Throughout history, various cultural, economic, social, and individual factors have influenced the trend in breastfeeding practices. At the beginning of the 20th century in Europe, the prevalence of initiation of breastfeeding was generally high, although regional differences especially between rural and urban areas existed (Thorvaldsen, Reference Thorvaldsen2008). For example, in Norway about two-thirds of mothers breastfed, and in Germany the prevalence of initiation of breastfeeding increased from 75% to 95% between 1910 and 1937 (Thorvaldsen, Reference Thorvaldsen2008). This increase was probably due to the progressive understanding of the health benefits of breastfeeding (Bryder, Reference Bryder2009). In post-World War II Europe, however, the prevalence of initiation of breastfeeding declined until the 1960s, partly because of economic reasons, including mothers joining the labor force on an unprecedented scale. Since the 1970s, however, the prevalence of initiation of breastfeeding has increased (Thorvaldsen, Reference Thorvaldsen2008; Yngve & Sjostrom, Reference Yngve and Sjostrom2001).
In the Netherlands, the prevalence of initiation of breastfeeding has displayed some variation since 1850. Infant mortality rates dropped from over 25% in 1850 to less than 10% in the 1920s, which has been linked to an increase in breastfeeding rates (Walhout, Reference Walhout2010), although numerous other factors obviously contributed as well. During World War II, breastfeeding rates dropped, presumably due to stress and malnutrition (Bulk-Bunschoten et al., Reference Bulk-Bunschoten, van Bodegom, Reerink, Pasker-de Jong and de Groot2001). Prevalence of initiation of breastfeeding increased after the war, then dropped to a low point in the early 1970s, and has been increasing ever since. At the beginning of 21st century in the Netherlands, 78–91% of mothers initiated breastfeeding (Lanting et al., Reference Lanting, Van Wouwe and Reijneveld2005; van Rossem et al., Reference van Rossem, Vogel, Steegers, Moll, Jaddoe, Hofman and Raat2010). However, after 6 months only 15% of infants are fully breastfed, that is, do not receive any formula milk, but may receive some other complementary foods (Lanting et al., Reference Lanting, Van Wouwe and Reijneveld2005); while in total, 31–33% are partially or fully breastfed, that is, receive some breastmilk in addition to other foods or formula (Lanting et al., Reference Lanting, Van Wouwe and Reijneveld2005; van Rossem et al., Reference van Rossem, Vogel, Steegers, Moll, Jaddoe, Hofman and Raat2010).
Mothers of twins initiate breastfeeding less often than mothers of singletons (McDonald et al., Reference McDonald, Pullenayegum, Chapman, Vera, Giglia, Fusch and Foster2012; Yokoyama et al., Reference Yokoyama, Wada, Sugimoto, Katayama, Saito and Son2006). This may be related to maternal health problems, complications in delivery, lower birth weight, or poorer sucking ability in twins and the stress related to caring for two infants instead of one. In general, stressful life experiences, abuse, low socioeconomic status, low birth weight of the baby, and complications during pregnancy or delivery are associated with a decrease in the likelihood of initiating breastfeeding or, once initiated, with low persistence in breastfeeding (Kendall-Tackett, Reference Kendall-Tackett2007; Li et al., Reference Li, Kendall, Henderson, Downie, Landsborough and Oddy2008).
