Dietary fat quality is a recognized determinant of nutritional health. It is important for children to consume adequate amounts and types of fats since fatty acid (FA) intake in childhood may have important consequences for growth and development( Reference Huffman, Harika and Eilander 1 ). Moreover, long-chain PUFA (LC-PUFA; namely EPA (20 : 5n-3), docosapentaenoic acid (DPA; 22 : 5n-3), DHA (22 : 6n-3) and arachidonic acid (AA; 20 : 4n-6)) are important for several physiological and developmental needs( Reference Huffman, Harika and Eilander 1 ). DHA and AA are vital structural elements of cell membranes and they are major components of the brain and retina. PUFA also play an important role in the synthesis of prostaglandins and growth hormones and in the biosynthesis of membrane components( Reference Huffman, Harika and Eilander 1 ). Additionally, one n-3 PUFA (α-linolenic acid (ALA; 18 : 3n-3)) and one n-6 PUFA (linoleic acid (LA; 18 : 2n-6)) are essential, that is they cannot be synthesized endogenously so they must be supplied in the diet( 2 ). A clinical deficiency of ALA or LA causes skin problems, neurological abnormalities and poor growth( 2 ). However, the Institute of Medicine states that ‘approximately 10 % of the total n-3 and n-6 PUFA (ALA and LA) intakes can come from n-3 and n-6 LC-PUFA’( 2 ) (p. 1325).
No biomarkers reflect absolute fat intake; however, the measurement of FA in biological samples reflects to some extent the proportional intake of FA( Reference Arab 3 ). The measurement of FA composition of red-blood-cell (RBC) membranes has been commonly used to assess PUFA intake in epidemiological studies( Reference Lucas, Proust and Blanchet 4 – Reference Lauritzen, Harslof and Hellgren 8 ). Indeed, although man is technically capable of endogenously synthesizing n-3 LC-PUFA from ALA, this conversion is very limited and so diet is the major source of PUFA( Reference Arab 3 ).
Inuit traditional foods comprise plants and animals harvested from the local environment, mainly marine mammals, fish and seafood, land mammals and game birds( Reference Kuhnlein, Soueida and Receveur 9 ). Traditional foods provide many benefits to Inuit people such as cultural, social, nutritional, psychological and economic well-being( Reference Kuhnlein and Receveur 10 ). However, as part of the rapid socio-cultural transition observed in Arctic Inuit populations, several studies have reported a decreased reliance on traditional foods over the last decades for the benefit of a more Western diet, especially in younger generations( Reference Kuhnlein, Soueida and Receveur 9 , Reference Egeland, Berti and Soueida 11 – Reference Gagné, Blanchet and Lauzière 15 ). The ratio between n-3 LC-PUFA and n-6 PUFA is known to be a strong indicator of seafood intake and thus is a good indicator of the relative consumption of traditional food v. imported food among Inuit( Reference Deutch, Pedersen and Hansen 16 ). So far, most evidence regarding the dietary transition among Inuit has been obtained from dietary studies, while analyses of biomarkers of nutritional exposures have been under-represented( Reference Zhou, Kubow and Egeland 6 ). Still, some authors have observed that Inuit have higher levels of LC-PUFA in their tissues than non-Inuit( Reference Innis, Kuhnlein and Kinloch 17 – Reference Dewailly, Blanchet and Gingras 19 ).
To the best of our knowledge, only one study described the RBC FA composition in Inuit children from the Canadian Arctic and this study was conducted more than 20 years ago( Reference Innis, Kuhnlein and Kinloch 17 ). The relationship between dietary sources of n-3 and n-6 PUFA and RBC n-3 and n-6 PUFA has never been assessed among Inuit children. The objectives of the present study were to assess dietary FA intakes and to examine the relationship between dietary sources of n-3 and n-6 PUFA and RBC membrane n-3 and n-6 PUFA composition in Inuit pre-school children attending child-care centres in Nunavik.
