Introduction
The increasing global prevalence of type 2 diabetes (T2D), a chronic health condition characterised by impaired insulin secretion and insulin resistance, highlights the urgent need for improved lifestyle intervention strategies. Milk products are available globally and have been associated with a range of health benefits, including reduced risk of T2D( Reference Kalergis, Yinko and Lan 1 , Reference Elwood, Givens and Beswick 2 ). In a recent meta-analysis, Tong et al. ( Reference Tong, Dong and Wu 3 ) reported that T2D risk could be reduced by 5 and 10 % for each one serving per d of total dairy and low-fat dairy products, respectively. A separate meta-analysis of seventeen cohort studies( Reference Aune, Norat and Romundstad 4 ) additionally demonstrated significant inverse associations between T2D risk and intake of total dairy products, low-fat dairy products and cheese. These findings suggest that dairy products may represent a promising lifestyle intervention strategy to aid in the prevention and management of T2D. However, intervention studies are currently limited and have shown mixed results( Reference Turner, Keogh and Clifton 5 ). Turner et al. ( Reference Turner, Keogh and Clifton 5 ) systematically reviewed ten randomised controlled trials, including three intervention studies that increased low-fat milk and/or yoghurt consumption per d, six that increased the number of total dairy product serves per d and one that increased either daily whey or casein protein consumption. The intervention durations ranged from 1 week( Reference Hoppe, Molgaard and Vaag 6 ) to 6 months( Reference Rideout, Marinangeli and Martin 7 – Reference Wennersberg, Smedman and Turpeinen 10 ). Four studies showed a positive effect on insulin sensitivity, one was negative and five had no effect. Methodological differences including variation in the type of dairy product consumed may be one explanation for the inconsistency in findings.
Knowledge of the properties of dairy products which may reduce T2D risk and the underlying mechanisms is important as it could assist in the development of improved prevention and management strategies for T2D, such as functional foods or nutraceuticals. Furthermore, such knowledge could assist in clarifying dietary guidelines regarding dairy product intake. Although a number of factors have been proposed and the exact mechanisms remain to be fully determined( Reference Hirahatake, Slavin and Maki 11 ), dairy protein has received recent interest as a dietary component that may aid in T2D prevention( Reference McGregor and Poppitt 12 ). Milk protein is comprised primarily of whey and casein proteins, which constitute about 20 and 80 % of the total protein fraction, respectively( Reference Jensen 13 ). Whey proteins can be further subdivided into α-lactalbumin, β-lactoglobulin, serum albumin, immunoglobulins, lactoferrin and proteose-peptone fractions, and caseins into four fractions: α-, β-, γ- and κ-caseins.
Milk-derived whey and casein proteins have been demonstrated to have a stimulating effect on insulin secretion in healthy, obese, pre-diabetic and T2D individuals( Reference Pal, Ellis and Dhaliwal 14 – Reference Manders, Hansen and Zorenc 21 ). Gunnerud et al. ( Reference Gunnerud, Ostman and Bjorck 17 ) reported that whey protein affected glycaemia, insulinaemia and plasma amino acids to a glucose load in a dose-dependent manner in twelve healthy individuals. A number of studies have similarly reported beneficial effects of whey protein on insulin secretion (for a review, see Pal & Radavelli-Bagatini( Reference Pal and Radavelli-Bagatini 18 )). While caseins have been less well studied, Hoefle et al. ( Reference Hoefle, Bangert and Stamfort 19 ) recently demonstrated that both 50 g sodium caseinate and 50 g whey protein isolate administered with maltodextrin equivalently increased insulin secretion by 96 % compared with maltodextrin alone in pre-diabetic adults. The enhanced insulin secretion was accompanied by a 21 % decrease in postprandial blood glucose following both protein meals. In a study of ten healthy males, a casein protein hydrolysate significantly accelerated protein digestion and absorption from the gut and enhanced postprandial insulin secretion and amino acid availability compared with its intact protein( Reference Koopman, Crombach and Gijsen 22 ). These findings add to an increasing body of work suggesting that the composition and/or source of protein ingested and subsequent digestion and absorption pattern may have a significant impact on the postprandial metabolic response( Reference Boirie, Dangin and Gachon 23 – Reference Claessens, Saris and Van Baak 27 ). The key mechanisms underlying such a response are not known but may be the result of the action of amino acids and/or bioactive peptides, either originally present in the protein or formed during gastrointestinal (GI) digestion( Reference Frid, Nilsson and Holst 28 ).
