Introduction
Calcifying pseudoneoplasm of the neuraxis (CAPNON) is thought to be a rare tumor-like lesion that can occur anywhere in the neuraxis, including the brain, spinal cord, meninges, and their adjacent tissue. Since CAPNON was first characteristically described in 1978, Reference Rhodes and Davis1 more than 100 cases have been reported in the literature. Reference Barber, Low, Johns, Rich, MacDonald and Jones2–Reference Lu, Yang, Reddy and Wang4 However, its prevalence is likely underestimated, since CAPNONs could be an incidental finding in asymptomatic patients. Reference Lu, Yang, Reddy and Wang4 In addition, its radiological features are nonspecific. Reference Yang, Reddy, Ellenbogen, Wang, Bojanowski and Lu3–Reference Aiken, Akgun, Tihan, Barbaro and Glastonbury8 CAPNON is diagnosed exclusively by pathological examination in the form of a fibro-osseous lesion with the following histopathological features: granular amorphous confluent cores with calcification/ossification, peripheral palisading spindle to epithelioid cells, variable fibrous stroma, and foreign-body reaction with multinucleated giant cells. Reference Yang, Reddy, Ellenbogen, Wang, Bojanowski and Lu3–Reference Kocovski, Parasu, Provias and Popovic6,Reference Qian, Rubio and Powers9,Reference McCarthy10 However, these histopathological features are variable and nonspecific as they may overlap with some other disease entities. Reference Yang, Reddy, Ellenbogen, Wang, Bojanowski and Lu3–Reference Abdaljaleel, Mazumder and Patel11 Although a few immunohistochemical stains have been described in some studies, Reference Yang, Reddy, Ellenbogen, Wang, Bojanowski and Lu3,Reference Lu, Yang, Reddy and Wang4 none of them is diagnostic or characteristic of CAPNONs.
The pathogenesis of CAPNON also remains unknown. Several hypotheses have been proposed, including a reactive process, Reference Barber, Low, Johns, Rich, MacDonald and Jones2–Reference Kocovski, Parasu, Provias and Popovic6 degenerative process, Reference McCarthy10 metabolic dysfunction, Reference Abdaljaleel, Mazumder and Patel11 metaplastic transformation, Reference Hubbard, Qaiser, Clark and Tummala12 and neoplasm. Reference Rhodes and Davis1 The hypothesis of a reactive process associated with inflammation and/or injury has been favored, Reference Yang, Reddy, Ellenbogen, Wang, Bojanowski and Lu3,Reference Lu, Yang, Reddy and Wang4 but there are no observational studies to support it. The most distinguishing feature of CAPNON is its granular amorphous cores that represent varying degrees of calcification/ossification over time. It is unclear what constitutes these cores. Also, it is unclear why CAPNON is located specifically in the “neuraxis” despite its predominantly mesenchymal features. The objectives of our present study were to answer these questions and identify immunohistochemical markers that could be used to diagnose CAPNON and examine its pathogenesis.
Materials and Methods
Participants
Ethics approval was granted by the Hamilton Integrated Research Ethics Board. All participants gave informed consent for the research project. We retrospectively examined CAPNON specimens of 11 patients (median age: 60 years; 7 women, 4 men) who were diagnosed in the last 15 years from the hospitals of McMaster University (Hamilton, Ontario, Canada), University of Montreal (Montreal, Quebec, Canada), and Western University (London, Ontario, Canada). The diagnosis of CAPNONs was made by pathological examination revealing the characteristic histopathological features (described in Introduction) and excluding other disease entities by histopathology and immunohistochemistry (IHC). Reference Yang, Reddy, Ellenbogen, Wang, Bojanowski and Lu3,Reference Lu, Yang, Reddy and Wang4,Reference Kocovski, Parasu, Provias and Popovic6,Reference Haji, Alturkustani, Parrent, Megyesi, Gulka and Hammond7
For comparison, we also examined the specimens of the following groups: intervertebral disc disease from 7 patients (median age: 68 years; 4 women, 3 men), hippocampal sclerosis (International League Against Epilepsy or ILAE type 1) from 16 patients (median age: 28 years; 4 women, 12 men), and null-cell pituitary adenoma (a neuroendocrine tumor) from 8 patients (median age: 62.5 years; 6 women, 2 men). These comparison groups were chosen, because the tissue of intervertebral disc disease and CAPNON shared some similar histological features such as calcification/ossification; CAPNONs contained inflammatory cell infiltrates, reminiscent of the immune cell infiltrates in hippocampal sclerosis the pathogenesis of which may involve an autoimmune process Reference Lu, Steve, Wheatley and Gross13 ; null-cell pituitary adenomas also contained immune cell infiltrates, Reference Lu, Adam, Jack, Lam, Broad and Chik14 and possibly as well neurofilaments that were found in some other neuroendocrine tumors, which were described and discussed later in this study.