Experimental methods
Study population and data collection
The methods have been described in detail elsewhere( Reference Gagné, Blanchet and Lauzière 15 ). Briefly, this research project was a component of the Nutrition Program implemented in child-care centres of Nunavik, northern Québec, Canada, which aims to reduce Fe deficiency and improve nutrition among Inuit pre-school children. Cross-sectional data used in the present paper were collected between 2006 and 2010. Parents of Inuit children aged 1–4 years and attending a child-care centre in one of the ten Nunavik communities visited were invited to participate. Information on the study was provided to parents individually, orally or through a DVD available in Inuktitut, English and French. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human participants were approved by the Research Ethics Board of the Centre Hospitalier Universitaire de Québec, Québec, Canada. Written informed consent was obtained from parents/caregivers for all participants.
A total of 245 children aged 11–54 months were recruited; forty-nine were excluded from the present analyses because they did not have a 24 h dietary recall or because it was incomplete, fourteen were excluded because they did not have an RBC FA measurement, fourteen more had neither a complete 24 h dietary recall nor an RBC FA measurement, and one was lacking information about early feeding (breast-feeding and use of infant formula), which left 167 children for the present analyses.
Dietary assessment
Dietary intakes of children were assessed through the 24 h recall method. Each recall was completed by a registered dietitian with the parent or primary caregiver, and with cooks and educators if the child had attended a child-care centre during the day of the recall( Reference Gagné, Blanchet and Lauzière 15 ). An initial 24 h dietary recall was obtained from each of the 167 participants. Interviews captured both weekend and weekday intakes. Three-dimensional graduated food model kits (Santé Québec) were used and information on cooking methods and brand names were obtained whenever possible. For seven participants, a standard portion was imputed based on the menu that was served because the research team did not find anyone at the child-care centre who could recall the quantity consumed by the child or his/her usual portion size. A second 24 h dietary recall was completed for more than 40 % of the children (n 73), with two of them held over the telephone and two containing imputed portions sizes for food eaten at the child-care centre. Although we attempted not to do so, a few recalls were held on consecutive days (n 8). Dietary recalls did not include specific information on nutritional supplements.
Computerization of the 24 h dietary recalls was done by registered dietitians at the Institut National de Santé Publique du Québec (INSPQ, Québec, QC, Canada) using the Micro Gesta software package version 1·1·56 (Micro Gesta, Québec, QC, Canada). This software is based on the Canadian Nutrient File( 20 ) for the nutrient content of foods. All recipes served as part of the Nutrition Program were added to the database. Also, the nutrient content of infant formulas and some new products was added manually to the database according to the information provided by manufacturers. We attributed 0 g, 447 g and 956 g of human milk to breast-fed children who had respectively 1–3, 4–6 and ≥7 feedings on the reference day( Reference Gagné, Blanchet and Lauzière 15 ).
Blood sampling and laboratory analyses
Blood samples (6 ml) were collected by venepuncture into a vial containing EDTA as the anticoagulant (BD 367863; Becton Dickinson and Co., Mississauga, ON, Canada). Blood samples were either kept at room temperature for a maximum of 20 min, kept in an insulated container with ice packs, or refrigerated at 4°C prior to processing. After centrifugation, RBC were isolated and aliquoted for FA analysis. Within 3 h of collection, aliquots were frozen and stored at −18/−20°C. Frozen aliquots were kept in insulated containers with ice packs during transportation to the University of Guelph (Guelph, ON, Canada). The FA composition was measured in RBC membranes after membrane purification, chloroform–methanol lipid extraction( Reference Folch, Lees and Sloane Stanley 21 ) and methylation of FA( Reference Morrison and Smith 22 ), followed by capillary GLC using a DB-23 column (39 m × 0·25 mm internal diameter × 0·25 μm thickness) in a Varian 3400 GC. FA composition is given as the percentage of individual FA relative to the total chromatogram (%FA).