The World Health Organization recommends breastfeeding the infant exclusively for 6 months, and partially, along with complementary foods until the child is 1–2 years old. Breastfeeding benefits the health of both the mother and the child. For example, breastfeeding has been found to be associated with a reduced risk of type 2 diabetes, breast and ovarian cancer, and postpartum depression (Ip et al., Reference Ip, Chung, Raman, Chew, Magula, DeVine and Lau2007). Although the evidence is mixed regarding several health outcomes, the strongest evidence has been found for the association between breastfeeding and reduced risk of breast cancer (Anothaisintawee et al., Reference Anothaisintawee, Wiratkapun, Lerdsitthichai, Kasamesup, Wongwaisayawan, Srinakarin and Thakkinstian2013; Bernier et al., Reference Bernier, Plu-Bureau, Bossard, Ayzac and Thalabard2000; Collaborative Group on Hormonal Factors in Breast Cancer, 2002; Ip et al., Reference Ip, Chung, Raman, Chew, Magula, DeVine and Lau2007; Lichtenstein et al., Reference Lichtenstein, Holm, Verkasalo, Iliadou, Kaprio, Koskenvuo and Hemminki2000). In addition, wider family, economic, and environmental advantages are associated with breastfeeding (Gartner et al., Reference Gartner, Morton, Lawrence, Naylor, O’Hare, Schanler and Eidelman2005). For instance, although it may represent a burden on the mother, it is free and naturally available without extra financial costs to the individual. Regarding health benefits, breastfeeding may be important for those who have genetic vulnerability to disease. For example, breastfeeding is related to lower risk of adult depression in offspring, especially among those with a higher genetic risk for psychopathology (Merjonen et al., Reference Merjonen, Jokela, Salo, Lehtimäki, Viikari, Raitakari and Keltikangas-Järvinen2012). Thus, breastfeeding may confer specific benefits to people who are at a specific genetic risk for disease. In addition, part of the heritability in disease, such as breast cancer, may be genetically mediated via lack of breastfeeding. Those who have inherited difficulties in breastfeeding or a tendency not to breastfeed may have higher risk of breast cancer, if they have not breastfed. On the other hand, not breastfeeding and breast cancer may be caused by same genetic or environmental factors without any causal link between each other, meaning that lack of breastfeeding does not cause breast cancer, but they both are caused by the same genetic or environmental factors. However, at present, little is known about the heritability of breastfeeding per se. The aim of the present article is therefore to establish the heritability of breastfeeding using data from twins, their sisters, and their mothers in the Netherlands.
There is one other study on the heritability of breastfeeding (Colodro-Conde et al., Reference Colodro-Conde, Sanchez-Romera and Ordonana2013), which included 390 pairs of Spanish female twins of whom 87.2% had breastfed at least one of their children. Forty-nine percent of the variance in initiation of breastfeeding of the firstborn child was due to additive genetic factors, while the remaining variance was explained by environmental factors unshared by the twins (Colodro-Conde et al., Reference Colodro-Conde, Sanchez-Romera and Ordonana2013). The absence of shared environmental influences means that twin resemblance in breastfeeding practice was due to genetic factors only. However, the classical twin design is limited in its power to detect shared environmental or dominant genetic effects (Posthuma & Boomsma, Reference Posthuma and Boomsma2000; Rietveld et al., Reference Rietveld, Posthuma, Dolan and Boomsma2003). An extended family design, as applied in this article, furnishes a more detailed account of the factors contributing to individual differences in initiation of breastfeeding.
Our aim is to study the heritability of initiation of breastfeeding in an extended twin design including twins, their sisters, and their mothers, that is, in twin-twin, twin-sister, and mother-daughter pairs. Including additional family members to the model allows us to separate the special twin shared environment, that is, environmental factors that are shared by the twins, but not with rest of the family, and will provide more power to detect possible shared environmental effects. In addition, it enables testing whether the prevalence of initiation of breastfeeding is lower among mothers of twins than in mothers who themselves are twins or sisters of twins. The women included in the sample varied greatly in age and were born in different time points during the 20th century. This allows us to study the prevalence of initiation of breastfeeding by birth cohort.
Materials and Methods
Participants
The present study is part of a large, ongoing, longitudinal twin-family study of the Adult Netherlands Twin Register (ANTR; Willemsen et al., Reference Willemsen, Vink, Abdellaoui, den Braber, van Beek, Draisma and Boomsma2013). Women with at least one biological child, and information on breastfeeding were included. Information on breastfeeding was reported in the eighth follow-up survey of ANTR. In total, data from 5,581 individuals from 4,570 families were analyzed, including 1,182 MZ twins, 1,048 DZ twins, 357 sisters, and 2,994 mothers of twins, with a maximum of 4 individuals per family (i.e., only data from one sister were selected. This was done to simplify data management). The sample included 337 complete MZ twin pairs, 129 complete DZ twin pairs, 168 twin-sister pairs, and 533 mother-daughter pairs (Table 1).