Histopathology and Immuno Histochemistry
The surgical resection or biopsy specimens were formalin fixed, routinely processed, paraffin-embedded, sectioned at 5 µm, and stained with hematoxylin and eosin (H&E) and by IHC. The H&E-stained slides were examined for histopathological features. Automated IHC was performed on tissue sections using the Dako Autostainer Link 48 and visualized with the Dako Envision Flex kit detection system (Dako, Carpinteria, CA, USA). Antibodies to the following were used: Neurofilament-light (NF-L; 2F11, Dako), Neurofilament-phosphorylated (NF-p; SMI 31, Covance, Berkeley, CA, USA), CD68 (KP1, Dako), CD8 (C8/144B, Dako), CD4 (4B12, Dako), glial fibrillary acidic protein (Z0334, Dako), Vimentin (V9, Dako), Desmin (D33, Dako), epithelial membrane antigen (E29, Dako), and synaptophysin (27G12, Leica). The antibodies listed here were only those immunostains described later in this study, while a few other immunostains and histological stains were also performed for the diagnosis of CAPNON in most cases. Reference Yang, Reddy, Ellenbogen, Wang, Bojanowski and Lu3,Reference Lu, Yang, Reddy and Wang4
Electron Microscopy
In three selected cases of CAPNONs, a small portion of the tissue was fixed in 2% glutaraldehyde, postfixed in 1% osmium tetroxide, dehydrated, and embedded in resin. Ultrathin sections were stained with uranyl acetate followed by lead citrate and examined with JEOL 1230 Transmission electron microscope (EM).
Immuno Histochemical Assessment
Immunohistochemical features of each case/section were reviewed and assessed. The positivity of NF-L and NF-p was scored using the following scheme: -, absent (except linear positivity of entrapped axons); +, <10%; ++, 10%–50%; +++, > 50% within the lesion cores. This IHC was not assessed in the control group of hippocampal sclerosis, ILAE type 1 or the control group of null-cell pituitary adenoma, due to the innate neurofilament (NF) positivity in the brain and posterior pituitary. Reference Lu, Steve, Wheatley and Gross13,Reference Lu, Adam, Jack, Lam, Broad and Chik14 The numbers of CD8+ cytotoxic T-cells and CD4+ T-cells were consistently counted in microscopic high-power fields (HPF, original magnification × 400), and the values represented sums of 10 consecutive HPFs in an area with the most frequent positive cells.
Statistical Analysis
Nonparametric statistical analysis was performed using GraphPad Prism 8 software. The 2-tailed Mann–Whitney U-test was used for comparisons between the groups of CAPNONs and one of the other diseases. P values < 0.05 were regarded as being statistically significant.
Results
All 11 CAPNONs exhibited the characteristic histopathological features (described in Introduction) that were diagnostic of CAPNON (Figures 1A–C, and 2A). The clinical and pathological characteristics of these patients were summarized in Table 1.

Figure 1: CAPNON magnetic resonance imaging and pathology. Head T1-weighted image with contrast shows a hypointense, focally calcified, enhancing lesion in the right cerebellopontine angle (A). The lesion exhibits amorphous to fibrillary cores, peripheral palisading cells, calcification/ossification (B), and multinucleated giant cells (C, arrows). Electron microscopy reveals the lesion cores containing fibrillary materials consistent with neurofilaments (D). The lesion cores demonstrate positive neurofilament-light (NF-L) immunostaining (E, F, with an inset of higher magnification) and negative neurofilament-phosphorylated (NF-p) immunostaining except occasional peripherally-located entrapped axons (G, arrows, versus F, arrows). The lesion contains focally scattered CD68+ macrophages (H), rare CD4+ T-cells (I), and focally frequent CD8+ T-cells (J). Original magnification, ×100 (B), ×200 (C, E, H–J), ×400 (F, G), and ×50,000 (D).