Statistical analyses
Statistical analyses were carried out using the SAS statistical software package version 9·2. A P value <0·05 was considered statistically significant. Frequencies (%) and means were used to describe the characteristics of participating children. To determine the distribution of usual dietary intakes, we used the Software of Intake Distribution Estimation (SIDE-IML; Iowa State University, Ames, IA, USA) to take into account the intra-individual variation in nutrient intakes. Nutrient intakes included intakes from foods and beverages. Fat and FA intakes were compared with the Acceptable Macronutrient Distribution Ranges (AMDR), which are expressed as a percentage of total energy intakes( 2 ). By determining the proportion of the group that falls below, within and above the AMDR, it is possible to assess population adherence to the recommendations and to determine the proportion of the population that is outside the recommended range( 2 ). For nutrients with an Adequate Intake (AI), methods for assessing nutrient adequacy are limited since the AI cannot be used to determine the proportion of individuals in a group with inadequate nutrient intakes( 23 ). However, population subgroups with mean intakes at or above the AI can be assumed to have nutritionally adequate diets( 23 ). Considering that only one participant was younger than 1 year of age and two participants were older than 3 years old, nutrient intakes of all participants were compared to recommendations for 1–3-year-old children.
Means were used to describe RBC FA composition and predictors of RBC n-3 and n-6 PUFA, ALA, EPA + DHA, LA and AA levels were investigated by standard linear regression. Independent variables were included in regression models if there was a priori evidence that they could be independent predictors or confounders of the outcomes. Selected variables were age, sex, breast-feeding status (never breast-fed/ever breast-fed/currently breast-fed), infant formula status (never consumed/ever consumed/currently consuming), consumption of traditional food item (yes/no) and dietary intake of the predicted PUFA during the day of the first 24 h dietary recall. In additional models, we further adjusted the RBC n-3 and n-6 PUFA models for energy intake and BMI-for-age Z-score (either separately or in combination), replaced breast-feeding status by breast-feeding duration, and used dietary intakes of LA and n-6 PUFA as predictors of RBC n-3 PUFA. All these models explained about the same proportion of the variance in RBC n-3 and n-6 PUFA levels. For brevity, the results of these additional analyses are not presented in the form of tables.
Results
The descriptive characteristics of the study population are shown in Table 1. The mean age of participating children was 25·1 (sd 9·7) months (range: 11–53 months), 40 % of them were currently breast-fed or consuming an infant formula, and 34 % ate at least one traditional food item during the period covered by the first 24 h dietary recall.
Table 1 Descriptive characteristics of the participants: Inuit children aged 11–53 months (n 167) attending child-care centres, Nunavik, northern Québec, Canada

*During the day of the first 24 h dietary recall.
Dietary intakes
Table 2 presents estimates of the usual intake distributions for fat and FA. Participating children had higher usual intakes of SFA and MUFA than PUFA (mean: 13·17 % of energy (%E), 11·44 %E and 4·45 %E, respectively). Their usual intake of n-6 PUFA was more than five times higher than their usual intake of n-3 PUFA (mean: 3·48 %E and 0·64 %E, respectively). The median n-6:n-3 PUFA was 5·81 and 21·0 % of the children had a ratio lower than or equal to 4:1. Also, 31·4 %, 47·9 % and 93·5 % of our participants fell outside the AMDR for fat, n-3 and n-6 PUFA, respectively. The vast majority of intakes tended to be below rather than above the recommended range. A minority of participants had adequate ALA and LA intake (40·4 % and 4·9 %, respectively).