The functional significance of milk protein-derived peptides for health is dependent on their bioavailability and the urgent need for an integrated approach to address the role and mechanism of action of milk protein-derived peptides in humans has been emphasised( Reference Nongonierma and FitzGerald 29 ). For pertinent recent reviews, see Nongonierma & FitzGerald( Reference Nongonierma and FitzGerald 29 ), Boutrou et al. ( Reference Boutrou, Henry and Sanchez-Rivera 30 ) and Nongonierma & FitzGerald( Reference Nongonierma and FitzGerald 31 ). From a glycaemic management perspective, in order to exert an effect on postprandial responses, bioactive peptides need to reach the intestine and/or bloodstream in an active form. The objective of the present review is to provide an overview of the literature on the bioavailability of milk protein-derived peptides, including studies that have identified milk protein-derived peptides in the GI tract and/or bloodstream, and to examine the evidence on potential mechanisms of action for glycaemic management, with a focus on human studies.
Milk protein-derived bioactives
Bioactive peptides are defined as specific protein fragments that have a beneficial impact on body functions and may ultimately influence health( Reference Kitts and Weiler 32 ). Milk protein-derived bioactive peptides are naturally present in a range of milk and dairy product-based foods( Reference Nongonierma and FitzGerald 31 ) and are inactive within the sequence of the parent protein but can be released during GI digestion, microbial fermentation or food processing( Reference Nongonierma and FitzGerald 31 , Reference Nakamura, Yamamoto and Sakai 33 , Reference Uenishi, Kabuki and Seto 34 ). If the structure of the bioactive peptide is known, peptides can be synthesised using a variety of methods including chemical synthesis, recombinant DNA technology and enzymic synthesis( Reference Gill, López-Fandiño and Jorba 35 ).
The identification of bioactive milk peptides was first reported over 60 years ago, when it was suggested that casein-derived phosphorylated peptides enhanced vitamin D-independent bone calcification in infants with rickets( Reference Mellander 36 ). Since then, knowledge of bioactive peptides has rapidly increased and peptides with various functions including opiate, anti-hypertensive, anti-thrombotic and immunomodulatory activities have been described( Reference Nongonierma and FitzGerald 29 , Reference FitzGerald and Meisel 37 – Reference Korhonen and Pihlanto 43 ). In addition to health-enhancing properties, it should be noted that some milk proteins also include antigenic peptides that induce allergy and this has been the focus of another line of research( Reference Hochwallner, Schulmeister and Swoboda 44 , Reference Tsabouri, Douros and Priftis 45 ). Milk allergenicity can be reduced by various processing methods including hydrolysis( Reference Tsabouri, Douros and Priftis 45 ).
The hormone-like activity of bioactive peptides is based on their amino acid composition and sequence( Reference Vermeirssen, Camp and Verstraete 46 , Reference Meisel 47 ). Bioactive peptides usually contain two to twenty amino acid residues but in some cases may consist of more than twenty amino acids( Reference Erdmann, Cheung and Schröder 48 ). For example, peptides with opioid agonistic and antagonistic activity include casoxins (κ-casein, f(58–61); Tyr-Pro-Tyr-Tyr) and β-casomorphins (consisting of four to nine amino acid fragments of bovine β-casein (f(60–68))( Reference Silva and Malcata 38 , Reference Chiba, Tani and Yoshikawa 49 , Reference Schanbacher, Talhouk and Murray 50 ). Peptides that inhibit angiotension I-converting enzyme (ACE) in the cardiovascular system can exert antihypertensive effects( Reference Vermeirssen, Camp and Verstraete 46 ), and are one of the most studied classes of bioactive peptides( Reference Foltz, Meynen and Bianco 39 ). Dipeptides, tripeptides and some longer-chain peptides have all been reported to have ACE-inhibitory activity( Reference Hernández-Ledesma, Amigo and Recio 51 , Reference Yamamoto, Maeno and Takano 52 ). The tripeptide Ile-Pro-Pro, for example, has been obtained from milk fermentation and has known anti-hypertensive activity( Reference Nakamura, Yamamoto and Sakai 33 ). Furthermore, many bioactive milk-derived peptides may have more than one functional role. For example, peptides from the sequence 60–70 of β-casein show opioid, ACE-inhibitory and immunomodulatory activities( Reference Meisel 53 ). In contrast to the aforementioned bioactivities the potential of milk-derived peptides for glycaemic management, however, have only been more recently explored and the structure and sequence of milk protein-derived peptides exerting bioactivity has received little investigation in human studies.
Importance of determining bioavailability
While promising systemic effects of both whey and casein proteins and/or their hydrolysates on blood glucose and insulin secretion in healthy, obese, pre-diabetic and T2D individuals imply a certain level of systemic availability, there is a dearth of information on the bioavailability of the bioactive peptides. These peptides must reach their target site or organ in an active form to be bioactive( Reference Vermeirssen, Camp and Verstraete 46 ).
Gastrointestinal digestion
Milk protein-derived peptides could produce local effects in the GI tract and/or enter intact into the bloodstream and exert systemic effects on glycaemic control. However, during the process of GI digestion, a number of factors may lead to the degradation of bioactive peptides, thereby limiting their bioavailability. To assist in understanding factors that influence the ability of a bioactive to reach its target site intact, the following paragraphs provide a brief overview of the processes involved in the GI digestion of proteins and are illustrated in Fig. 1.