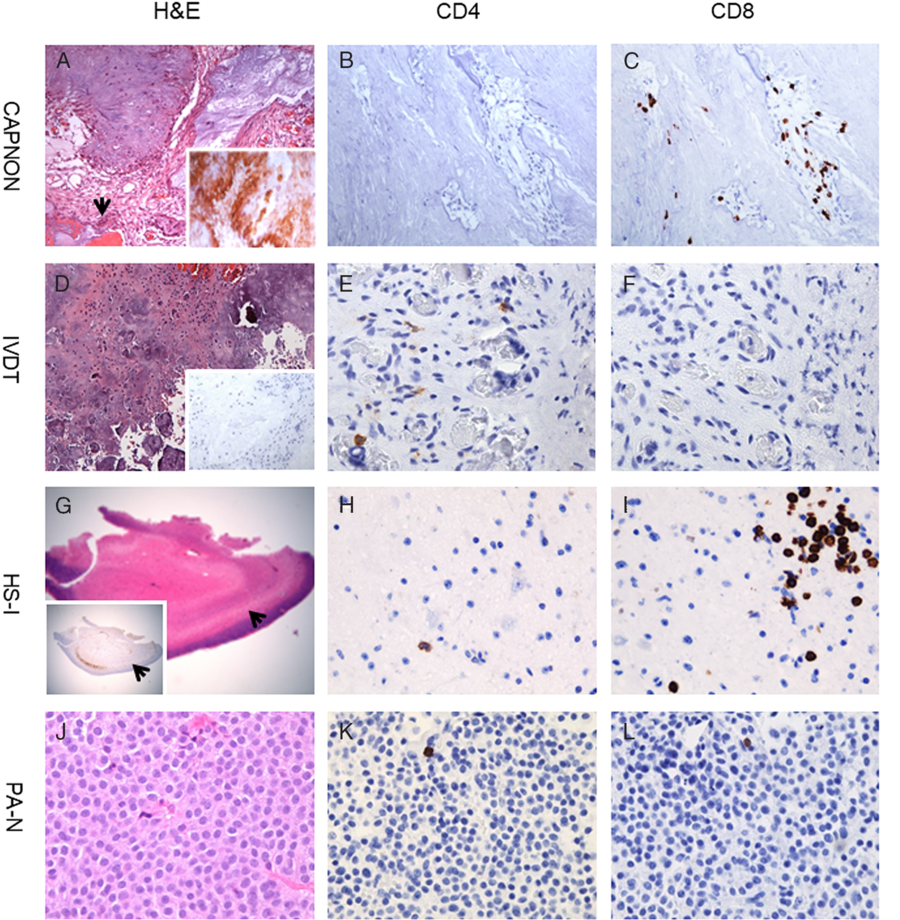
Figure 2: T-cells in CAPNON and other diseases. CAPNON (A) with positive neurofilament-light immunostaining (inset in A) shows no to rare CD4+ T-cells (B) and focally frequent CD8+ T-cells (C). IVDT (D) with negative neurofilament-light immunostaining (inset in D) exhibits rare CD4+ T-cells (E) and no CD8+ T-cells (F). HS-I (G) with Neu-N immunostain demonstrating neuronal loss in the hippocampus CA1 subfield (arrows; inset in G) demonstrates rare CD4+ T-cells (H) and focally frequent CD8+ T-cells (I). PA-N (J) shows rare CD4+ T-cells (K) and CD8+ T-cells (L). Original magnification, ×15 (G), ×100 (D), ×200 (A), and ×400 (B, C, E, F, H, I, J–L). CAPNON, calcifying pseudoneoplasm of the neuraxis; HS-I, hippocampal sclerosis ILAE type 1; IVDT, intervertebral disc disease tissue; PA-N, null-cell pituitary adenoma.
Table 1: Clinical and pathological characteristics of participants

Abbreviations: CAPNON, Calcifying pseudoneoplasm of the neuraxis; CT, computed tomography; F, female; HS-I, hippocampal sclerosis, type I; GTR, gross total resection; IHC, immunohistochemistry; IVDT, intervertebral disc disease tissue; M, male; mos, months; MRI, magnetic resonance imaging; ND, not done; N/A, not applicable; PA-N, pituitary adenomas, null cell; T1WI, T1-weighted imaging; T2WI, T2-weighted imaging; yrs, years.
IHC NF-L/NF-p: -, absent (except linear positivity of peripherally-located entrapped axons); +, < 10%; ++, 10–50%; +++, > 50% of the lesion cores.