Table 2 Distributions of usual intakes of fat and FA among participants: Inuit children aged 11–53 months (n 167) attending child-care centres, Nunavik, northern Québec, Canada
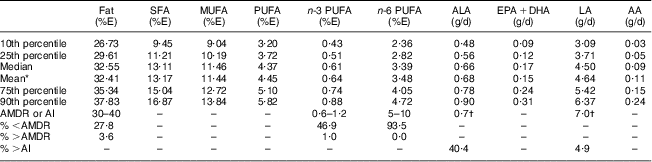
FA, fatty acid; %E, percentage of energy intake; ALA, α-linolenic acid, 18 : 3n-3; EPA, 20 : 5n-3; DHA, 22 : 6n-3; LA, linoleic acid, 18 : 2n-6; AA, arachidonic acid, 20 : 4n-6; AMDR, Acceptable Macronutrient Distribution Range; AI, Adequate Intake.
*Arithmetic mean.
†Approximately 10 % of the total can come from longer-chain n-3 or n-6 PUFA.
Red-blood-cell fatty acid composition
As indicated in Table 3, the most abundant RBC FA were SFA followed by PUFA and MUFA (40·14 %FA, 35·64 %FA and 24·22 %FA, respectively). The two major SFA were 16 : 0 and 18 : 0, whereas the major MUFA was 18 : 1. LA and AA were the major n-6 PUFA (15·70 %FA and 10·11 %FA, respectively), whereas DHA and DPA were the major n-3 PUFA (2·67 %FA and 1·54 %FA, respectively). ALA and EPA contributed less than 1 % of the total RBC FA (0·30 %FA and 0·84 %FA, respectively). Mean RBC DPA:EPA and n-6:n-3 PUFA were 2·23 and 6·32, respectively.
Table 3 RBC membrane FA composition (%FA) of Inuit children aged 11–53 months (n 167) attending child-care centres, Nunavik, northern Québec, Canada
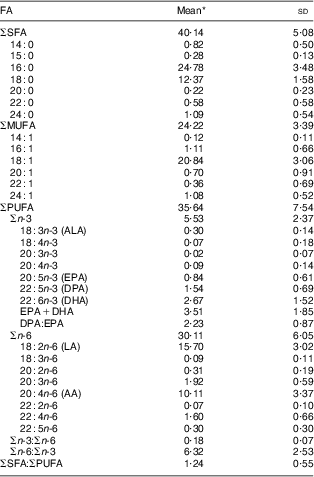
RBC, red-blood-cell; FA, fatty acid; %FA, percentage of individual FA relative to the total chromatogram; ALA, α-linolenic acid; DPA, docosapentaenoic acid; LA, linoleic acid; AA, arachidonic acid.
*Arithmetic mean.
Multivariate models
Table 4 presents multivariate regression models explaining RBC n-3 and n-6 PUFA, ALA, EPA + DHA and LA concentrations. The consumption of at least one traditional food item during the initial 24 h dietary recall (yes/no) and breast-feeding status (never breast-fed/ever breast-fed/currently breast-fed) were significant positive predictors of RBC n-3 PUFA (P = 0·0080 and P = 0·0243, respectively). Older children also tended to have higher RBC n-3 PUFA (P = 0·0528), whereas sex, infant formula status (never consumed/ever consumed/currently consuming) and n-3 PUFA dietary intakes during the day of the first 24 h dietary recall were not associated with RBC n-3 PUFA. Neither LA nor n-6 PUFA dietary intakes were associated with RBC n-3 PUFA, nor were n-3 PUFA dietary intakes associated with RBC n-6 PUFA (data not shown). RBC n-6 PUFA concentrations were positively explained by breast-feeding status and n-6 PUFA dietary intakes during the day of the first 24 h dietary recall (P = 0·0222 and P = 0·0108, respectively), whereas no significant associations were found with age, sex and infant formula status. These models accounted for 11–13 % of the variance in RBC n-3 and n-6 PUFA concentrations. Further adjusting for energy intake or BMI-for-age Z-score did not improve models and these variables were not significantly associated with RBC n-3 or n-6 PUFA concentrations (data not shown). Finally, when breast-feeding status was replaced by breast-feeding duration in these models, a positive and significant association was observed with RBC n-3 PUFA but not with RBC n-6 PUFA (data not shown). Models explaining RBC ALA, EPA+DHA and LA concentrations were very similar to the models explaining n-3 and n-6 PUFA except for age, which was significantly associated with RBC EPA+DHA levels (P = 0·0306). None of the models tested for RBC AA level reached statistical significance.
