of contacts (referred to as ‘contact group 1’ in this paper), it was decided that the rest of the school year should be offered screening, even though they had less than 8 h of contact per week with the index case and their exposure was mainly outside the classroom, for example in the library, assembly hall or dining hall. In this paper we refer to this group as ‘contact group 2’.
Contact group 2 included a further 191 children who were offered screening in October 2014. The same consent form, questionnaire and screening methods were used for the contact group 2 as previously used for group 1. On this occasion, 16 out of 191 (8.4%) students did not attend for screening. Of the 175 who were screened, 10 were diagnosed with LTBI and one with active non-infectious TB, a yield of 6.3% (Table 1). Unfortunately there were no specimens available for culture or strain typing for the child with active TB. The diagnosis of active TB was based on positive TST and IGRA tests, abnormal chest X-ray findings and corresponding symptoms. This child belonged to an ethnic group with high prevalence of TB.
A small number of staff members were also offered screening but are not included in the analysis due to small numbers and the focus of this paper is on the children.
Methods
Factors associated with TB infection or disease were analysed using a cohort study design. The study population was all students who attended screening (n = 271). A case was defined as anyone diagnosed with active TB disease or latent TB infection after screening. Other variables were included in the analysis to investigate the association with a positive screening result. Variables included: contact group 1 or 2, sex, ethnicity, country of birth (recoded into birth in a high TB prevalence, i.e. ⩾40 new cases/100 000 population annually, or low TB prevalence country, i.e. <40 new cases/100 000 population annually), ever lived overseas, previous contact with someone with TB and contact with someone from overseas (either a long-term visitor from overseas, or had travelled overseas to visit family/friends).
All analysis was carried out using STATA 13 (Stata Corp LP, College Station, TX, USA). Chi-squared or Fisher's exact test (differences between two proportions) was used to analyse the differences between the two contact groups. Odds ratios (ORs) were calculated using logistic regression. Exposure variables with a P-value of 0.2 or less in univariable analysis were included in a multivariable logistic regression model, along with those considered significant a priori. We used a backwards stepwise approach to identify a final model, eliminating variables with a P value >0.05, as determined by the likelihood ratio test, and testing for evidence of interaction. Variables with the highest P value were removed sequentially provided there was no evidence of confounding. Multivariable analysis is presented as an adjusted OR with 95% confidence intervals. A two-tailed P-value of 0.05 or lower was considered statistically significant. Contacts for whom there was missing information on factors included in the multivariable model were excluded from the analysis. Ethnicity was available for 197/271 contacts (73%), and country of birth known for 235 (87%).
Results
Two hundred and ninety-one students were included in the analysis. Of these 36 met the case definition for active or latent TB infection; a prevalence (prev) of active TB or infection from screening of 12.4%. Of these, 25 (prev: 26%) were identified in initial screening (contact group 1) and 11 (prev 6.3%) in additional screening (contact group 2). Contact groups 1 and 2 were similar in terms of the demographics collected (Table 2).
Table 2. Comparison of variables for contact group 1 vs. contact group 2
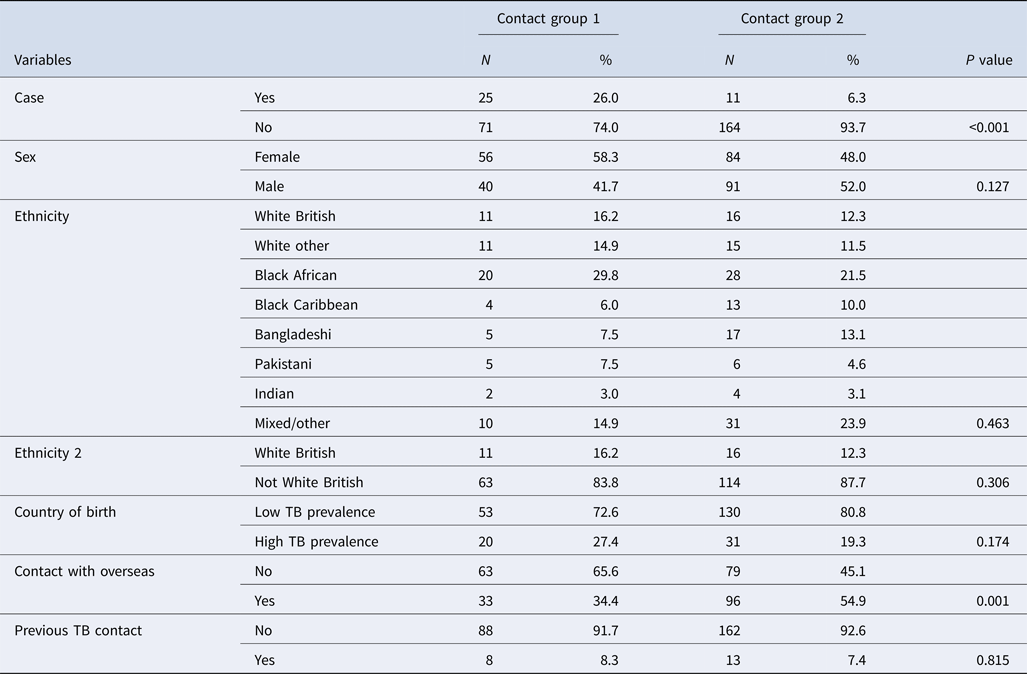
In the univariable analysis only being in contact group 1 was statistically significantly associated with being a case (OR 5.25, 95% CI 2.33–12.45, P value <0.001) (Table 3).