TABLE 1 Number of Individual Twins, Sisters and Mothers and Complete Twin-Twin, Twin-Sister, and Mother-Daughter Pairs from 4,570 Families

Zygosity was determined on the basis of parent/self-report items about physical resemblance between twins. In a subsample, zygosity was determined on the basis of measured genetic polymorphisms. The agreement between the item-based zygosity assessment and the DNA assessment was over 96% (Willemsen et al., Reference Willemsen, Vink, Abdellaoui, den Braber, van Beek, Draisma and Boomsma2013). Zygosity information was available for 2,139 twins (including 466 complete twin pairs) of whom 1,182 were MZ and 957 DZ. Twins with unknown zygosity (N = 91) all came from incomplete twin pairs.
Main Variables
The twins, sisters, and mothers provided information on breastfeeding for each of their children on the basis of a six-category item (1 = never breastfed, 2 = less than 2 weeks breastfed, 3 = 2–6 weeks breastfed, 4 = 6 weeks–3 months, 5 = 3–6 months, 6 = more than 6 months breastfed). For the present study, we recoded the data to define a binary phenotype comprising ever (scored 1) versus never breastfed (scored 0). This phenotype is associated with greater reliability after a long recall period (Li et al., Reference Li, Scanlon and Serdula2005). In addition, we clustered the information for multiple children, so those who breastfed at least one child for any duration were coded as ‘ever breastfed’ (1) and those who never breastfed any of their children as ‘never breastfed’ (0).
Birth cohort (the birth year of participant) ranged from 1911–1991 and was recoded into a four category variable, with values 0 = born before 1955, 1 = 1955–1964, 2 = 1965–1974, 3 = 1975 or later. The categorized variable explained 80% of the variance in the original birth cohort variable.
Statistical Analysis
Prevalence of initiation of breastfeeding, birth cohort, and maternal age at first childbirth were summarized using SPSS 21. Next, thresholds, twin-twin, twin-sister, and mother-daughter correlations, and genetic and environmental variances were estimated using a liability-threshold model in R 3.1.1 (http://www.r-project.org) using the SEM package OpenMx 1.4 (Boker et al., Reference Boker, Neale, Maes, Wilde, Spiegel, Brick and Fox2011).
In the liability-threshold model, the dichotomous breastfeeding variable is assumed to have an underlying continuous, standard normally distributed liability with a threshold that divides the liability into the two discrete categories (Falconer & Mackay, Reference Falconer and Mackay1996; Neale et al., Reference Neale, Eaves and Kendler1994). The value of the threshold determines the probability of the two outcomes. For instance, a threshold value of 0 on the standard normal liability implies the probabilities 0.5 (0; never breast fed) and 0.5 (1; ever breast fed). To account for cohort effects on the probability of initiation of breastfeeding, we added a cohort (coded 0 to 3) to model the threshold as a linear regression function of the cohort (threshold = b0 + b1* cohort). In this regression model, the intercept (b0) was constrained to be equal for twins, sisters, and mothers, so that the variation in threshold is a function of cohort only. We then tested the regression model, in which the intercept was constrained to be equal in twins and sisters (b0t1), but different in the mothers of twins (b0t2; see Figure 1), and the model in which the intercept was free to differ between twins, sisters, and mothers of twins. Cohort was expected to have the same effect for twins, sisters, and mothers, and thus b1 was constraint to be equal between twins, sisters, and mothers in all the models.
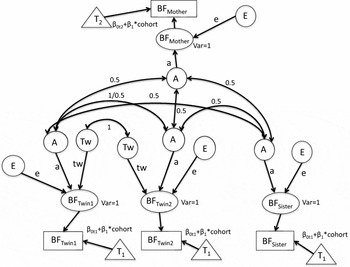
FIGURE 1 The full path diagram with additive genetic (A), specific shared twin environment (Tw), and unshared environment (E). Intercepts for liability thresholds (βot1 and βot2) are constrained to be equal between twins and sisters (βot1) and different for mothers (βot2) to estimate different thresholds for mothers of twins (T2) versus twins and their singleton sisters (T1). The threshold intercept as depicted varies between mothers and daughters. We also fitted a model with a single threshold intercept. The cohort's slope (β1) for liability threshold for initiation of breastfeeding threshold is constrained to be equal between twins, sisters and mothers, that is, the cohort effect is the same for twins, sisters, and mothers. Phenotypic variances are constrained to be 1 and to be equal between MZ and DZ.
