1 Introduction
Schizophrenia represents one of the leading public health issues in psychiatry. The median (10–90 percentile) of point prevalence of schizophrenia was found to be 460 (190–1000), and the incidence was found to be about 15.0 (7.7–43.0)/100,000 in a systematic review that included studies from several regions of the World Reference McGrath, Saha, Chant and Welham[1]. A comprehensive study including data from member states of the European Union (EU-27) plus Switzerland, Iceland and Norway Reference Wittchen, Jacobi, Rehm, Gustavsson, Svensson and Jonsson[2] estimated that the prevalence of psychotic disorders is 1.2% in the EU population, and the estimated number of persons affected in 2011 was 5 million.
Existing antipsychotics can achieve full remission only in about 30% of schizophrenia patients and about 20–30% are resistant Reference Steeds, Carhart-Harris and Stone[3] showing a significant unmet need. Antipsychotics cause typical adverse effects (e.g. extrapyramidal symptoms, altered glucose metabolism) especially when administered in combinations Reference Frankenburg and Frankenburg[4]. Both the high rate of resistance and the need for adverse effect treatment result in an additional burden on health systems.
Individuals with schizophrenia use a substantial amount of healthcare services. This condition imposes a significant economic burden on both the patients and their families, and on society as a whole Reference Mancuso, Specchia, Lovato, Capizzi, Cadeddu and Ferriero[5]. The interpretation of the cost-of-illness studies for schizophrenia can be difficult, due to the diversity in study design, reporting and the change in prices. The most recent systematic literature review, published in 2017, gives a comprehensive overview on the global economic burden of schizophrenia Reference Jin and Mosweu[6] from a societal perspective. The study shows high differences in the total societal cost across the globe varying from $US5818 in Thailand to $US 94,587 in Norway. We believe that a more detailed overview of the direct healthcare costs among schizophrenia patients in Europe might provide additional insight into the cost drivers and the factors explaining the variation of treatment costs across patient groups within a given country. This information can highlight certain aspects of the disease and processes of care where improvements are needed, and thus inform those involved in the planning of healthcare services and prioritizing research.
The development of systems of mental healthcare in Western Europe is characterized by a common trend toward deinstitutionalization, less inpatient treatment and improvement of community services Reference Becker and Kilian[7]. The structure and capacity planning of inpatient care has been changed dynamically in recent years, leading to the strengthening of outpatient care provisions which reduce hospital bed days. The development of health care provision in the Central- and Eastern European (CEE) countries shows more inconsistencies. Recently, mental health policy began to change, new mental health legislation focusing on human rights was taken into effect and a deinstitutionalization process took place. However, in some CEE countries decrease in the number of psychiatric beds was not accompanied by adequate development of outpatient care and so it is often limited to drug prescription [Reference Dlouhy8, Reference Winkler, Krupchanka, Roberts, Kondratova, Machu and Hoschl9]. Consequently, in some countries recent trends in pharmaceutical therapies may have more influence on the direct costs of schizophrenia.
Our review included relatively recent papers published from 2010. Our aim was to balance between the requirements of HTA agencies preferring up-to-date data and to have sufficient information to draw conclusion. When initial date for inclusion was selected we considered that major policy and treatment changes with potentially significant impact on direct health care cost, including deinstitutionalization of patients, shift toward generic and/or long acting injectable drugs were implemented earlier than 2010. Hence the period since 2010 could reasonable be considered fairly homogenous period in the management of schizophrenia.
The objective of this overview was to provide an overview on the magnitude of the impact of schizophrenia on the healthcare system in Europe and to gain a better understanding on the most important factors influencing the variation of costs. More, specifically the review aims to address the following questions:
• What is the total direct healthcare cost per patient with schizophrenia in European countries?
• What is the relationship between European countries’ economic wealth (GDP per capita) and direct healthcare cost per patient with schizophrenia?
• What are the most important factors associated with the variations in cost of schizophrenia across patient groups?
2 Methods
2.1 Databases and literature search strategy
The systematic literature search was conducted and reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement Reference Panic, Leoncini, de Belvis, Ricciardi and Boccia[10], an external quality control benchmark. The literature search was performed in 19th January 2017 using MEDLINE (via Scopus), EMBASE (via Scopus) and Cochrane Database of Systematic Reviews.
The search strategy was built up as a combination of search strings related to the economic burden of schizophrenia. The detailed search strategy with number of hits can be found in Appendix A. The literature search was limited to English language papers published between 2011 and 2017. Due to the overlap of coverage between the databases, search results were de-duplicated first, followed by a title and abstract-based screening conducted by two independent reviewers (T.A., G.K). Disagreements were resolved by a third, principal researcher (A.T.Z.).