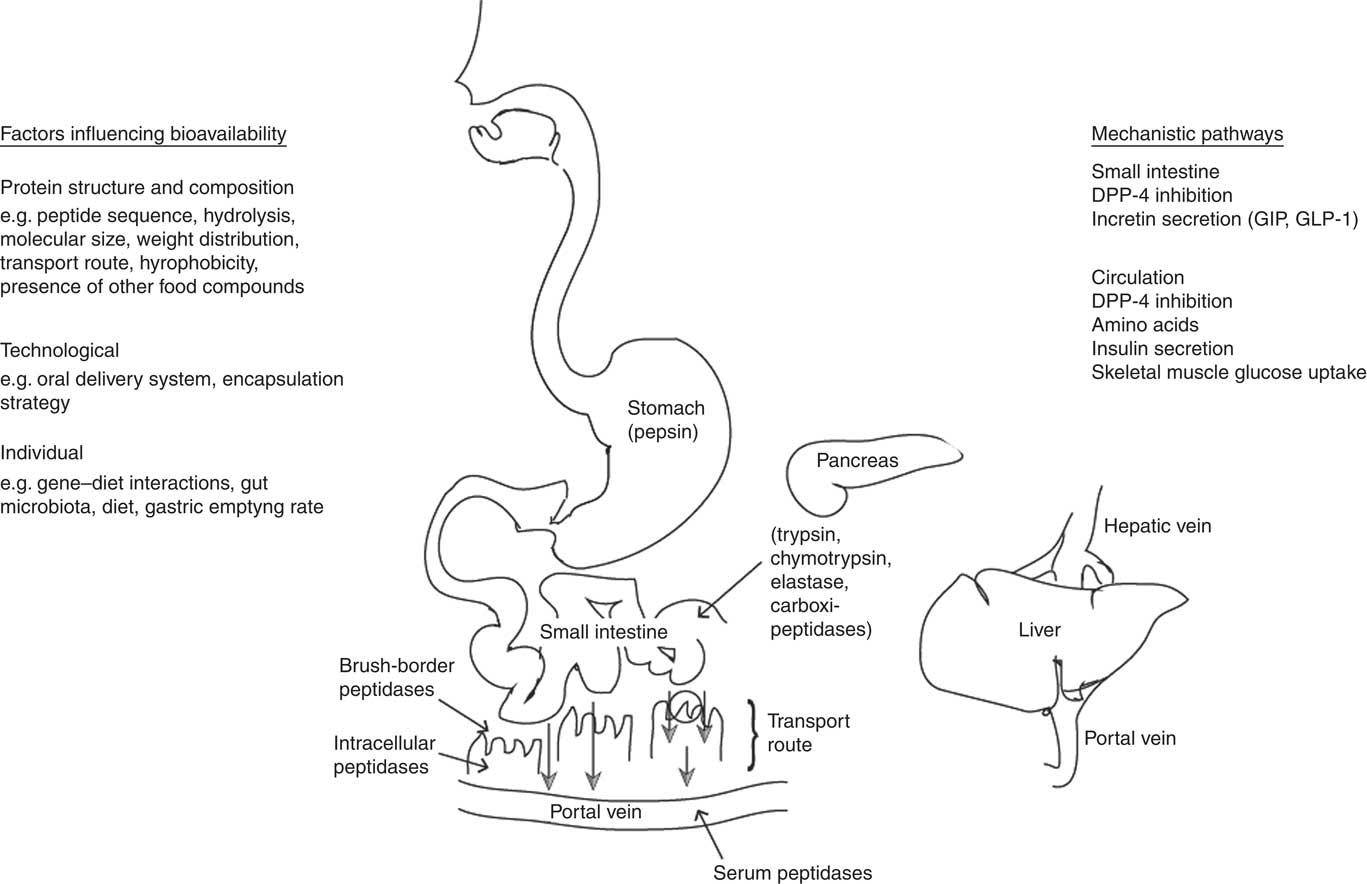
Fig. 1 Representation of the processes involved in the gastrointestinal digestion, absorption and passage into the blood of ingested milk proteins. To be bioavailable, bioactive peptides must escape degradation from intestinal or serum peptidases and be transported intact to the target site or organ. Different transport systems for intestinal absorption of peptides into the blood have been described. Smaller peptides are transported by a specific peptide transporter, whereas oligopeptides are generally transported by transcellular and paracellular pathways. Once present in the small intestine, or absorbed into the bloodstream, bioactive peptides could act via various potential mechanisms to influence glycaemic control. DPP-4, dipeptidyl peptidase-4; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1.