Table 4 Multivariate linear regression of factors explaining RBC membrane n-3 and n-6 PUFA concentrations in Inuit children aged 11–53 months (n 167) attending child-care cntres, Nunavik, northern Québec, Canada
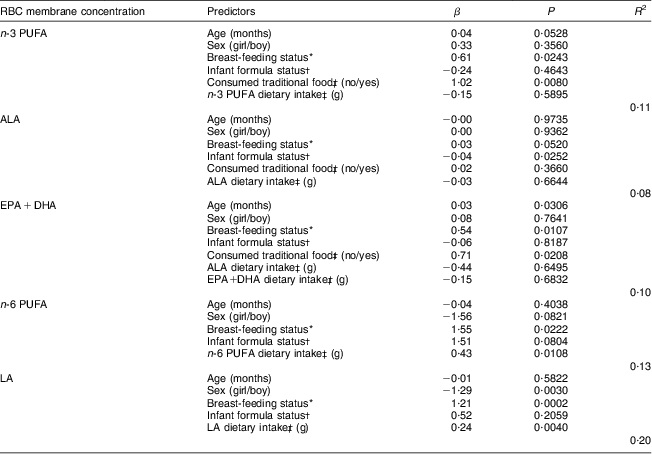
RBC, red-blood-cell; ALA, α-linolenic acid; LA, linoleic acid.
*Never breast-fed/ever breast-fed/currently breast-fed.
†Never consumed/ever consumed/currently consuming.
‡During the day of the first 24 h dietary recall.
Discussion
Dietary intakes
A significant proportion (31 %) of our participants had a usual fat intake that fell outside the AMDR set at 30–40 %E for children aged 1–3 years. This proportion was at the lower end of those observed in representative groups of Canadian and US children of the same age (23–50 %E)( Reference Butte, Fox and Briefel 24 – Reference Troiano, Briefel and Carroll 27 ). Also, in agreement with these studies, we found that more than a quarter of our participants (27·8 %) had a usual fat intake below the AMDR, with only a small proportion above the higher limit of the AMDR. According to the Institute of Medicine, there is no evidence that the macronutrient composition of the diet affects childhood growth if energy and protein requirements are met( 2 ). This seems to be the case among our participants as only 1·4 % had a protein intake below the Estimated Average Requirement (H Turgeon O'Brien, D Gagné, É Vaissière et al., unpublished results). Moreover, the mean fat intake (32 %E) in the present study was in the range of values reported in a systematic review of fat and FA intakes of children and adolescents around the world (23–40 %E)( Reference Harika, Cosgrove and Osendarp 28 ). Mean usual intakes of SFA and MUFA were also similar to levels observed in a representative sample of 1–3-year-old Canadian children( 25 ), whereas the usual intake of PUFA was slightly higher among our participants (4·5 %E v. 3·8 %E, respectively). Mean usual intakes of SFA and PUFA were also within the range observed by Harika et al. among children worldwide (1·0–15·2 %E and 3·5–9·7 %E, respectively)( Reference Harika, Cosgrove and Osendarp 28 ). When compared with a representative group of Inuit adults who participated in the Nunavik Inuit Health Study (NIHS), total fat and MUFA contributed a similar proportion to energy intake but PUFA contributed a slightly lower proportion to energy intake (4·5 %E v. 5 %E in NIHS, respectively)( Reference Blanchet and Rochette 13 ). In the same way, n-3 and n-6 PUFA usual dietary intakes of participants in the present study were almost half those in NIHS( Reference Blanchet and Rochette 13 ). However, our participants had the same median n-6:n-3 PUFA dietary intake ratio( Reference Blanchet and Rochette 13 ). The sum of dietary intakes of EPA + DHA (0·15 g/d) was similar to that observed in a group of 3–5-year-old Canadian children from Vancouver (0·14 g/d)( Reference Innis, Vaghri and King 29 ) and within the range observed in children worldwide (0·05–0·15 g/d)( Reference Harika, Cosgrove and Osendarp 28 ). Concerning essential FA, the proportion of children with adequate usual intakes (>AI) of ALA and LA in the present study was lower than in Canadian children and Canadian Inuit adults (ALA: 40 %, 69 % and 75–90 %, respectively; LA: 5 %, 21 % and 2–29 %, respectively)( 30 – Reference Kuhnlein, Receveur and Soueida 32 ). Altogether, these results reiterate that Inuit pre-school children rely less on traditional foods than Inuit adults( Reference Gagné, Blanchet and Lauzière 15 ) and indicate that their FA dietary intakes are similar to those of children worldwide.