Based on the visual inspection of the twin-family correlations, we initially fitted an ATwE model, including additive genetic (A), unshared environmental (E), and special twin shared environmental influences (Tw), that is, representing common influences shared only by the twins (see Figure 1). The expected correlations between the relatives equal:
-
a2 + tw 2 for MZ twin pairs,
-
0.5a2 + tw 2 for DZ twin pairs,
-
0.5a2 for twin-sister pairs, and
-
0.5a2 for mother-daughter pairs,
where a2 is the proportion of variance explained by genetic factors, and tw 2 is the proportion of variance that is explained by correlated environmental influences in twin sisters. As mentioned above, liability variance is constrained at 1, and thus the estimate for e2 (the proportion of variance explained by unique environmental factors) is given by 1-a2 or 1-a2-tw 2, depending on the model.
Results
Table 2 shows the descriptive statistics of the sample. Mean age of the participants was 49.29 (SD = 11.36, ranging from 19 to 97) years at the time of reporting on their breastfeeding, while they were 27.92 (SD = 4.14) years of age on average when their oldest child was born. 4,177 (74.8%) had breastfed at least one of their children (the percentage was lower in the mothers of twins: 68.3%), and the average number of children was 2.72 (SD = 1.21).
TABLE 2 Descriptives: 5,581 Individuals from 4,570 Families

SD = standard deviation.
The probability of initiating breastfeeding increased with birth cohort. Table 3 shows first the fitting of the saturated threshold model (threshold = b0 + b1*cohort). We tested whether the threshold is equal between all family members. In the best fitting threshold model, where the twins and sisters had an equal intercept, but mothers of twins had a different intercept, the intercept is b0t1 = -0.80 for twins and sister and b0t2 = -0.42 for mothers, and the slope is b1 = -0.078. Thus, given our coding of cohort as 0,1,2,3, the thresholds are -0.799, -0.877, -0.955, and -1.033 for twins and sisters, and -0.422, -0.500, -0.578, and -0.656 for mothers, in cohorts 0 to 3, respectively. The corresponding prevalences are 0.79, 0.81, 0.83, and 0.85 for twins and sisters, and 0.66, 0.69, 0.72, and 0.74 for mothers. Thus, among those who were born before 1955, 79% of twins and sisters and 66% of mothers of twins had initiated breastfeeding with at least one of their children, while among those born 1975 or after, the prevalence of initiation of breastfeeding was already 85% and 74%, respectively.
TABLE 3 Model Fitting on Initiation of Breastfeeding

Best fitting models are bolded (the smaller AIC is, the better fitting the model is); -2LL = -2 log likelihood; df = degrees of freedom; AIC = Akaike Information Criterion; χ2 = chi squared; b0t1 = intercept for threshold for twins; Δdf = change in degrees of freedom; p value = statistical significance; b0t2 = separate intercept for threshold for mothers; b0t3 = separate intercept for threshold for sisters; b1 = regression slope for cohort on the threshold; a1 = unstandardized additive genetic path estimate; tw1 = unstandardized shared twin environment path estimate; e1 = unstandardized unshared environmental path estimate; A = standardized estimate of additive genetic variance; Tw = standardized estimate of special shared twin environmental variance; E = standardized estimate of unshared environmental variance; ATwE: genetic (A), shared twin environment (Tw) and unique environment (E); AE: no shared twin environment (Tw).