To be transported intact to the target site or organ, bioactive peptides must escape degradation during digestion. In the stomach a low pH initiates hydrolysis, and gastric pepsin, an endopeptidase secreted by the stomach mucosa, cleaves proteins into large peptides. The generated peptides pass into the small intestine, where they are further cleaved by pancreatic proteases into free amino acids and oligopeptides. Oligopeptides are then further hydrolysed by brush-border peptidases into dipeptides, tripeptides and free amino acids. For example, the enzyme dipeptidyl peptidase-4 (DPP-4) cleaves dipeptides from the N-terminus of oligopeptides with proline or alanine in the penultimate position( Reference Aertgeerts, Ye and Tennant 54 ).
Different transport systems for the intestinal absorption of peptides have been described. Smaller peptides are transported by a specific peptide transporter (PepT1)( Reference Foltz, Meynen and Bianco 39 , Reference Shimizu 55 , Reference Shimizu 56 ) located in the brush-border membrane. Oligopeptides on the other hand can be transported by transcytosis (vesicle-mediated transcellular transport)( Reference Shimizu 55 ) and paracellular( Reference Shimizu 55 , Reference Pappenheimer, Dahl and Karnovsky 57 ) pathways. The paracellular pathway is known to be a non-degradative transport route and is suggested to be the main mechanism for transport of intact peptides( Reference Shimizu 56 ). Whichever the route, a variety of proteins and peptides even in small amounts could be absorbed and demonstrate physiological function( Reference Shimizu 56 ).
Milk protein-derived peptides identified in the gastrointestinal tract and plasma
The hypothesis that some small peptides escape degradation and are transported from the intestinal lumen into the circulation is gaining acceptance( Reference Regazzo, Mollé and Gabai 58 ). Although, this may be due in part to an increasing number of in vitro studies describing the transepithelial transport of bioactive peptides( Reference Regazzo, Mollé and Gabai 58 – Reference Foltz, Cerstiaens and van Meensel 62 ), there is some evidence of milk protein-derived peptides identified in the GI tract and bloodstream in human subjects (for an overview, see Table 1). Indeed, 30 years ago, Svedberg et al. ( Reference Svedberg, de Haas and Leimenstoll 63 ) reported that considerable amounts of β-casomorphin-7, a milk opioid peptide with seven amino acids, were present in the small intestine of healthy young volunteers following the ingestion of 1 litre of bovine milk. Following these observations, Chabance et al. ( Reference Chabance, Marteau and Rambaud 64 ) later examined casein peptide release and passage into the blood during digestion of milk or yoghurt in six healthy adults. Participants consumed either 500 ml of skimmed milk or yoghurt in random order and intestinal samples were collected over 4 h. On another three test days, blood samples were collected over 8 h following the consumption of 500 ml water, skimmed milk or yoghurt. After milk or yoghurt ingestion, many peptides derived from casein, including α-, β- and κ-caseins, were detected in the stomach, smaller peptides were detected in the duodenum and two long peptides, the κ-caseinoglycopeptide (cCGP) f(106–117) and the N-terminal peptide f(1–23) of αs1-casein were detected in the plasma. Moreover, cCGP was present for 8 h in the plasma after both milk or yoghurt ingestion. As cCGP was also found in the stomach for 4 h after milk ingestion, one explanation is that κ-casein digestion resulted in a consistent flow of peptides being progressively released into the plasma. The presence of proline residues might also have protected cCGP from proteolysis( Reference Chabance, Marteau and Rambaud 64 ). More recently, in a cross-over study Foltz et al. ( Reference Foltz, Meynen and Bianco 39 ) examined the bioavailability of Ile-Pro-Pro (a whey-derived tripeptide with known high in vitro ACE-inhibitory activity( Reference Wu, Aluko and Nakai 65 )) present in a lactotripeptide-enriched yoghurt beverage compared with a whey protein isolate placebo (containing no free Ile-Pro-Pro). Plasma concentrations of Ile-Pro-Pro were elevated after both beverages, suggesting that the tripeptide may have been generated by luminal and brush-border peptidases during the placebo treatment and absorbed intact into the bloodstream.
Table 1 Summary of studies that have identified milk protein-derived peptides in the gastrointestinal tract and/or circulation in human subjects

cCGP, κ-caseinoglycopeptide; CMP, caseinomacropeptide; ACE, angiotension I-converting enzyme.
* Number and sex of participants are given in parentheses where described in the original paper.
† Milk protein source as described in the original paper.