Studies were included if the population of interest had a clinical diagnosis of schizophrenia at any age and the study contained any cost or resource use data related to the treatment of schizophrenia. No restrictions on study interventions, comparators and study outcomes were used during the systematic literature review. Search results were considered in two steps. Initially, titles and abstracts of all articles were screened using the following exclusion criteria: (1) article is without abstract; (2) article is not English language paper; (3) article is editorial, letter or review; (4) article is case study/case series or the total sample number is <100; (5) article does not report data relevant to the research topic. Due to the potential limitations of electronic search strategies, reference lists of the excluded reviews were also checked for additional relevant studies. Articles deemed relevant were checked for eligibility in full-text. Publications were excluded if met any of the following criteria (1) in language other than English; (2) conference abstract or study protocol; (3) no European focus; (4) no relevant data; (5) data collection closed before the end of 2005 (i.e. if a study did not present any cost or resource use related from the period after December 31, 2005); (6) the number of the included persons < 100; (7) review article; (8) data referred from other included articles.
2.2 Data extraction
A standardized data extraction form was developed and then checked for suitability. The following information was extracted from each included study: (1) the first author and year of publication; (2) the study perspective (i.e., societal, healthcare, third party payer or patient as sub-perspectives); (3) epidemiological approach (i.e., prevalence or incidence based); (4) study design (i.e., prospective or retrospective); (5) the country; (6) the cost calculation method: bottom-up (assessing the individual cost of persons with schizophrenia) or top-down (using national or regional statistics to withdraw the cost of the disease); (7) the year of analysis, pricing year and currency; (8) the diagnostic criteria for schizophrenia; (9) the characteristics of the study sample; (10) data on healthcare utilization (i.e., resource use data on inpatient care); and (11) data on direct healthcare costs (i.e., the resource consumption in the healthcare sector associated with the provision of healthcare interventions: e.g. the cost of hospital stays, outpatient visits and drugs). Costs and resource use estimates were extracted with regards to the related follow-up period, unit, and currency.
Data was retrieved per study arm from included studies that had a comparative design investigating differences in the cost of treatment across study groups. Based on these subgroup analyses we identified important factors and cost drivers that explain the variation of treatment costs across patient groups with schizophrenia.
2.3 Reporting cost estimates
To compare costs across studies, the costs were extrapolated to calculate annual costs per patient where necessary. Cost data reported in US dollars were converted into euro (€) using the average US dollar/euro exchange rate reported by the European Central Bank (https://www.ecb.europa.eu/stats/policy_and_exchange_rates/key_ecb_interest_rates/html/index.en.html) for the year of costing. Cost estimates were reported in two ways, reflecting the different purpose of our analyses: (1) The direct healthcare cost of schizophrenia was compared to other country specific figures such as population or GDP in the given year. In these analyses cost estimates were considered for the given year, because inflating cost to a specific year would have distorted the ratios included in the analyses (e.g. expenditure per 100,000 head, total cost per GDP or cost per patient to GDP per capita ratio). The value of the denominator can be influenced by several other factors (such as GDP growth or population change) which are irrelevant in our context, therefore we omitted uplifting costs and other indicators (GDP, population) to a specific year. (2) We also compared the cost per patient across countries. In order to ensure comparability of the results costs were inflated to a common year (2012, representing the latest year of costing among the included studies).
2.4 Assessment of methodological quality of included studies
A quality assessment of the included studies was performed with the checklist used by Larg et al. Reference Larg and Moss[11]. The purpose of the assessment was to check the validity of study methods considering the disease-specific cost and resource-use estimation. The checklist consists of 3 sections; general analytical framework (11 checking questions), used methodology (7 checking questions) and conducted analysis and reporting (10 checking questions). Each included article was assigned an overall score calculated as the share of yes answers divided by the total number of ‘yes’, ‘no’ or ‘unclear’ answers. The detailed checklist can be found in Appendix B.
3 Results
3.1 Search result
After deduplication, a total of 1076 titles and abstracts were reviewed, and 77 full articles were retrieved, from which 66 papers were identified through the screening process and 11 papers were included from the reference lists of published review/overview papers [Reference Jin and Mosweu6, Reference Achilla and McCrone12–Reference Vieta, Badia, Álvarez and Sacristán25]. Of these, 23 met the predefined inclusion criteria and were included. A flow diagram of the systematic literature search, based on the PRISMA template Reference Panic, Leoncini, de Belvis, Ricciardi and Boccia[10], is presented in Fig. 1.