In the best fitting saturated model, we estimate the tetrachoric correlations, that is, the correlations among the family members at the level of the liability. The MZ twin correlation was estimated at 0.72 (95% confidence interval: 95% CI being 0.57–0.84), the DZ twin correlation at 0.56 (0.26–0.77), the twin-sister correlation at 0.21 (-0.14–0.52), and the mother-daughter correlation at 0.27 (0.10–0.43). Since the phenotypic correlation in MZ twins (rMZ) was not perfect (rMZ < 1), unshared environment (including measurement error) contributed to the variance of initiation of breastfeeding. Furthermore, since the DZ twin correlation (rDZ) is larger than half of the MZ twin correlation (rDZ > 0.5 rMZ), shared environment may be significant. Additionally, shared environment appears to be twin specific, because the twin-sister correlation (0.21) is smaller than half of the DZ twin correlation (0.56) and the mother-daughter correlation (0.27) is equal to the twin-sister correlation.
The threshold and genetic model fitting results are presented in Table 3. As stated above, the best fitting threshold model includes two thresholds (one for twins and sisters and one for mothers of twins). Based on the tetrachoric correlations, first, an ATwE model with additive genetic effects (A), special twin shared environmental effects (Tw) and unique environmental effects (E) was fitted to the data (Akaike information criterion (AIC) = -5,119.74). Second, we fitted a nested AE model to test whether the specific shared twin environment can be dropped out of the model without significantly reducing the fit of the model statistically. In the model with two thresholds (one for twins and sisters and one for mothers of twins), the specific twin environment can be dropped out of the model without significantly reducing the fit of the model statistically (AIC = -5,118.20, χ2(1) = 3.55, p = .06 for AE model). However, because the one threshold model is more parsimonious than the model with two thresholds, we also tested genetic model fit with only one threshold (AIC = -5,050.12 for ATwE and AIC = -5,047.27 for AE). Based on the AIC values, these could be rejected, because the smaller the AIC, the better the model.
The AE model with two threshold intercepts is the best fitting model, with standardized heritability and unique environmental estimates for initiation of breastfeeding being 0.70 (0.67–0.73) and 0.30 (0.27–0.33), respectively. Thus, 70% of individual differences, that is, the variance in the liability to initiate breastfeeding is explained by genetic factors. Unshared environment, on the other hand, explained 30% of the variance, that is, individual differences in the liability of initiation of breastfeeding.
Discussion
We studied the prevalence of the initiation of breastfeeding and its heritability in the Netherlands among mothers born between 1911 and 1991. Prevalence of initiation of breastfeeding increased with a mother's year of birth. In addition, the prevalence of initiation of breastfeeding was lower in mothers of twins than in twins and their sisters. In mothers born before 1955, 66% of mothers of twins and 79% of mothers who themselves were twins or sisters of twins had initiated breastfeeding, while in mothers born 1975 or later, 74% of mothers of twins and 85% of mothers who themselves were twins or sisters of twins, had initiated breastfeeding with at least one of their children.
The lower prevalence of initiation of breastfeeding among mothers of twins than among mothers of singletons has been observed before (McDonald et al., Reference McDonald, Pullenayegum, Chapman, Vera, Giglia, Fusch and Foster2012; Yokoyama et al., Reference Yokoyama, Wada, Sugimoto, Katayama, Saito and Son2006) and may be related to the stressfulness and difficulty of rearing two infants instead of one. Not breastfeeding is related to maternal perceived stress and health (Mezzacappa et al., Reference Mezzacappa, Guethlein and Katkin2002). Mothers may feel safer not to initiate breastfeeding with twins to ensure that also other people can help in feeding them. Mothers may also be worried whether they can produce enough milk. Formula and bottle-feeding increase an infant's weight more rapidly than breastfeeding (Gibbs & Forste, Reference Gibbs and Forste2014), and because twins are born with a lower birth weight, mothers may want to compensate for this with formula feeding. In addition, premature or low birth weight twins have poorer sucking ability, which is related to lower probability to initiate breastfeeding (Yokoyama et al., Reference Yokoyama, Wada, Sugimoto, Katayama, Saito and Son2006).