The use of intrinsically labelled milk proteins is one method that allows for a greater understanding of the digestion and absorption of dietary milk protein-derived peptides( Reference Mahé, Roos and Benamouzig 66 ). In a formative study using 15N-intrinsically labelled milk proteins, Ledoux et al. ( Reference Ledoux, Mahe and Dubarry 67 ) detected milk caseinomacropeptide (CMP) in the jejunum of human subjects after ingestion of a 15N-labelled casein, whey protein or yoghurt meal, indicating that CMP (a sixty-four-amino acid peptide released after κ-casein hydrolysis) resisted hydrolysis by pancreatic enzymes. This supports the earlier observations of Chabance et al. ( Reference Chabance, Marteau and Rambaud 64 ) that milk-derived peptides could exert a physiological function in humans as both CMP and CMP-derived peptides have been identified as important putative bioactive sequences( Reference Ledoux, Mahe and Dubarry 67 ). The presence of other peptides or presence of milk-derived peptides in the bloodstream was not examined in that study, however. Only relatively recently has an extensive characterisation of the peptides present during digestion been made possible with the use of liquid chromatography coupled with MS( Reference Boutrou, Henry and Sanchez-Rivera 30 ). Using this technique in combination with 15N-casein or -whey protein, Boutrou et al. ( Reference Boutrou, Gaudichon and Dupont 68 ) detected and sequenced a total of 356 peptides following casein ingestion and 146 peptides following whey ingestion in jejunal effluents of human subjects. A large number of peptides from β- and αs1-casein covering almost the whole sequence of both proteins were identified. However, few peptides from minor proteins such as κ-casein, lactoferrin, and serum albumin were identified, possibly due to differences in protein structure or composition( Reference Boutrou, Gaudichon and Dupont 68 ). The majority (72 %) of whey-derived peptides detected were from β-lactoglobulin and were generally of larger size (nine to fifteen amino acid residues) compared with casein (six to nine amino acid residues). β-Casein was identified as the most important precursor of peptides, including bioactive peptides such as β-casein f(60–66) and f(108–113). These peptides are known to have opioid and antihypertensive properties, respectively, and the amounts detected were suggested to be sufficiently high to exert a biological effect( Reference Boutrou, Gaudichon and Dupont 68 ). For example, at 2 h after casein ingestion, the estimated amount of β-casomorphin-7 was 17 mmol/l and the half-maximal inhibitory concentration (IC50) of β-casomorphin-7 for an opioid agonist activity is 3–100 mmol/l( Reference Boutrou, Gaudichon and Dupont 68 , Reference Yoshikawa, Suganuma and Takahashi 69 ). In contrast, none of the whey-derived peptides were identified as bioactive based on previous literature( Reference Boutrou, Gaudichon and Dupont 68 ). A limited number of animal studies have also identified sequences of bioactive peptides present in the GI tract, with the majority deriving from casein and few from whey proteins (for a comprehensive review, see Boutrou et al. ( Reference Boutrou, Henry and Sanchez-Rivera 30 )). Whether these peptides also reach the bloodstream in a concentration sufficient to exert a biological effect has not been established, and is particularly relevant for further study.
Potential factors influencing bioavailability of milk protein-derived peptides
An increased knowledge of the factors that influence bioavailability of bioactive peptides in humans is necessary to understand which are most important physiologically. Several studies have investigated this in in vitro experiments. However, as highlighted by Foltz et al. ( Reference Foltz, van der Pijl and Duchateau 70 ), such an approach cannot fully characterise the poor absorption, distribution, metabolism and excretion (ADME) properties of peptides which can result in low bioavailability in humans. A limited number of studies have been undertaken in human subjects. Evidence that the level of CMP recovered after whey, casein or yoghurt ingestion depended on its transit rate in the lumen( Reference Ledoux, Mahe and Dubarry 67 ) indicates that the rate of GI transit may have a significant role in determining the bioavailability of bioactive peptides. Boirie et al. ( Reference Boirie, Dangin and Gachon 23 ) have classed intact micellar casein and whey as ‘slow’ and ‘fast’ proteins, respectively, based on their digestion and absorption patterns. They found whey to produce a high, fast and transient increase of amino acids, whereas casein ingestion induced a prolonged plateau of moderately increased amino acids in healthy volunteers( Reference Boirie, Dangin and Gachon 23 ). Casein proteins clot at low pH, whereas whey proteins do not, which may cause casein to aggregate into a gel resulting in slower release of amino acids( Reference Boirie, Dangin and Gachon 23 , Reference Mahé, Roos and Benamouzig 71 , Reference Morifuji, Ishizaka and Baba 72 ). Hydrolysed casein on the other hand has been shown to be absorbed more rapidly than intact casein and approaches a similar rate to whey absorption( Reference Koopman, Crombach and Gijsen 22 , Reference Calbet and Holst 73 ). Hydrolysis of proteins, especially casein, may consequently affect bioavailability of peptides and hence metabolic parameters( Reference Bendtsen, Lorenzen and Gomes 74 ).