Ultrastructural Features of CAPNON
EM in three representative cases (Cases 1, 2, and 6 in Table 1) revealed that the CAPNON cores contained fibrillary materials, mostly ranging from 8 to 13 nm in diameter with the average of about 10 nm (Figure 1D), morphologically consistent with NFs.
Neurofilament Positivity in CAPNON
To investigate the EM finding of fibrillary materials, we performed IHC with NF and other antibodies. NF-L was positive in the cores of all 11 CAPNON cases (Figures 1E, and 2A; Table 1); in comparison, no NF-L positivity was found in seven cases of intervertebral disc disease tissues (Table 1; Figure 2D). To further delineate the NF subunit in CAPNONs, we also examined NF-p IHC in four representative cases (Table 1). There was no NF-p positivity in the CAPNON cores, but the periphery of CAPNON lesions exhibited occasional NF-p+ and NF-L+ entrapped axons (Figure 1G versus 1F). The CAPNON cores stained negative for the other intermediate filaments, including glial fibrillary acidic protein, vimentin, desmin, and synaptophysin (not shown) in several representative cases. Epithelial membrane antigen immunostaining was limited at the periphery (likely due to the leptomeningeal involvement) or completely negative in these CAPNONs, consistent with our previous reviews. Reference Yang, Reddy, Ellenbogen, Wang, Bojanowski and Lu3,Reference Lu, Yang, Reddy and Wang4
T-cell Infiltrates in CAPNON
Given the reported autoimmunity to NF-L Reference Puentes, van der Start and Boomkamp15,Reference Puentes, van der Star and Victor16 and comorbidity of CAPNON and immune-mediated diseases in at least two patients of this series (Cases 6 and 7 in Table 1), we further examined immune cell infiltrates in 11 cases of CAPNONs. All CAPNONs showed some CD68+ macrophages, and variable infiltration of CD8+ cytotoxic T-cells with rare to no CD4+ T-cells (Figures 1H–J, 2A–C, and 3).

Figure 3: T-cells in CAPNON compared with other diseases. CAPNONs (n = 11) show significantly more CD8+ T-cells with less CD4+ T-cells and a decreased CD4/CD8+ ratio, compared with IVDT (intervertebral disc disease tissue; n = 7), HS-I (hippocampal sclerosis, ILAE type 1; n = 16) and PA-N (null-cell pituitary adenoma; n = 8). In the box graphs, values represent the median and 25/75 percentile, with the horizontal lines inside each box indicating the median and whiskers demonstrating 5/95 percentile, from the patients in each group. ***, p < 0.001 (versus CAPNONs) by the 2-tailed Mann–Whitney U-test; ns, not significant (versus CAPNONs); n/a, not applicable (because of 0 in some IVDT cases).
The amount of CD4+/CD8+ T-cells within CAPNONs was quantified and compared to that of intervertebral disc, hippocampal sclerosis ILAE type 1, and null-cell pituitary adenoma. The infiltration of CD8+ T-cells was significantly greater in the group of 11 CAPNONs than that of 7 intervertebral disc disease tissues (p < 0.0001; Figure 2D versus 2A, and 2F versus 2C), and that of 8 null-cell pituitary adenomas (p = 0.0002; Figure 2J versus 2A, and 2L versus 2C), but not significantly different from that of 16 intra-axial lesions of hippocampal sclerosis ILAE type 1 (p = 0.05; Figure 2G versus 2A, and 2I versus 2C). There was no significant difference (p > 0.05) in the infiltration of CD4+ T-cells between the groups of CAPNONs and any one of the other diseases (Figure 2E, H, K versus 2B). While the ratio of CD4+/CD8+ T-cells is normally greater than 1.5 in the blood Reference McBride and Striker17 and higher in the brain tissue, Reference Hansen, Schwing and Önder18 this ratio in the group of CAPNONs (median: 0.066) was significantly lower than that in the group of null-cell pituitary adenomas (median: 3.214; p < 0.0001), but not significantly different from that in the group of hippocampal sclerosis ILAE type 1 (median: 0.085; p = 0.584; Figure 3). This suggests that CAPNON, like hippocampal sclerosis ILAE type 1, may be an immune-mediated process. Reference Lu, Steve, Wheatley and Gross13
Discussion
Our present work is one of the largest CAPNON case series published to date, and the first case–control/observational study to examine biomarkers in the diagnosis and pathogenesis of CAPNON. This study provides several novel observations, including the aggregation of NF-L in the CAPNON granular amorphous cores, entrapment of the axons at the CAPNON periphery, and the infiltration of CD8+ cytotoxic T-cells with a decreased ratio of CD4/CD8+ T-cells in CAPNONs. Our findings suggest that IHC of NF-L and CD4/CD8+ may serve as the markers to diagnose CAPNON in clinical/pathological practice and investigate the pathogenesis of CAPNON.