Fig. 1 The flow diagram of the systematic literature search.
3.2 Summary of included studies
Seventeen studies had two or more study arms comparing different patient groups, while six studies had a non-comparative design. The majority of the included studies assessed both the costs and healthcare utilization of schizophrenia, while six studies examined only resource use in association with the disease. The classification of each study is presented in Appendix C.1.
Five studies had societal [Reference Ekman, Granström, Omérov, Jacob and Landén26–Reference Pletscher, Mattli, von Wyl, Reich and Wieser30], nine had healthcare system [Reference Baandrup, Sørensen, Lublin, Nordentoft and Glenthoj31–Reference Zaprutko, Nowakowska, Kus, Bilobryvka, Rakhman and Pogłodziński39], and nine had a third-party-payer perspective [Reference Esposti, Sangiorgi, Mencacci, Spina, Pasina and Alacqua40–Reference Zeidler, Slawik, Fleischmann and Greiner48]. Eighteen studies had a retrospective [Reference Ekman, Granström, Omérov, Jacob and Landén26–Reference Baandrup, Sørensen, Lublin, Nordentoft and Glenthoj31, Reference Hirjak, Hochlehnert, Thomann, Kubera and Schnell35, Reference Sicras-Mainar, Maurino, Ruiz-Beato and Navarro-Artieda37–Reference Jacobs, Gutacker, Mason, Goddard, Gravelle and Kendrick42, Reference Pillay and Moncrieff44–Reference Zeidler, Slawik, Fleischmann and Greiner48], five studies had a prospective design [Reference Bernardo, San, Olivares, Dilla, Polavieja and Gilaberte32–Reference Herbild, Andersen, Werge, Rasmussen and Jürgens34, Reference Sarlon, Heider, Millier, Azorin, König and Hansen36, Reference Novick, Haro, Bertsch, Anand, Jemiai and Haddad43], and all studies had a prevalence based approach. Although a publication date since 2010 was necessary for any study to be included according to the criteria, the oldest data analyzed in a study was from 1998 Reference Pillay and Moncrieff[44] and the latest data was from 2013 [Reference Hirjak, Hochlehnert, Thomann, Kubera and Schnell35, Reference Szkultecka-Debek, Miernik, Stelmachowski, Jakovljević, Jukić and Aadamsoo38, Reference Szkultecka-Debek, Miernik, Stelmachowski, Jakovljevic, Jukic and Aadamsoo45]. The countries covered by the studies are summarized in Appendix C.2.
From the 23 studies, 3 did not specify the disease classification criteria used to identify patients with schizophrenia. Fifteen studies used ICD-10 [Reference Ekman, Granström, Omérov, Jacob and Landén26–Reference Baandrup, Sørensen, Lublin, Nordentoft and Glenthoj31, Reference Hirjak, Hochlehnert, Thomann, Kubera and Schnell35, Reference Szkultecka-Debek, Miernik, Stelmachowski, Jakovljević, Jukić and Aadamsoo38–Reference Jacobs, Gutacker, Mason, Goddard, Gravelle and Kendrick42, Reference Pillay and Moncrieff44, Reference Uggerby, Nielsen, Correll and Nielsen46, Reference Zeidler, Slawik, Fleischmann and Greiner48], while 5 papers applied DSM-IV-based patient inclusion criteria [Reference Bernardo, San, Olivares, Dilla, Polavieja and Gilaberte32, Reference Cortesi, Mencacci, Luigi, Pirfo, Berto and Sturkenboom33, Reference Sarlon, Heider, Millier, Azorin, König and Hansen36, Reference Sicras-Mainar, Maurino, Ruiz-Beato and Navarro-Artieda37, Reference Van Der Lee, De Haan and Beekman47]. Differences were found when considering the included ICD-10 codes. From the 15 studies using ICD-10, 4 were restrictive by limiting their study population to only those patients who had disease diagnoses ICD – F20 [Reference Frey28, Reference Jacobs, Gutacker, Mason, Goddard, Gravelle and Kendrick42, Reference Uggerby, Nielsen, Correll and Nielsen46, Reference Zeidler, Slawik, Fleischmann and Greiner48], while the remaining 11 studies applied a broader disease definition, admitting codes varying from F20 to F29 [Reference Ekman, Granström, Omérov, Jacob and Landén26, Reference Esposti, Sangiorgi, Ferrannini, Spandonaro, Turi and Cesari27, Reference Olesen, Gustavsson, Svensson, Wittchen and Jonsson29–Reference Baandrup, Sørensen, Lublin, Nordentoft and Glenthoj31, Reference Hirjak, Hochlehnert, Thomann, Kubera and Schnell35, Reference Szkultecka-Debek, Miernik, Stelmachowski, Jakovljević, Jukić and Aadamsoo38–Reference Evensen, Wisloff, Lystad, Bull, Ueland and Falkum41, Reference Pillay and Moncrieff44].