The molecular size, weight distribution and other properties of peptides, such as hydrophobicity, can determine the major transport route for peptides( Reference Shimizu, Tsunogai and Arai 75 ) which in turn can also influence bioavailability. Of the two long peptides that Chabance et al. ( Reference Chabance, Marteau and Rambaud 64 ) identified in the blood after milk or yoghurt ingestion, some common characteristics were identified suggesting the possibility that both peptides possess common transport pathways for intestinal absorption. Both peptides are the result of two major chymosin cleavage sites, highly hydrophilic, located on the surface of milk micelles, in part homologous, in part situated in β-turns and not rapidly destroyed by plasma proteases. Of the peptides that Boutrou et al. ( Reference Boutrou, Gaudichon and Dupont 68 ) identified in the jejunum of human subjects they noted that most contained at least two proline residues, consistent with evidence that proline-containing peptides are resistant to degradation( Reference FitzGerald and Meisel 76 ). In addition to a high proline content, other characteristic features of β-casomorphins include their hydrophobic character and the presence of tyrosine on the N-terminus( Reference Brantl, Teschemacher and Henschen 77 , Reference Teschemacher 78 ). Bioavailability also appears to increase with decreasing peptide chain length( Reference Roberts, Burney and Black 79 ). For example, Foltz et al. ( Reference Foltz, Meynen and Bianco 39 ) observed that levels of dipeptides were greater than tripeptides in the plasma after whey ingestion.
Differences in whey and casein protein structure and the dairy matrix can make an impact on the bioaccessibility of peptides and subsequently bioavailability. Whey proteins are highly ordered globular proteins, whereas caseins have a relatively open and disordered structure which makes caseins highly susceptible to proteolysis( Reference Power, Nongonierma and Jakeman 80 ). Moreover, the presence of other food compounds could influence the susceptibility to peptidase degradation and intestinal transport( Reference Charman, Porter and Mithani 81 ). For example, a substantially greater amount of carotenoids was found to be absorbed when salads were consumed with a full-fat compared with a reduced-fat salad dressing( Reference Brown, Ferruzzi and Nguyen 82 ). Fat slows gastric emptying and the presence of lipid products within the duodenum induces secretion of biliary and pancreatic fluids that can impact on absorption( Reference Charman, Porter and Mithani 81 ). Individual differences as a result of factors such as diet, gene–diet interactions( Reference Abdullah, Cyr and Labonté 83 ), gut microbiota( Reference Claesson, Jeffery and Conde 84 ) or the oral mastication process( Reference Rémond, Machebeuf and Yven 85 ) could also influence the effects of dairy product intake on physiological outcomes and hence interindividual variation in bioavailability. Moreover, oral delivery systems of peptides and technological factors such as encapsulation strategies of bioactives may influence bioavailability and there is great potential for integrative studies between food scientists and drug delivery researchers in this area( Reference Brayden and Baird 86 ) when bioactive sequences are identified. In the GI tract of mini-pigs, three times as many peptides were identified after ingestion of heated milk compared with rennet gels, highlighting the role that the dairy product matrix can have on peptide availability( Reference Barbé, Le Feunteun and Rémond 87 ). Collectively, these findings illustrate the wide range of factors (see Fig. 1) that may determine whether an ingested milk-derived protein could exert a physiological effect.
Mechanisms for glycaemic management
Knowledge of the potential mechanistic pathways by which milk protein-derived peptides may enhance glycaemic management is also important to assist in enhancing their bioavailability and produce greater health benefits. Several mechanisms have been proposed whereby milk protein-derived amino acids and peptides might exert insulinotropic effects, including via direct effects on insulin secretion and indirect effects on incretin gut peptides, illustrating the potential for milk protein-derived peptides to enhance glycaemic management via multiple pathways (Fig. 1). However, whether intact peptides elicit the insulinotropic activity and the specific peptide sequences involved remains unclear.
Mechanisms
Amino acids
Several amino acids can regulate insulin secretion from pancreatic β-cells( Reference Newsholme, Brennan and Bender 88 ), and an increase in plasma amino acids has been associated with an enhanced insulin response( Reference Nilsson, Stenberg and Frid 89 , Reference Calbet and MacLean 90 ). Milk proteins contain a rich source of amino acids and postprandial amino acid patterns have been consistently found to correlate with enhanced insulin responses to the ingestion of milk proteins and/or their hydrolysates in human subjects. For example, Nilsson et al. ( Reference Nilsson, Stenberg and Frid 89 ) demonstrated that there was a significantly higher postprandial insulin AUC after a whey drink when compared with other animal and vegetable protein sources in twelve healthy individuals. The essential amino acids leucine, isoleucine, valine, lysine and threonine were found to show pronounced postprandial increases in plasma after the whey drink, and also showed the strongest correlation with the insulin response. Moreover, the postprandial glucose AUC was reduced by 57 % after the whey drink compared with the bread reference meal. Calbet & MacLean( Reference Calbet and MacLean 90 ) also observed that the insulin response was closely related to the increase in plasma amino acids, particularly leucine, isoleucine, valine, phenylalanine and arginine after a whey protein hydrolysate. In that study the whey protein hydrolysate elicited a peak insulin response four times greater than that evoked by a cows’ milk solution containing complete proteins or by glucose in six healthy individuals. Others have similarly demonstrated that casein hydrolysates, compared with intact casein, show a more rapid and increased postprandial amino acid availability and increased insulin response( Reference Koopman, Crombach and Gijsen 22 , Reference Deglaire, Fromentin and Fouillet 91 ). These findings collectively suggest that the postprandial composition and pattern of amino acid availability could be important factors determining the insulin response to milk proteins. The mechanisms by which specific amino acids enhance insulin secretion are varied and have been comprehensively reviewed elsewhere (see Newsholme et al. ( Reference Newsholme, Brennan and Bender 88 , Reference Newsholme, Bender and Kiely 92 , Reference Newsholme and Krause 93 )).