Red-blood-cell fatty acid composition
RBC n-6 and n-3 PUFA were higher in our participants than in US children aged 1–11 years (30·11 %FA v. 24·93 %FA and 5·53 %FA v. 3·81 %FA, respectively)( Reference Orton, Szabo and Clare-Salzler 7 ). Although RBC PUFA composition of Danish adolescents was similar to that of our participants, the former had higher levels of LC-PUFA( Reference Lauritzen, Harslof and Hellgren 8 ). Canadian Inuit children living on Broughton Island (formerly in Northwest Territories, now in Nunavut) and aged 0–5 years in 1985 (n 13) had probably higher levels of n-3 LC-PUFA, although it is uncertain because concentrations of RBC FA were reported for phosphatidylcholine and phosphatidylethanolamine separately( Reference Innis, Kuhnlein and Kinloch 17 ).
The ratio of n-6 to n-3 PUFA has been used as an indicator of dietary Westernization among Indigenous people, with higher ratios indicating lower consumption of traditional foods( Reference Simopoulos 33 ). In our study, we observed a high n-6:n-3 PUFA, confirming our previous observation on the dietary transition and the lower reliance upon traditional foods( Reference Gagné, Blanchet and Lauzière 15 ). Moreover, Zhou et al. suggested that a high DPA:EPA might be a novel indicator of a diet containing a larger proportion of land animals than of both fish and marine mammals( Reference Zhou, Kubow and Egeland 6 ). We observed a higher DPA:EPA than the ones reported by Zhou et al. in all the communities they surveyed, which would suggest that our participants may have consumed more land animals than fish and marine mammals. If we look more closely, we can see that this high ratio is explained by lower RBC EPA levels among our participants rather than higher levels of DPA. Therefore, this result seems to be more indicative of a low consumption of fish and marine mammals rather than of a high consumption of land animals. Indeed, when considering multiple indicators, we conclude that these young Inuit children eat a lower proportion of traditional food and more specifically a lower amount of fish and marine mammals than Inuit adults.
Multivariate models
In the regression models, we showed that breast-feeding status (never breast-fed/ever breast-fed/currently breast-fed) was positively associated with RBC n-3 and n-6 PUFA concentrations. This is in agreement with the positive association observed between breast-feeding duration and RBC DHA and EPA levels in Danish adolescents( Reference Lauritzen, Harslof and Hellgren 8 ). When replacing breast-feeding status by breast-feeding duration, we observed a positive and significant association with RBC n-3 PUFA but not with RBC n-6 PUFA. This result may suggest that breast-feeding duration may be indicative of a more traditional lifestyle containing more n-3 LC-PUFA than n-6 LC-PUFA after weaning( Reference Kuhnlein, Chan and Leggee 34 ).