Table 3. Distribution of cases, by sex and exposure variables
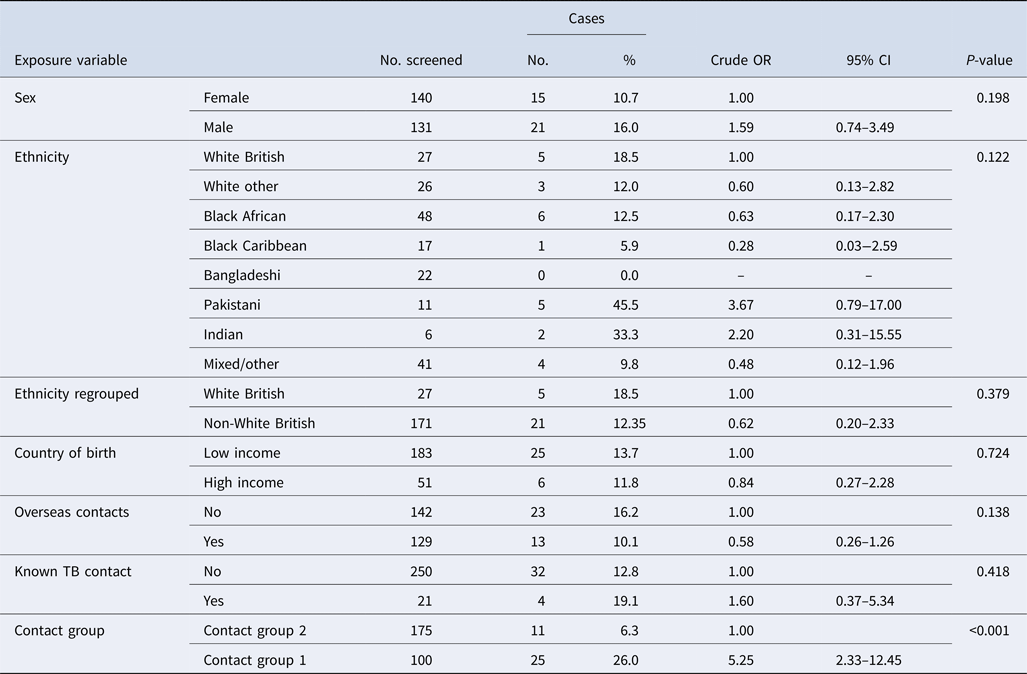
In the multivariable model after adjusting for sex and ethnicity (white British or non-white British) contact group 1 remained significantly associated with being a case (adjusted OR 4.40, 95% CI 1.81–10.71, P value 0.001) (Table 4). Being male was also associated with increased odds of being infected. Although ethnicity (white British or non-white British) was not independently associated with being a case, it was included in the model as a confounder.
Table 4. Multivariable analysis results

Discussion
In this paper we describe the outcome of two rounds of an extensive TB contact screening in a secondary school and estimated the background prevalence of latent TB infection by calculating yield among two groups of students, one with significant and the other with limited exposure to an index case. We also compared the factors associated with being a case in the two groups.