Incretin secretion
Milk protein-derived bioactive peptides may also have an effect on postprandial glycaemia and insulinaemia via effects on the incretin gut peptides glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Approximately 50–70 % of the insulin secretory response to an oral glucose load may be due to the stimulatory effect of gut-derived incretin peptides, although this effect is lost or greatly impaired in individuals with T2D( Reference Holst and Gromada 94 – Reference Holst, Vilsboll and Deacon 96 ). Following release into the bloodstream in response to glucose or nutrient ingestion GLP-1 and GIP bind to G-protein-coupled receptors on the pancreatic β-cell membrane to stimulate the release of insulin( Reference Thorens 97 , Reference Yabe and Seino 98 ). However, both GLP-1 and GIP are rapidly degraded in vivo by the enzyme DPP-4 and they have a short half-life of less than 2 min( Reference Mentlein, Gallwitz and Schmidt 99 ). Bioactive peptides could therefore potentially act to influence postprandial incretin hormone responses by either directly stimulating an increase in GLP-1 and GIP secretion or inhibiting DPP-4 in the GI tract or bloodstream.
Increasing evidence indicates that milk-derived proteins can alter postprandial incretin hormone responses in healthy, lean, overweight/obese and T2D individuals( Reference Hoefle, Bangert and Stamfort 19 , Reference Hall, Millward and Long 25 , Reference Frid, Nilsson and Holst 28 , Reference Nilsson, Stenberg and Frid 89 , Reference Calbet and MacLean 90 , Reference Nilsson, Holst and Bjorck 100 – Reference Chungchunlam, Henare and Ganesh 106 ). The majority of evidence points to an enhanced GIP response to intact whey protein( Reference Hoefle, Bangert and Stamfort 19 , Reference Hall, Millward and Long 25 , Reference Frid, Nilsson and Holst 28 , Reference Nilsson, Stenberg and Frid 89 , Reference Nilsson, Holst and Bjorck 100 , Reference Ma, Stevens and Cukier 102 ), although there is some disparity in findings( Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 107 , Reference Holmer-Jensen, Hartvigsen and Mortensen 108 ). Similarly, much of the evidence points to an enhanced GLP-1 response to intact whey protein when compared with a variety of reference meals( Reference Ma, Stevens and Cukier 102 , Reference Akhavan, Luhovyy and Panahi 104 – Reference Chungchunlam, Henare and Ganesh 106 , Reference Bowen, Noakes and Clifton 109 ) and when compared with intact casein( Reference Hall, Millward and Long 25 , Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 110 , Reference Juvonen, Karhunen and Vuori 111 ). Incretin responses to milk proteins in their hydrolysed form have been less well studied. However, in a seminal study examining postprandial responses to whey and casein proteins and their hydrolysates, Calbet & Holst( Reference Calbet and Holst 73 ) found that both whey and casein hydrolysates increased the GIP response during the first 20 min of gastric emptying compared with intact proteins in healthy individuals. Further, Bendtsen et al. ( Reference Bendtsen, Lorenzen and Gomes 74 ) more recently demonstrated that the protein form had a significant influence on the postprandial GLP-1 response pattern in healthy overweight and obese individuals. The GLP-1 response was enhanced at 15 min after ingestion of intact casein, but at 60 and 90 min after ingestion of a casein hydrolysate, when compared with intact whey and intact casein. These findings suggest that the protein form has a significant influence on the postprandial incretin response pattern, and that while intact casein does not appear to elicit much effect, casein hydrolysates have been shown to have some effect on incretin responses.
Dipeptidyl peptidase-4 inhibition
DPP-4-inhibitory properties of milk proteins have been increasingly demonstrated in vitro (for a comprehensive review, see Power et al. ( Reference Power, Nongonierma and Jakeman 80 )). DPP-4 is a membrane-spanning enzyme expressed on several cell types, and is also found in a soluble circulating form( Reference Power, Nongonierma and Jakeman 80 ). Casein-derived hydrolysates, and β-caseins in particular, have generally shown a significantly higher proportion of peptide sequences with DPP-4-inhibitory activity( Reference Power, Nongonierma and Jakeman 80 , Reference Lacroix and Li-Chan 112 ). However, as noted by Power et al. ( Reference Power, Nongonierma and Jakeman 80 ), if the DPP-4-inhibitory activity observed in vitro is to translate to humans it is essential that the bioavailability of these peptides is established.