The details of the cost studies included are presented in Table 1. Note that irrespective of the perspective described in the study (e.g. societal, healthcare system etc.) only direct healthcare costs were presented in our review, making data more comparable across studies.
In total 13 articles had good quality with overall scores ranging between 72% and 97%. There was no article with lower total scores than 46% or lower score than 33% in any of the domains. Data presented in lower quality articles were concerned acceptable to be included in the analysis.
3.3 Direct healthcare cost of schizophrenia
3.3.1 Total cost per country
Estimates for total direct healthcare costs for European countries were provided in five studies [Reference Frey28–Reference Pletscher, Mattli, von Wyl, Reich and Wieser30, Reference Sarlon, Heider, Millier, Azorin, König and Hansen36, Reference Evensen, Wisloff, Lystad, Bull, Ueland and Falkum41] presented in Table 2. According to Olesen Reference Olesen, Gustavsson, Svensson, Wittchen and Jonsson[29] the total European cost of schizophrenia in 2010 was €29 billion. The data suggest that the total cost of schizophrenia relative to the country population is threefold higher in Germany and Norway compared to France. The proportional direct cost of schizophrenia relative to the GDP is the highest in Germany (0.28%), although the EU27 countries plus Iceland, Norway and Switzerland show a similar percentage (0.21%) on average. This might be due to the relatively high prevalence (1.2%) reported by Olesen Reference Olesen, Gustavsson, Svensson, Wittchen and Jonsson[29] compared to prevalence estimates in other studies. Comparability of data is limited due to the difference in the year of costing, cost calculation method and the estimated prevalence.
3.3.2 Cost per patient
Cost estimates from 8 studies [Reference Ekman, Granström, Omérov, Jacob and Landén26, Reference Dlouhy28–Reference Pletscher, Mattli, von Wyl, Reich and Wieser30, Reference Sarlon, Heider, Millier, Azorin, König and Hansen36, Reference Zaprutko, Nowakowska, Kus, Bilobryvka, Rakhman and Pogłodziński39, Reference Esposti, Sangiorgi, Mencacci, Spina, Pasina and Alacqua40, Reference Van Der Lee, De Haan and Beekman47] considering 8 countries and 1 study referring to the total EU27 supplemented by Iceland, Norway and Switzerland are presented in Table 3 in terms of cost in € and the proportion of inpatient, outpatient, drug and other costs. The review revealed large differences in the total direct healthcare cost of schizophrenia among countries. The average annual direct medical cost per patient was estimated to be €5800 Reference Olesen, Gustavsson, Svensson, Wittchen and Jonsson[29] in Europe. The annual cost per patient ranged from €533 in Ukraine Reference Zaprutko, Nowakowska, Kus, Bilobryvka, Rakhman and Pogłodziński[39] to €13,704 in the Netherlands Reference Van Der Lee, De Haan and Beekman[47]. Notably drug costs contributed to less than 25% of the direct healthcare cost per patient in every country, while inpatient costs were the largest component of health service costs in the majority of the countries.
Ukraine is classified as a lower-middle-income country according to the World Bank Country Classification,1 while all other countries included in the analyses are classified as high-income countries. This difference is reflected in the direct healthcare cost of schizophrenia in Ukraine compared to the EU countries listed in Table 3.
To improve the cross-country comparability of the costs of schizophrenia, we plotted the results from studies providing a direct per capita cost estimate against the GDP per capita estimate of the respective country (we limited this comparison to studies using samples representative of a larger population of the given county). A moderate relationship was found between costs and economic wealth: GDP per capita explains about half of the variation in cost per patient estimates (see R 2 in Fig. 2).