NF is a neuronal cytoplasmic protein highly expressed in axons, where they contribute to the cytoskeleton and facilitate axonal transport. Reference Puentes, van der Start and Boomkamp15,Reference Puentes, van der Star and Victor16,Reference Gaetani, Blennow, Calabresi, Di Filippo, Parnetti and Zetterberg19,Reference Khalil, Teunissen and Otto20 Mammalian NF is composed of three major subunits based on their molecular weight: NF-light (NF-L, ∼70 kDa), medium (NF-M, ∼160 kDa), and heavy (NF-H, ∼200 kDa, or NF-p). The levels of NF-L increase in cerebrospinal fluid and blood proportionally to the degree of axonal damage in a variety of neurological disorders, including inflammatory, neurodegenerative, traumatic, and cerebrovascular diseases. Therefore, NF-L has been considered as a promising diagnostic and prognostic biomarker of axonal damage in these neurological diseases. Reference Gaetani, Blennow, Calabresi, Di Filippo, Parnetti and Zetterberg19,Reference Khalil, Teunissen and Otto20 NF-L corresponds to the IHC 2F11 antibody, directed against ~70 kDa protein. Reference van Muijen, Ruiter, van Leeuwen, Prins, Rietsema and Warnaar21,Reference Miettinen, Virtanen and Talerman22 In our present study, the finding of NF-L (rather than NF-p) in all CAPNON cores indicates that CAPNONs contain NF-L, and suggests that IHC with NF-L can aid the diagnosis of CAPNONs, along with histological examination.
The IHC positivity of NF-L is also seen in neuroendocrine tumors in a few sites including cutaneous tissue, gastrointestinal tract, breast, lung, and mediastinum, Reference Astarloa, Sánchez-Franco, Cacicedo and García-Villanueva23–Reference Miettinen, Lehto, Dahl and Virtanen25 as well as the testis and testicular germ-cell tumors. Reference Miettinen, Virtanen and Talerman22 The presence of NF-L in the testis and testicular germ-cell tumors has been attributed to the involvement of neural tissue. Reference Miettinen, Virtanen and Talerman22 Our present study has identified NF-L+ (and NF-p+) entrapped axons at the periphery of CAPNONs, in addition to the aggregation of NF-L in the CAPNON cores, which supports the involvement of neural tissue in the formation of CAPNONs. In the neuraxis, CAPNONs can occur either in the central nervous system (intra-axial, brain, or spinal cord parenchymal tissue) or peripheral nervous system (extra-axial, nerves), and their occurrence may be associated with preferential entrapment of the adjacent neural tissue that is presumably the source of NF-L in CAPNONs. In extra-axial CAPNONs, there are NF-L debris and fragmented axons that may be identified at the lesion periphery (as demonstrated in Figure 1F). The CAPNON cores could be formed with the aggregation of tissue debris containing NF-L from the damaged axons, which then calcify or ossify over time; and the surrounding tissue components of CAPNONs are likely reactive in nature. This hypothesis of CAPNON formation due to axonal entrapment from the adjacent neural tissue may explain the specific location of “neuraxis” for CAPNON.
The clinical manifestations of CAPNONs may be highly variable, ranging from a subclinical lesion found incidentally on pathological examination to marked tumor-like mass effect, mainly depending on the lesion location, size, and comorbidity (Table 1). Reference Rhodes and Davis1–Reference Qian, Rubio and Powers9 Intracranial CAPNONs most commonly manifest with headaches and/or seizures (in approximately 1/3 of the cases) due to the localized mass effect. Reference Barber, Low, Johns, Rich, MacDonald and Jones2 Incidental CAPNONs are not very uncommon, especially when they occur in association with other lesions including tumors. Reference Rhodes and Davis1,Reference Lu, Yang, Reddy and Wang4 CAPNONs have been also occasionally reported in collision with tumors such as dysembryoplastic neuroepithelial tumor, low-grade gliomas, meningioma, and lipomas, as well as rheumatoid nodules. Reference Lu, Popovic, Provias and Cenic28 In these cases of CAPNONs with coexisting or collision lesions, the presentation of CAPNONs may be overwhelmed by that of the other lesions.
