Nucleation is the initial stage of crystallization, a process in which individual building blocks (e.g., atoms and molecules) assemble into various crystalline structures, or “polymorphs.” Polymorphs usually exhibit different physiochemical properties due to their diverse structures, and hence are suitable platforms to demonstrate “structure–function” relationships. Therefore, understanding the nucleation and crystallization mechanisms leading to distinct polymorphs is critical. Now, by using cryo-transmission electron microscopy (cryo-TEM), a group of researchers—from the Centre Nacional de la Recherche Scientifique–Université Grenoble Alpes, France; Technische Universiteit Eindhoven, The Netherlands; and the Vrije Universiteit Brussel, Belgium—has directly observed how the protein glucose isomerase nucleates as different polymorphs. Their findings were recently published in Nature (doi:10.1038/nature25971).
“One of the most elusive moments to observe the crystallization process is the nucleation stage, when a nucleus, or crystalline embryo, is formed,” say the authors. To study the nucleation processes of glucose isomerase, they rapidly froze the solutions containing the protein molecules and nucleation initiators at different time intervals during the nucleation. This process stopped the nucleation and allowed direct imaging of the morphologies of the resultant crystalline structures using state-of-the-art cryo-TEM.
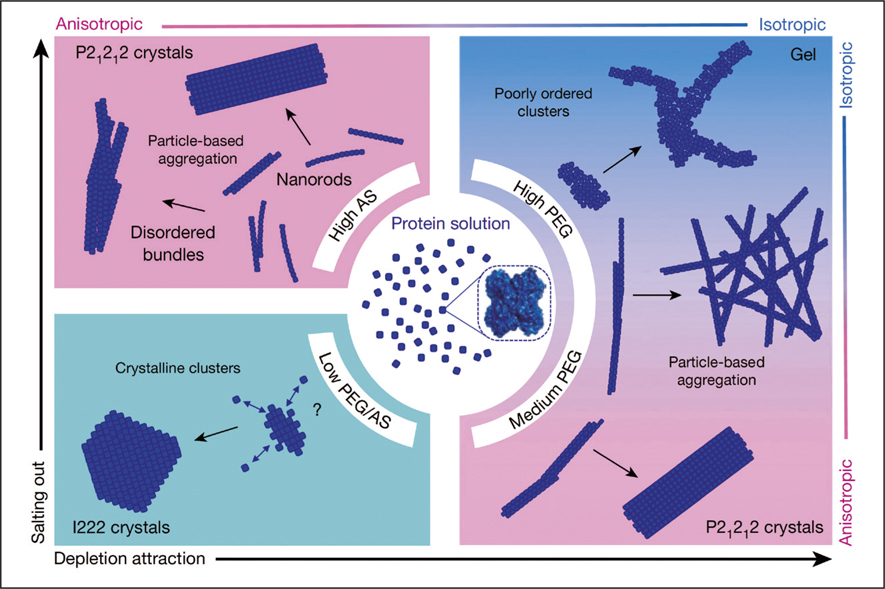
Schematic illustration depicting the pathways that glucose isomerases take to nucleate into different polymorphs depending on the type and concentration of the nucleation initiators. AS: ammonium sulfate; PEG: poly(ethylene glycol). Both AS and PEG are nucleation initiators. Credit: Nature.
The researchers discovered that the type and the concentration of the nucleation initiators determined the pathways by which the protein molecules nucleated. Twenty seconds after mixing the isomerases with 1.5 M ammonium sulfate, the former assembled into nanorods of ∼1.7 nm width and ∼12 nm length. Within the next 15 min to 30 min, these nanorods combined into dimers, trimers, and eventually into bundled long fibers (∼800 nm in length) or loosely attached fiber aggregates. In the meantime, prismatic-shaped and rhombic-shaped nanocrystals were also detected. Additionally, the researchers witnessed a similar nucleation pattern when 4.5% (w/v) poly(ethylene glycol) (PEG) was used as the nucleation initiator, but no nanorods were seen at early stages. When the PEG concentration was further increased to 7% (w/v), only protein gels composed of disorderly arranged protein clusters were obtained. These contrasting behaviors were attributed to the inter-molecular interactions (isotropic repulsion and anisotropic attraction) among the individual protein molecules, which can be altered by nucleation initiators of different compositions and concentrations.
The pathways revealed in this study do not involve a metastable dense liquid intermediate state as reported in previous studies, and thus deviates from the current understanding of protein nucleation. Yuki Kimura of Hokkaido University, Japan, says, “Their achievements not only present a deeper understanding of nucleation of a protein material, but also open up opportunities for controlling polymorphs. I believe we have advanced into a new stage of the nucleation study [since this work is published].” Kimura was not involved in this study.
“In a nutshell, our results are advancing the fundamental understanding of nucleation and polymorph selection. These insights are not only relevant for macromolecules but can also be translated to other crystal-forming substances including pharmaceutical compounds,” the authors say. “Now we are very interested to apply the same workflow to other protein molecules, because we are convinced, considering the complex nature of macromolecules, that many more unexpected findings will pop up along the (nucleation path) way.” The research team is also searching for alternative strategies to control polymorph selection.