3.4 Inpatient care related resource use
Average length of stay shows heterogeneity across the studies (see Table 4). Where total group-specific data were available, the duration of hospital stay ranged between 32.62 Reference Frey[28] and 107.7 days Reference Pillay and Moncrieff[44], representing a threefold difference. UK-specific length of stay was reported by 2 studies [Reference Jacobs, Gutacker, Mason, Goddard, Gravelle and Kendrick42, Reference Pillay and Moncrieff44] that show a twofold difference; the average length of stay was 107.7 and 47.7 days. This might be explained by the different disease definitions used in the two papers. Comparative studies show that young age Reference Frey[28], antipsychotic polypharmacy Reference Baandrup, Sørensen, Lublin, Nordentoft and Glenthoj[31] and oral antipsychotics (compared to LAI antipsychotics) Reference Esposti, Sangiorgi, Ferrannini, Spandonaro, Turi and Cesari[27] also tend to increase length of hospitalization. In Eastern Europe, the average duration ranges between 15.5 (Estonia) and 33.9 days (Serbia) according to Szkultecka-Debek Reference Szkultecka-Debek, Miernik, Stelmachowski, Jakovljević, Jukić and Aadamsoo[38]. Greatest number of admission frequency (mean 3.7/month) was associated with the use of LAI antipsychotics Reference Bernardo, San, Olivares, Dilla, Polavieja and Gilaberte[32]. This outstanding value may be explained by several reasons: (1) a small subgroup sample size of 7 persons; (2) unfavorable baseline characteristics of the examined patient population; (3) psychiatric and general hospitalization frequencies were examined separately and the latter – that we also report in our review – may include a greater extent of services than hospitals usually provide in the form of inpatient care.
3.5 Factors associated with the variations in the direct healthcare costs
Seven comparative studies were identified investigating substantial factors that explain the differences of treatment costs among patient groups with schizophrenia. A large sample German study Reference Zeidler, Slawik, Fleischmann and Greiner[48] found that the annual overall treatment cost of hospitalized patients (€16,073) was threefold higher compared to the non-hospitalized group (€3754). Direct healthcare costs were also found to be strongly related to the global assessment of functioning (GAF) score of the patient in a study with a large sample size in Sweden Reference Ekman, Granström, Omérov, Jacob and Landén[26]. The study highlights that patients with serious to severe impairment (GAF score < 50) have 236% (€9315 per patient per year) higher treatment costs compared to those with no or slight symptoms (GAF score ≥ 70). According to the study of Sicras-Mainar et al. Reference Sicras-Mainar, Maurino, Ruiz-Beato and Navarro-Artieda[37], patients with negative symptoms used 23% (€382 per patient per year) more healthcare resources over a period of 12 months, regarding especially primary care assistance (compared to patients without negative symptoms). In another German study Reference Frey[28], that analyzed a large patient sample, the difference in the excess resource use attributable to schizophrenia for elderly (aged > 65 years) patients was found to be 66% higher (€18,007) than the excess cost (€10,530) attributed to schizophrenia in the overall population in 2008. In a cost-consequence study Esposti (2012) found that the mean cost per patient one year after switching to depot-injection (LA-risperidone) from oral antipsychotic decreased by 9% (€557) compared to the year before. The incremental cost of polypharmacy compared to monotherapy calculated based on the data presented by Baandrup et al. Reference Baandrup, Sørensen, Lublin, Nordentoft and Glenthoj[31] was €7893 and €4594 in 2007 and 2008, respectively. Van Der Lee Reference Van Der Lee, De Haan and Beekman[47] found that compliance to elective pharmaceutical therapy is associated with less acute treatment events, inpatient care and reduced costs of healthcare for patients with schizophrenia, accounting for €5700 to €9300 savings per patient annually. The factors investigated by the included comparative studies are summarized in Table 5.
Table 1 Annual direct healthcare cost of schizophrenia per patient per study group.