Several demographic, psychosocial, cultural, attitudinal, and societal changes are suggested to have influenced the increase in the prevalence of the initiation of breastfeeding. For example, more educated (Bartels et al., Reference Bartels, van Beijsterveldt and Boomsma2009; Sutherland et al., Reference Sutherland, Pierce, Blomquist and Handa2012; van Rossem et al., Reference van Rossem, Oenema, Steegers, Moll, Jaddoe, Hofman and Raat2009) and non-smoking mothers (Lanting et al., Reference Lanting, Van Wouwe and Reijneveld2005) are more likely to initiate breastfeeding, while older mothers are more successful in breastfeeding, if initiated (Merjonen et al., Reference Merjonen, Jokela, Pulkki-Råback, Hintsanen, Raitakari, Viikari and Keltikangas-Järvinen2011; Sutherland et al., Reference Sutherland, Pierce, Blomquist and Handa2012; van Rossem et al., Reference van Rossem, Oenema, Steegers, Moll, Jaddoe, Hofman and Raat2009). Both maternal education and age at childbirth have increased with later cohorts (Baird et al., Reference Baird, Collins, Evers, Leridon, Lutz and Velde2010; Mills et al., Reference Mills, Rindfuss, McDonald and Velde2011), while smoking has decreased (Vink & Boomsma, Reference Vink and Boomsma2011). Dutch mothers stop breastfeeding mainly because of insufficient breastfeeding technique and maternal working obligations (Lanting et al., Reference Lanting, Van Wouwe and Reijneveld2005). Higher education of the parents, non-smoking, having a full-time job, being primiparous and home-delivery of full-term infant increase the probability of initiation of breastfeeding, and higher education, non-smoking, working less than 16 hours per week and delivering the infant at home are associated with longer breastfeeding duration (Lanting et al., Reference Lanting, Van Wouwe and Reijneveld2005). Thus, several societal factors, such as baby-friendly working environment, possibility for maternity/paternity leaves, equal opportunity for education and work, development in health care system, possibility for family planning, support for mothers and their families and teaching the best techniques for breastfeeding, may increase the possibilities and willingness to breastfeed and enhance the development of better parent-child relationship (Yngve & Sjostrom, Reference Yngve and Sjostrom2001).
In the best fitting model, heritability explained 70% of individual differences in initiation of breastfeeding, while 30% was explained by unshared environmental experiences. The heritability found for individual differences in initiation of breastfeeding in the present study was higher than in previous research in Spain, in which 49% of the variance in initiation of breastfeeding of the firstborn child was explained by genetic factors (Colodro-Conde et al., Reference Colodro-Conde, Sanchez-Romera and Ordonana2013). Although this is lower than the present estimate (70%) in the current study, it is similar to the heritability in the one threshold model, or in the model including twin-specific environment in the present Dutch data. In addition, we should recognize that in the Spanish study, initiation of breastfeeding of the firstborn child was studied, while in the present paper initiation of breastfeeding of any of the children was studied. Based on a visual inspection of the correlations and their confidence intervals from the saturated model, one could have expected twin-specific shared environment to be found. Although not statistically significant, reduction of the fit of the final model, where the twin specific shared environment is dropped out, approaches statistical significance (p = .06). Note, however, that confidence intervals are estimated one at a time while twin-sister, DZ and mother-daughter correlations are not independent. Thus, simple visual inspection of the confidence intervals does not really suffice, and the appropriate test is the likelihood ratio test of the special twin common environment parameter. The drop of the twin specific environment from the final model may reflect limited power, although the group of mother-daughter pairs (533 complete pairs), for example, is not very small. In the second best fitting model, which included twin specific shared environment, explaining 26% of individual differences in the initiation of breastfeeding, heritability still explained 48% of individual differences in the initiation of breastfeeding.