Having identified several DPP-4-inhibitory peptides in gouda-type cheese, Uenishi et al. ( Reference Uenishi, Kabuki and Seto 34 ) performed an oral glucose test in rats to evaluate the effects of synthesised β-casein f70–77, the peptide with the highest DPP-4-inhibitory activity in the cheese. Administration of the synthesised peptide resulted in a significant reduction in blood glucose AUC, leading the authors to speculate that the peptide was absorbed directly into the bloodstream; however, this was not examined. Although limited, there is some evidence of an enhanced DPP-4-inhibitory activity in response to whey proteins in both animal models and human subjects. In mice, Gunnarsson et al. ( Reference Gunnarsson, Winzell and Deacon 113 ) observed that whey protein significantly enhanced both the insulin and the GIP response to glucose. They investigated whether these changes were associated with differences in DPP-4 activity and observed a significant reduction in DPP-4 activity in the duodenum – the main site of GIP synthesis, but not in the distal gut or plasma. One explanation is that protein fragments generated after whey protein digestion may serve as endogenous inhibitors of DPP-4 in the proximal gut( Reference Drucker 114 ). These findings were extended by Jakubowicz et al. ( Reference Jakubowicz, Froy and Ahrén 105 ) in a randomised cross-over clinical trial in human subjects with T2D. After a whey compared with water preload consumed before a breakfast meal, postprandial glucose levels were reduced by 28 %, insulin was increased by 105 % and both total GLP-1 and intact GLP-1 were higher by 141 and 298 %, respectively. However, there were no significant differences in plasma DPP-4 activity. Although intestinal DPP-4 activity was not assessed, one explanation may be that whey protein served as an endogenous inhibitor of DPP-4 in the proximal small intestine, but not in the plasma. Alternatively, amino acids and bioactive peptides generated during whey protein GI digestion may have directly stimulated L cells to secrete GLP-1 and other incretin hormones. Further studies are needed to examine the structure and sequence of casein- and whey-derived peptides with potential DPP-4-inhibitory activity in human subjects.
Skeletal muscle glucose uptake
Bioactive peptides may also act to regulate glucose uptake in skeletal muscle. Morifuji et al. ( Reference Morifuji, Koga and Kawanaka 115 ) found several dipeptides identified in whey protein hydrolysates, including Ile-Val, Leu-Val, Val-Leu, Ile-Ile, Leu-Ile, Ile-Leu and Leu-Leu, to stimulate glucose uptake in isolated skeletal muscle in vitro. Furthermore, in diabetic rats, treatment with β-casomorphin-7 lowered blood glucose and increased the expression of GLUT-4, the principal glucose transporter that mediates glucose uptake in muscle( Reference Han, Zhang and Wang 116 ). Further studies are needed to examine whether glucose uptake in skeletal muscle has a mechanistic role in the glycaemic management effects of milk protein-derived peptides in humans.
Conclusions and future directions
The current evidence base has established beneficial effects of milk proteins and/or their hydrolysates on blood glucose, insulin and incretin responses. Several mechanisms of action whereby milk proteins may exert bioactivity for glycaemic management have been proposed including enhanced amino acid availability, promotion of insulin and incretin secretion, DPP-4 inhibition and skeletal muscle glucose uptake. Milk-derived bioactive peptides may therefore offer a more diverse glycaemic management strategy compared with more targeted pharmacological approaches. However, despite this knowledge, it is currently relatively unknown as to which components of milk proteins (for example, peptide fractions, amino acids or interactions among them and gut endogenous proteins) mediate their physiological effects in humans. Moreover, while the acute effects of milk proteins are apparent, it is important for future studies to establish the long-term effects.
In order for milk protein-derived peptides to exert biological effects in humans, they must first be liberated from milk proteins by fermentation of milk, food processing and/or GI digestion. In addition, bioactive peptides must reach the small intestine and/or bloodstream intact, avoiding degradation from intestinal brush-border or serum peptidases. Various peptides have been identified in the GI tract and bloodstream after milk-protein ingestion, providing valuable insights into the potential bioavailability of milk protein-derived peptides. β-Casein-derived peptides in particular have both been identified in the GI tract in human subjects and shown to have beneficial effects for glycaemic management in vitro. However, the evidence base is limited and further work is needed to extensively characterise peptides present in the GI tract and bloodstream after milk-protein ingestion and to fully determine their functional significance in terms of glycaemic management. The availability of stable isotope labelling and recent analytical techniques now makes such extensive characterisation feasible and holds substantial potential for enhancing understanding of the structure–activity and bioavailability of milk protein-derived peptides in humans. Such knowledge is critical to improve the isolation and production of effective bioactive peptides for use in glycaemic management. Overall, while much more work is needed, milk protein-derived peptides appear to hold significant promise for future use as part of a functional food matrix or nutraceutical for glycaemic management.
Acknowledgements
The present review was supported by Enterprise Ireland through Food for Health Ireland.
All authors contributed to and approved the final version of the manuscript.
E. D. currently works for Glanbia but has no conflicts of interest. K. H. and L. B. declare no conflicts of interest.