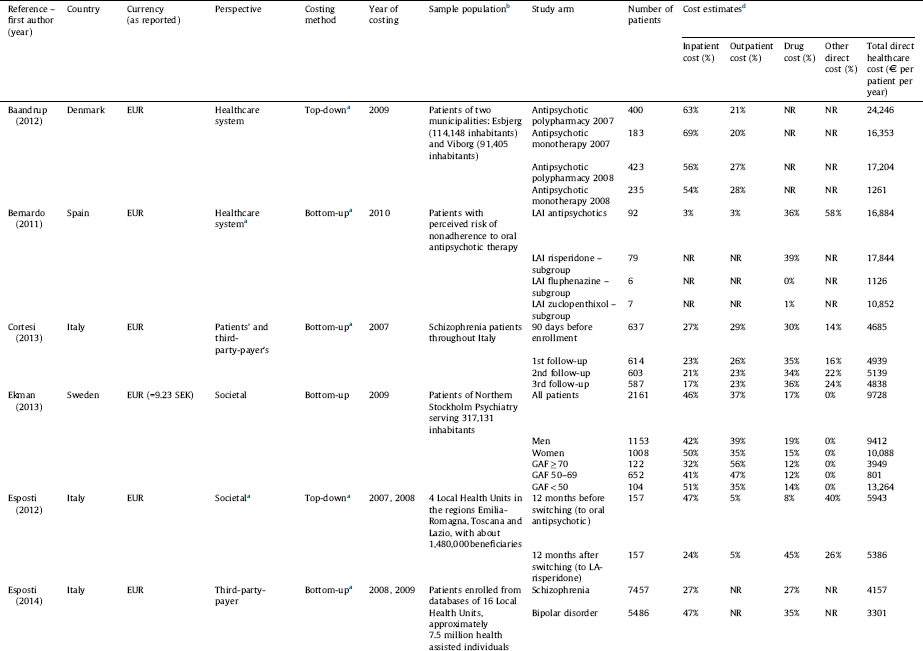


a Assumed based on the presented methodology since information was not explicitly stated in the study.
b Studies not presenting estimations for the total population or using a non-representative sample were excluded.
c Converted from USD to EUR (1 EUR = 1.33 USD as of 2010, annual average exchange rate, European Central Bank).
d The table represents the ratio of inpatient, outpatient and drug cost compared to the total cost, while other costs (such as community care, GP etc.) were omitted due to incomparability of data across studies. As a result the cost elements presented here do not necessarily add up to 100%.
Table 2 Total annual direct healthcare cost of schizophrenia by countries.
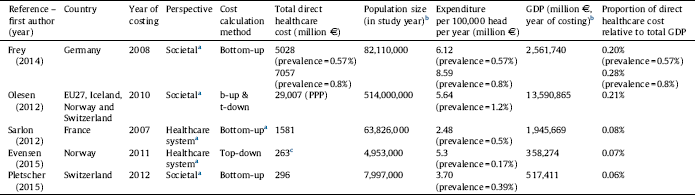
a Assumed based on the presented methodology of this review since information was not explicitly stated in the study.
b Source: Eurostat: http://ec.europa.eu/eurostat/data.
c Total mental health treatment cost, converted from USD, 1 EUR = 1.39 USD, as of 2011, annual average exchange rate, European Central Bank.
Table 3 Average annual direct healthcare cost of schizophrenia per patient by countries.

a Assumed based on the presented methodology since information was not explicitly stated in the study.
b Health specific harmonised index of consumer prices (HICPs) was used to inflate all costs to the common year of 2012.
c Originally reported data referred to 3 years.
d Originally reported data referred to 6 month.
Table 4 Hospitalization – resource use by study group where data was available.



NR: not reported.
a Mean value.
b Excess resource use attributable to schizophrenia.
c ATT: average treatment effect on the treated.

Fig. 2 The association between GDP per capita and average direct healthcare cost per patient. Note: The line depicts the best fit based on the linear regression of annual direct healthcare costs on actual GDP per capita in € in the given year. The size of the bubbles reflects the sample size of the study. The data for EU27 plus Iceland, Norway and Switzerland study are marked with a triangle because no data was available on the sample size.
4 Discussion
The direct healthcare cost of schizophrenia was investigated in a limited number of cost studies, which were considered to be of good quality and representative for a country population. The analysis provides evidence for an association between a direct per capita cost estimate and a GDP per capita estimate of various countries. The review highlights that despite the modest prevalence of schizophrenia in Europe – varying between 0.4 and 1.2% – [Reference Wittchen, Jacobi, Rehm, Gustavsson, Svensson and Jonsson2, Reference Goldner, Hsu, Waraich and Somers49–Reference Saha, Chant, Welham and McGrath51] the total cost of the disease is substantial in the investigated countries due to the high cost per patient. The review also identified important cost drivers and factors associated with the variation in the treatment cost.