In the model explaining RBC n-3 PUFA, dietary n-3 PUFA intake was not significantly associated. This is in agreement with Hoyos et al., who did not observe a significant association between n-3 PUFA dietary intake and plasma n-3 PUFA in 18- and 36-month-old Australian children( Reference Hoyos, Almqvist and Garden 35 ). On the contrary, we observed that the consumption of traditional foods during the 24 h dietary recall period was associated with higher RBC n-3 PUFA levels. This may suggest that having consumed a traditional food may be a better indicator of a traditional diet than n-3 PUFA or EPA + DHA dietary intakes when assessed with a 24 h dietary recall. Indeed, we have previously hypothesized that it might be harder to report traditional food serving sizes of young children as they are often served or taken by hand, one mouthful at a time, from a centre dish, with other family members (or child-care centre staff) participating in the service and that this could lead to misestimating traditional food intakes( Reference Gagné, Blanchet and Lauzière 15 ). The positive association between the consumption of traditional food and RBC n-3 PUFA is also in accordance with results from Lucas et al., who observed that intakes of most Inuit traditional foods (marine mammals, fish, land animals and game-bird eggs) were correlated with RBC n-3 LC-PUFA in Nunavik adults( Reference Lucas, Proust and Blanchet 4 ). Young et al. also reported a positive association between the consumption of traditional foods and plasma EPA and DHA among adults( Reference Young, Gerrard and O'Neil 18 ). In the present study, age also tended to be significantly associated with RBC n-3 PUFA levels and was significantly associated with RBC EPA+DHA, which is agreement with increased plasma n-3 LC-PUFA observed in 36- and 60-month-old children compared with the same children at age 24 months( Reference Guerra, Demmelmair and Toschke 36 ). Sex and infant formula status were not significantly associated with RBC n-3 PUFA. It has been hypothesized that intake of LA or n-6 PUFA may blunt the tissue incorporation of n-3 PUFA( Reference Hoyos, Almqvist and Garden 35 , Reference Hibbeln, Nieminen and Blasbalg 37 ) but their intakes were not associated with RBC n-3 PUFA in our study. We did not observe any relationship between BMI-for-age Z-score and RBC n-3 PUFA levels; this differs from Burrows et al. who had suggested a role for n-3 LC-PUFA in the development of obesity as they observed lower levels of RBC EPA+DHA among obese Australian children compared with non-obese( Reference Burrows, Collins and Garg 38 ). As for RBC n-6 PUFA levels, they were positively associated with breast-feeding status as mentioned above and, as expected, with n-6 PUFA dietary intakes. In 18- and 36-month-old Australian children, dietary n-6 PUFA were also positively associated with plasma n-6 PUFA levels( Reference Hoyos, Almqvist and Garden 35 ). Age, sex and infant formula status were not associated with RBC n-6 PUFA concentrations.
Limitations
When interpreting the present results, one ought to keep in mind the limits of the study. First, to estimate usual dietary intakes, SIDE requires that individuals must be independent. This was not always true in the present study as some siblings were recruited. Unfortunately, this information was not collected, preventing us from adjusting for this variable. Also, as reported elsewhere( Reference Gagné, Blanchet and Lauzière 15 ), children in the present study had a lower energy intake compared with a representative group of Canadian children of the same age group participating in the 2004 Canadian Community Health Survey (CCHS). Even though parents in the CCHS were believed to have overestimated the dietary intakes of their children( 39 ), there is a possibility that energy intake was under-reported in our participants and this could have increased the prevalence of inadequate nutrient intakes in our study( Reference Gibson 40 ). Moreover, we may have induced a selection bias since children included in the present analyses (i.e. those with a complete set of variables) represent 68·2 % of enrolled participants.