The heritability implies the importance of genetic factors on initiation of breastfeeding. However, the study of genetic background of breastfeeding in humans is scarce. For example, oxytocin related genes are potential causal factors affecting breastfeeding, and the oxytocin gene SNP (OXT rs2740210) has been shown to moderate the effect of early adversity on duration of breastfeeding (Jonas et al., Reference Jonas, Mileva-Seitz, Girard, Bisceglia, Kennedy and Sokolowski2013). In the future, both molecular and quantitative genetic studies on breastfeeding may reveal the genetic variants behind the heritability of breastfeeding behavior. Knowing the genetics of breastfeeding helps us to understand more clearly how breastfeeding is related to disease risks and psychological functioning. For example, lack of breastfeeding is associated with an increased risk for breast cancer (Lichtenstein et al., Reference Lichtenstein, Holm, Verkasalo, Iliadou, Kaprio, Koskenvuo and Hemminki2000). At this point it is not known whether this is a causal link or whether this is explained by a third factor, that is, the same genes or environmental factors cause both lack of breastfeeding and risk of breast cancer. The link between breastfeeding and breast cancer could be clarified with more advanced models, such as with a bivariate model of the heritability of breastfeeding and breast cancer, or with discordant MZ twin pairs, where environmental factors are compared between MZ twins with different phenotypes, that is, one was breastfed and the other was not breastfed, one has breast cancer and the other does not have breast cancer. These are suggested to be helpful methodologies in revealing causal effects (Zwijnenburg et al., Reference Zwijnenburg, Meijers-Heijboer and Boomsma2010). In addition, studying the heritability in other populations with quantitative genetic methods is needed to ensure whether the findings are only valid in the Netherlands or if these can be generalized globally to different cultural, societal and geographical environments. For example, in risky environments, parenting efforts are reduced because of the fact that the costs may be greater than the benefits (Quinlan & Quinlan, Reference Quinlan and Quinlan2007). Thus the heritability of breastfeeding might change according to riskiness of the environment.
The following potential limitations should be considered when interpreting the results of the present study. The age range of the participants was wide (mean age 49 years), thus they had to recall their breastfeeding behavior retrospectively from several years earlier. Recalls of both initiation and duration of breastfeeding history by mothers have been found to be quite reliable and valid even after long recall periods, although reliability of breastfeeding duration starts to decrease after 3 years (Li et al., Reference Li, Scanlon and Serdula2005). However, the measure of ever breastfed versus never breastfed is more reliable even after long recall than, for example, exact duration of exclusive breastfeeding, and it can be expected to have sufficient reliability. An advantage in the present study is the availability of information of breastfeeding from twins, their sisters, and their mothers, which gives us a more accurate picture of the heritability and environmental effects than a classical twin design (Posthuma & Boomsma, Reference Posthuma and Boomsma2000). However, results should still be replicated with a bigger sample size to ensure more power and the possibility of finding shared familial environment and/or cohort effects on the heritability (i.e., gene-environment interactions).
To conclude, the present study showed an increasing trend for initiation of breastfeeding in later cohorts, meaning that a larger proportion of mothers wants to breastfeed and at least tries to breastfeed their infants in the later cohorts. The willingness of mothers to breastfeed can be supported by being offered maternity leave and breastfeeding friendly workplaces, as well as providing education and support on breastfeeding practices. In addition, mothers of twins had a lower prevalence in initiation of breastfeeding than mothers of singletons, indicating that mothers of twins should receive more attention and support when they are willing to breastfeed. Heritability explained 70% of the variance in initiation of breastfeeding any children, when the difference in the prevalence of initiation of breastfeeding between mothers of twins and mothers who themselves are twins or sisters of twins was taken account. The heritability is higher than previously found when studying heritability of initiation of breastfeeding the firstborn child in twins in Spain. Thus, the differences in prevalence of initiation of breastfeeding in mothers of twins and mothers of singletons, as well as among different birth cohorts, should be taken account when studying breastfeeding and its heritability.
Acknowledgments
This work was supported by The Academy of Finland Projects 258578 (P.M., principal investigator Mirka Hintsanen) and 265869 (principal investigator Liisa Keltikangas-Järvinen) and Alfred Kordelin Foundation (P.M.). Analyses were done on data collected by the Netherlands Twin Register, VU University, Amsterdam, which is supported by multiple grants, including funding from the Netherlands Organization for Science (NWO), the European Research Council (ERC-230374), and the EMGO Institute for Health and Care Research.
Supplementary Material
To view the supplementary material for this article which contains the R-SCRIPT used for analyzing the data, please visit http://dx.doi.org/10.1017/thg.2015.5.