Hospital stay represents the most important direct cost of schizophrenia. The share of hospitalization cost among direct medical costs varied from 27% to 92% depending on the country being considered. The large difference in the share of hospitalization cost among direct medical costs might be explained by substantial differences in the analyzed healthcare systems. The comparability of the data regarding the hospital admissions and inpatient stays are very limited due to several reasons. There are substantial differences in the analyzed healthcare systems, such as health policy objectives and resources devoted to mental health care. There are also dissimilarities across the countries in their basic conceptual approach and preferences regarding the management of a disease. Furthermore socio-cultural factors (living alone, addictive behaviors, role of caregivers etc.) or educational differences and resource issues can also explain the differences observed in the results of inpatient admission indicators. However, no obvious differences in the impatient care related resource use could be detected based on the identified surveys in the contrast of Western versus Eastern European countries, probably because the most significant development in the psychiatric care of Eastern European countries took place in deinstitutionalization [Reference Dlouhy8, Reference Winkler, Krupchanka, Roberts, Kondratova, Machu and Hoschl9] similar to the trend in the Western European countries.
The high costs of inpatient care also highlight the importance of preventing relapses that need hospitalizations. Treatment costs were strongly related to the global assessment function (GAF) score of the patient, suggesting that improvements in global functioning e.g. by means of more effective treatment for severe symptoms might reduce hospitalizations and the cost of schizophrenia. Another study Reference Salize, McCabe, Bullenkamp, Hansson, Lauber and Martinez-Leal[52], investigating the cost drivers of schizophrenia in six European countries, suggests that each additional negative symptom (PANSS) increases the total healthcare costs by 1.5%. However, evidence on the impact of negative symptoms on schizophrenia related to healthcare costs is still scarce. The notable difference in the excess resource use attributable to schizophrenia for elderly (aged > 65 years) patients supports the view that the growing share of elderly people implies substantially higher treatment costs for economies in Europe. One of the most important predictors of hospitalization was found to be patient persistence with the assigned drug treatment, relatively modest interruptions of continuity of care may be associated with worse outcome and higher health care costs [Reference Zaprutko, Nowakowska, Kus, Bilobryvka, Rakhman and Pogłodziński39, Reference Van Der Lee, De Haan and Beekman47]. Several factors were identified in the reviewed studies that can influence non-adherence and non-persistence with antipsychotic therapy. In the real-world clinical practice setting, depot formulations of typical antipsychotics were shown to be more effective than oral formulations [Reference Novick, Haro, Bertsch, Anand, Jemiai and Haddad43, Reference Esposti, Sangiorgi, Ferrannini, Spandonaro, Turi and Cesari27] when prescribed for non-persistent outpatients previously receiving oral therapy, as indicated by increased persistence and therefore less hospitalization due to relapses. Another important factor associated with non-compliance of patients was polypharmacy Reference Baandrup, Sørensen, Lublin, Nordentoft and Glenthoj[31]. Combining treatment with multiple antipsychotics could increase the risk of distressing side effects, lead to greater non-compliance and reduce the level of functioning, which again could increase the risk of readmission and give rise to higher costs of treatment. Discontinuation of treatment was found to be responsible for a large proportion of treatment costs in hospitalized patients with schizophrenia, therefore improving persistence and adherence in antipsychotic therapy can thus lead to cost savings by reducing the frequency and duration of hospital stay.
Table 5 Factors associated with incremental costs of schizophrenia among different patient groups.

GAF: global assessment of functioning.
a Compared to patients without schizophrenia.
Based on the above findings, substantial improvement in the allocation of financial resources could potentially be achieved by increasing investment in the following areas: (1) reducing the number of hospitalizations e.g. by increasing the efficiency of outpatient care; (2) working out interventions targeted at specific symptoms; (3) improving patient persistence and adherence in antipsychotic therapy.