Another limit of our study includes the use of a 24 h dietary recall to estimate FA dietary intakes. The 24 h dietary recall method is a simple, rapid and well-established tool to assess the average intake of groups( Reference Willett 41 ). We collected a second recall for a significant proportion of the children to improve the accuracy of individual intake estimates and take intra-individual variability into account. However, in multivariate models, we had to use only the first 24 h dietary recall because we did not have enough recalls per children to represent usual dietary intakes on an individual basis. For example, sixty-four recalls per adults are necessary to estimate individual usual intakes of total fat( Reference Basiotis, Welsh and Cronin 42 ). Therefore dietary intakes included in regression models may not represent the usual intakes of children. Moreover, our results also depend on the Canadian Nutrient File for data analysis( 20 ) which has its own limits. Indeed, the version used did not cover all foods for n-3 and n-6 PUFA, and there might be more variation among wild animals resulting in higher or lower dietary intakes than estimated by the Canadian Nutrient File. Furthermore, a measure of dietary intakes covering a longer period of time would have been optimal to compare with exposure reflected by the RBC FA concentrations. The fact that we used a 24 h dietary recall could have lowered the strength of the relationships between dietary intakes and RBC levels, especially for n-3 LC-PUFA. However, using an FFQ covering a longer period of time was difficult with our population as some children had only commenced eating solids during the last 12 months, which was the period covered by the FFQ; limiting our capacity to use it. The uncertainty in the present study is therefore presumed to be mainly related to the dietary assessment, while assessment of RBC membranes reflects the general intake over the lifespan of RBC, or ∼120 d( Reference Arab 3 , Reference Hodson, Skeaff and Fielding 43 ). Still, FA in RBC membranes are under the control of both genetic and other modifying factors such as disease and nutritional status, although RBC PUFA respond to differences in dietary intake more so than do RBC SFA( Reference Arab 3 , Reference Hodson, Skeaff and Fielding 43 ).
Conclusions
Findings from the present study confirm that diet is a major determinant of RBC FA composition in Canadian Inuit. Indeed, n-6 PUFA dietary intake assessed with a 24 h dietary recall and breast-feeding status explained part of the variation of RBC n-6 PUFA levels among these Inuit pre-school children. Also, even if n-3 PUFA dietary intake was not significantly associated with RBC n-3 PUFA levels, the consumption of traditional food during the initial 24 h dietary recall and breast-feeding status were identified as significant predictors. The present findings also highlight the fact that Inuit pre-school children are not consuming enough n-3 LC-PUFA for optimum health and corroborate the intergenerational trend towards younger Inuit now depending more on store-bought foods than their parents. This is of concern, as the replacement of a traditional diet with a Western diet may increase the risk of CVD( Reference Bersamin, Luick and King 5 , Reference Zhou, Kubow and Egeland 6 ) and have detrimental consequences on growth and development( Reference Huffman, Harika and Eilander 1 ). However, it is important to recognize that store-bought food choices – but not store-bought food per se – influence FA profiles( Reference Kuhnlein, Receveur and Soueida 32 ). These observations call for actions to increase traditional food intake among Inuit children and to help them and their parents make healthier store-bought food choices.
Acknowledgements
Sources of funding: This study was made possible through funding by the Aboriginal Affairs and Northern Development Canada Canada-Northern Contaminants Program, Kativik Regional Government and Health Canada. Aboriginal Affairs and Northern Development Canada Canada-Northern Contaminants Program, Kativik Regional Government and Health Canada had no role in the design, analysis or writing of this article. Conflicts of interest: The authors declare that they have no conflicts of interest. Authors’ contributions: R.B. conducted the statistical analyses, interpreted the results and prepared the first draft of the manuscript; J.L. contributed to the interpretation of the results and participated in the drafting of the manuscript; D.G., C.V., H.T.O. and P.A. were responsible for the study idea and design; H.T.O. contributed to the interpretation of the data and drafting the manuscript. All authors read and approved the final manuscript. Acknowledgements: The authors are grateful to the Nunavik parents and children for their participation. They also wish to thank all educators, cooks and directors of child-care centres in Nunavik, who were very supportive of the project.