Being screened in contact group 1, i.e. having had significant exposure to index case, was significantly associated with being a case after adjusting for important variables. We concluded that the 6.3% yield in contact group 2 either is similar to or higher than the background prevalence rate of latent TB infection, thus well below the widely accepted threshold of 10%.
When using the principle of ‘stone in the pond’, the screening yield is compared to the background prevalence or ‘expected’ rates for TB infection. Any difference between the expected and observed prevalence in a contact screening exercise could suggest recent transmission [Reference Veen7].
The screening results indicated recent transmission had occurred among the children in contact group 1 and MIRU-VNTR molecular genotyping provided further evidence of transmission, as the index case and one other child had indistinguishable strains of M. tuberculosis. The children in contact group 1 had at least 8 h per week of contact with the index case. Of these, 26% were diagnosed with LTBI. In contrast, contact group 2 who had less exposure to the index case had a yield of 6.3%. The two contact groups have similar demographic and TB risk profiles. The results of the multivariable logistic regression analysis suggests the only significant factor associated with being infected in this incident was in the degree of exposure to the index case (adjusted OR 4.74 for students in contact group 1 being infected compared to students in group 2). It can therefore be concluded that the high rate of TB infection in contact group 1 is likely to be as a result of exposure to the index case and contact group 2 had been minimally affected by the index case. However, as we cannot rule out any transmission from the index case to children in contact group 2, the background prevalence of LTBI in this group of children could be even less than the 6.3% yield in contact group 2. These findings suggest the expected background levels of positive LTBI screening results in secondary school-aged children in a high-incidence area of inner city London is significantly below 10% and likely to be less than 6%.
We reviewed the published literature, including those describing findings of school contact screening exercises, in the UK [Reference Howard8–Reference Williams10] or other low-incidence countries [Reference Curtis11–Reference Phillips, Carlile and Smith14]. These publications report yields ranging between 4.5% and 43%, but we could not find any papers reporting on the background prevalence of LTBI in the UK school children or any evidence to suggest a 10% LTBI rate could be expected in children not recently infected by an index case at school. Following a contact screening investigations at a secondary school in Birmingham, Caley et al. [Reference Caley15] calculated the risk of being infected with TB in children in the same school year as the index case compared to those in other school years. Using data presented in this paper, we calculated the TB yield in the two groups. The students who were not in the same year as the index case had a yield of 3.7% compared to 29.9% in those in the same year. Although we have no further demographic or TB risk factor information for the two groups, the results further support our assumption that the background LTBI rates in England are significantly lower than 10%.
Limitations
We presented findings of only one extensive TB screening exercise in this paper which suggests the currently accepted threshold for expected rates of TBI are significantly overestimated. Further epidemiological investigations including point prevalence studies are needed to provide a robust evidence on current prevalence of latent TB in London in different age and socioeconomic groups. Routinely collected data could also provide opportunities for further observational studies but there are currently no requirements for collecting standardised and detailed demographic and risk factor information on individual contacts of TB cases in England. Although information might be collected locally in some areas, they are not routinely and consistently collated.
The background prevalence rates of latent TB are not static and likely to change over time. For example they will decline with decreasing TB incidence rates, as described in The Netherlands between 1956 and 1990 [Reference Veen7], and rates are likely to be lower in communities or areas with low rates of active TB compared to those with higher rates. The above incident provides an estimate for high prevalence inner city areas and background rates are likely to be lower in other parts of England. The rates of latent TB infection are also likely to be different in children compared to adults.
Conclusion
In this paper, we demonstrated the background prevalence of latent TB infection among secondary school children in a deprived inner city London borough with very high rates of active TB is well below the 10% threshold frequently used to assess recent transmission. Our findings indicate the background rates are likely to be less than 6.3% in high incidence areas for active TB and possibly lower in low incidence areas in England.
Further studies, including point prevalence studies, will provide robust evidence on the background rates of latent TB in the UK. The use of routine data, such as those collected during extensive screening of contacts, could provide further evidence, provided information is gathered and recorded systematically, preferably on a centralized database. Further discussion is required regarding the use of the 10% estimate during contact tracing.
Acknowledgement
With thanks to NHS London local TB services and PHE London TB Extended contact tracing team (LTBEx).
Conflict of interest
None.