Our results are comparable with previous reviews presenting data on the cost of schizophrenia. The cost per patient data in Gustavsson's study Reference Gustavsson, Svensson, Jacobi, Allgulander, Alonso and Beghi[53] regarding France (€7068) is the same as the cost reported in the current review, while the data for Germany (€5848) is about half of the amount (€12,251) reported recently by Frey Reference Frey[28]. The study conducted by Salize Reference Salize, McCabe, Bullenkamp, Hansson, Lauber and Martinez-Leal[52] shows a high variation in costs compared to our review. The costs presented for Sweden (€21,020), Germany (€16,868) and Switzerland (€36,978) are notably higher than in the studies included in our review, which may be due to different costing methodologies. Salize Reference Salize, McCabe, Bullenkamp, Hansson, Lauber and Martinez-Leal[52] included sheltered accommodation in total costs which represented a large proportion of the overall costs in these countries. This cost was not taken into account in our review considering it is not a direct medical cost. The review conducted by Chong et al. Reference Chong, Teoh, Wu, Kotirum, Chiou and Chaiyakunapruk[54] gives detailed information on the methodological issues of COI studies and presents only aggregated data that does not include cost per patient. The most recent systematic literature review was published in 2017, shortly after our literature search was completed. Jin et al. included nine studies [Reference Ekman, Granström, Omérov, Jacob and Landén26, Reference Frey28, Reference Pletscher, Mattli, von Wyl, Reich and Wieser30, Reference Sarlon, Heider, Millier, Azorin, König and Hansen36, Reference Evensen, Wisloff, Lystad, Bull, Ueland and Falkum41, Reference Behan, Kennelly and O’Callaghan55–Reference Oliva-Moreno, López-Bastida, Osuna-Guerrero, Montejo-González and Duque-González58] from European countries adopting a societal perspective. The studies published in 2010 or later [Reference Ekman, Granström, Omérov, Jacob and Landén26, Reference Frey28, Reference Pletscher, Mattli, von Wyl, Reich and Wieser30, Reference Sarlon, Heider, Millier, Azorin, König and Hansen36, Reference Evensen, Wisloff, Lystad, Bull, Ueland and Falkum41] were also identified by us, however we included in our review further studies that used healthcare perspective. Our findings related to cost drivers were similar: age, global assessment of functioning score, and hospitalizations were found to be important factors associated with higher costs. In addition we identified based on the results of the included comparative studies that continuation of treatment, persistence [Reference Esposti, Sangiorgi, Ferrannini, Spandonaro, Turi and Cesari27, Reference Baandrup, Sørensen, Lublin, Nordentoft and Glenthoj31, Reference Van Der Lee, De Haan and Beekman47] and negative symptoms Reference Sicras-Mainar, Maurino, Ruiz-Beato and Navarro-Artieda[37] also play significant role in influencing the total direct healthcare cost of schizophrenia.
The review has some limitations. Only papers in English language were selected for the review. Inclusion of further publications in other languages may improve the validity of major conclusions. Large differences could be identified in the total direct healthcare cost of schizophrenia among countries. Besides the diverse economic conditions of the investigated countries this might also be due to the different methodological approaches used in the studies. Several studies sampled patients from inpatient facilities and thus probably included the most severely impaired and expensive patients [Reference Ekman, Granström, Omérov, Jacob and Landén26–Reference Frey28, Reference Bernardo, San, Olivares, Dilla, Polavieja and Gilaberte32, Reference Hirjak, Hochlehnert, Thomann, Kubera and Schnell35, Reference Sicras-Mainar, Maurino, Ruiz-Beato and Navarro-Artieda37, Reference Zaprutko, Nowakowska, Kus, Bilobryvka, Rakhman and Pogłodziński39]. Studies used different approaches (top-down, bottom-up and econometric) for the estimation of resource consumption. Although each method is valid and appropriate, the resources allocated to the disease were identified differently, which can lead to disparities in the estimation of costs. The included cost-of-illness studies had a variety of perspectives, each of which included slightly different cost items leading to different and wide range of results for the illness of schizophrenia. These perspectives measured costs to the society, healthcare system and third-party payers. Each perspective provides useful information about the costs to a particular group, although it is weakening the comparability of our findings. Purchasing power parity was not used in the analyses because in our view it does not explain the healthcare related price differences across countries appropriately (e.g. it is not used in the external reference pricing system neither). However, the association between GDP per capita and average direct healthcare cost per patient was investigated in this study to expose in what extent the economic wealth of a country explains the variation in the cost per patient.
There is a lack of generalizable information on the impact of emergence of new treatment, therefore we think that further research is needed to collect more evidence on the effects of new-generation medications.
Central and Eastern European countries are short of up-to-date COI studies. No information is available on all European countries therefore no comprehensive comparisons and analyses could be made on the cost of schizophrenia. There were only a few studies that gave estimations on country-specific total direct healthcare cost by taking into account the population and disease prevalence.
5 Conclusion
Our overview, focusing on the factors influencing the direct health care costs of schizophrenia in European countries could identify several major factors (hospitalization, symptoms, age, persistence and adherence) that have substantial impact on direct healthcare costs of schizophrenia indicating potential solutions that could contribute to a more efficient allocation of available treatment resources.
Funding
This work was financially supported by Gedeon Richter Plc. (Budapest, Hungary)
Disclosure of interest
The authors declare that they have no competing interest. The review was performed for scientific purposes only, the financial support provided by Gedeon Richter had no influence on the outcome of this work. The content of this paper, as well as the views and opinions expressed therein are those of the authors’ and not the organizations that employ them.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.eurpsy.2017.10.008.










Comments
No Comments have been published for this article.