Although the pathogenesis of CAPNON needs further investigation, we propose an autoimmune-mediated process in the pathogenesis of CAPNONs, based on the following observations: (i) our findings of CD8+ cytotoxic T-cell infiltration with a decreased ratio of CD4/CD8+ T-cells; Reference McBride and Striker17,Reference Hansen, Schwing and Önder18 (ii) no difference in the infiltration of CD8+ cytotoxic T-cells or the CD4/CD8+ ratio between the specimens of CAPNONs and hippocampal sclerosis ILAE type 1, likely an autoimmune process Reference Lu, Steve, Wheatley and Gross13 ; (iii) CAPNONs containing NF-L, an autoimmune target identified in several neurological diseases, and the aggregation of NF-L likely associated with an autoimmune process in CAPNONs. Reference Puentes, van der Start and Boomkamp15,Reference Puentes, van der Star and Victor16 A recent case report showed a high ratio of infiltrating M2 (CD163+, alternatively activated)/M1 (CD68+/IBA1+, classically activated) macrophages and a high uptake of 11C-methionine positron emission tomography in an intra-axial CAPNON associated with an adjacent lipoma and agenesis of the corpus callosum, supportive of an activated immune response. Reference Inukai, Shibahara and Hotta26 We also reported a similar case (case 8 in Table 1) with immune cell infiltrates and inflammatory response. Reference Lu, Berthelet and Bojanowski27 Another recent case report from our team demonstrated collision lesions of CAPNON and rheumatoid nodules in a patient with systemic lupus erythematous (an autoimmune disease; case 6 in Table 1) as well as transitional lesions from rheumatoid nodules to CAPNON lesions. Reference Lu, Popovic, Provias and Cenic28 These case reports further support our hypothesis of an immune-mediated process in CAPNONs.
The limitations of our present study include the lack of molecular studies such as the western blotting to confirm the presence of NF-L corresponding to IHC in CAPNONs. As the preoperative diagnosis of CAPNONs was challenging, no fresh or frozen tissue had been collected from our cases of CAPNONs. Nevertheless, IHC positivity of NF-L can help with the diagnosis of CAPNON. Another limitation is the relatively small number of cases presented here partially due to the rarity and diagnostic challenges of CAPNONs. Future studies in a larger number of cases may be needed to verify our present findings in this study.
Conclusion
The expression of NF-L and a decreased ratio of CD4/CD8+ T-cells can aid the diagnosis of CAPNON. We propose that the pathogenesis of CAPNON is likely immune-mediated, with the aggregation of NF-L and immune cell infiltrates. Since both NF and T-cells have been considered for the therapeutic targets, Reference Gaetani, Blennow, Calabresi, Di Filippo, Parnetti and Zetterberg19,Reference Khalil, Teunissen and Otto20,Reference Kallies, Zehn and Utzschneider29 these immunohistochemical markers may not only be diagnostic but also potentially therapeutic.
Acknowledgements
The authors thank Ms. Bruna Capretta (Hamilton General Hospital) for administrative assistance and Dr. Lee Cyn Ang (Western University) for his advice on the present study. The present study included seven case reports published by our authors, Reference Yang, Reddy, Ellenbogen, Wang, Bojanowski and Lu3,Reference Lu, Yang, Reddy and Wang4,Reference Kocovski, Parasu, Provias and Popovic6,Reference Haji, Alturkustani, Parrent, Megyesi, Gulka and Hammond7,Reference Lu, Berthelet and Bojanowski27,Reference Lu, Popovic, Provias and Cenic28 and two previously published case series which served as controls for CAPNONs, Reference Lu, Steve, Wheatley and Gross13,Reference Lu, Adam, Jack, Lam, Broad and Chik14 but there is no duplication of the principal data or demonstration between this study and our other publications.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Statement of Authorship
KY and JQL designed the study. KR, BHW, AC, JP, SP, WHY, FB, MWB, RH, and JQL provided patient data and analysis. KY and JQL extracted the data and wrote the manuscript. All authors critically reviewed and approved the final manuscript.






