INTRODUCTION
The emergence of the first pandemic of influenza for 40 years proved less severe than had been anticipated in many governments' plans [Reference Donaldson1, Reference Bishop, Murnane and Owen2]. As a result, most people [Reference Pebody3] who were infected with pandemic influenza A(H1N1) suffered a short, self-limiting illness with no complications [4]. A proportion, however, suffered a serious illness resulting in hospitalization [Reference Jain5, 6]. For some this involved time in a critical care facility [6–Reference Perez-Padilla10].
Early clinical reports and, later, analysis of aggregated data from around the world in the first phase of the new pandemic, provided insight into the nature of the complications that were arising particularly in children and younger adults [Reference Perez-Padilla10–14]. This age profile for severe disease was different to seasonal influenza, which affects those aged ⩾65 years disproportionately [Reference Simonsen15]. Key risk factors (such as pre-existing medical conditions, younger age and pregnancy) for hospitalization following infection with pandemic influenza A(H1N1), were reported for Mexico, North America and Australasia [Reference Jain5, Reference Webb7, Reference Dominguez-Cherit8, Reference Baker, Kelly and Wilson16].
Uncertainties remain about the risks for hospitalization at the population level and for people with particular pre-existing conditions. Information on these risks is essential for assessing the adequacy of health service capacity (particularly of critical care) as well as clinical and public health interventions. The pandemic influenza A(H1N1) virus is expected to return in the Northern and Southern hemisphere 2010 influenza seasons [17].
We have gathered and analysed data on patients hospitalized with pandemic influenza A(H1N1) within a whole country during the main period of disease activity with the aim of clarifying some of these key uncertainties.
METHODS
A surveillance system for all hospital in-patients with pandemic influenza A(H1N1) in England was established in September 2009. It was designed to identify and quantify the risk factors for severe illness and to detect trends in virus behaviour. Hospitals already participating in a research project on pandemic influenza A(H1N1) were not invited to contribute to this surveillance system, to minimize the reporting burden on clinicians.
Consultant microbiologists in each hospital were asked to submit a standardized dataset for any case of pandemic influenza A(H1N1) admitted to their hospital. A case was defined as any person formally admitted to hospital (regardless of duration of stay) who had laboratory confirmation [by polymerase chain reaction (PCR) testing] of pandemic influenza A(H1N1) during or prior to their hospital admission.
The dataset included: demographic information (date of birth, sex), pre-existing medical conditions (by organ system, pregnancy and immunosuppression), treatment (antiviral medication use), dates of admission and discharge (to hospital and to critical care if relevant), and complications, as well as patient identifiers [National Health Service (NHS) number, hospital number, name]. Missing data were excluded from the denominator where appropriate.
Data gathering was via a secure web-based portal, which prompts clinicians for missing data fields. Incomplete records were followed up by telephone or by linking with other laboratory and field data. Additional cases were identified through the Health Protection Agency's (HPA) regional microbiology network.
Explicit ethical approval was not sought as this data collection was part of routine pandemic surveillance. Surveillance was carried out under the NHS Act 2006 (section 251), which provides statutory support for disclosure of such data by the NHS, and their processing by the HPA, for communicable disease control.
Prevalence of pre-existing medical conditions
The population prevalence of specific pre-existing medical conditions (excluding pregnancy) was estimated from information provided by English general practitioners (GPs) to the Department of Health-HPA (DoH-HPA) influenza vaccine uptake monitoring system [Reference Gates18]. For the population aged 6 months to 64 years, a breakdown by individual pre-existing condition is available (based on data provided by 96·2% of all English GP practices) [Reference Pebody3]. For the population aged ⩾65 years, the number of people with a pre-existing medical condition was extrapolated from data provided by 79·4% of GP practices (provisional data provided by the DoH). A breakdown by pre-existing condition was not available for people aged ⩾65 years.
The point prevalence of pregnant women was estimated using the sum of the published number of maternities (births and stillbirths) and an estimate of the number of miscarriages and abortions each year [19]. An estimate of the female population of childbearing age (15–44 years) was used to estimate the annual number of miscarriages or abortions, assuming a 4% abortion/miscarriage rate [20]. To calculate the point prevalence of pregnant women, 9/12 of the annual number of maternities (assuming a pregnancy of 9 months' duration) was added to 3/12 of the annual number of miscarriages or abortions (assuming a mean duration of 3 months). The number of pregnancies in each trimester was calculated, assuming that maternities were divided equally between trimesters and that all miscarriages and abortions occurred in the first trimester.
Estimated hospitalization rates for cases of pandemic influenza A(H1N1)
Estimated hospitalization rates for cases of symptomatic pandemic influenza A(H1N1) within the population were calculated for the period 1 April 2009 to 6 January 2010. A 1-week lag period was assumed from disease onset to hospital admission.
Throughout the pandemic, the HPA provided estimates of the total number of symptomatic cases of pandemic influenza A(H1N1). This cumulative estimate was used as the denominator to calculate the estimated hospitalization rates for cases with pandemic influenza A(H1N1). The method of estimating the number of symptomatic cases incorporates the number of people consulting their GP with influenza-like illness, the number using a national internet and telephone-based system to obtain antiviral medication (the National Pandemic Flu Service), the proportion of each of these groups with laboratory-confirmed pandemic influenza A(H1N1) in a tested sample (the positivity rate) and an estimate of the proportion of those with symptomatic illness in the population who do not seek medical attention via either of these routes [21]. Positivity and consultation rates were stratified by age, providing case estimates by age group. Upper and lower estimates around the central estimate reflect the uncertainty inherent in estimating case numbers.
Estimated hospitalization rates for cases of pandemic influenza A(H1N1) were calculated using the central estimate of cases as the denominator. The upper and lower estimates of cases were used to calculate lower and upper estimates, respectively, of the estimated hospitalization rate for cases. A 95% confidence interval (CI) was calculated around these estimates to account for the uncertainty around the observed number of hospitalizations. The ranges presented in this paper refer to the upper 95% confidence limit of the upper estimated rate and the lower 95% confidence limit of the lower estimated rate.
In addition to the calculating the estimated hospitalization rates for cases of pandemic influenza A(H1N1), estimated hospitalization rates for the population were calculated. The estimated hospitalization rate for the population was estimated by dividing the number of hospital admissions due to pandemic influenza A(H1N1) by the population. Population estimates were taken from the Office for National Statistics for 2007 [20].
To estimate the number of clinical cases with different pre-existing conditions, it was assumed that the risk of acquiring pandemic influenza A(H1N1) was the same for the general population and for those with pre-existing medical conditions. It was assumed that infants aged <6 months represent half the cases aged <1 year.
Relative risk
Pooled Mantel–Haenszel age-adjusted relative risks were calculated for each pre-existing condition with the exception of pregnancy. The denominator data were available in the age groups 6 months to <16 years and 16–64 years. Consequently, children have been defined as being aged <16 years. The relative risk for pregnancy was calculated by comparing the hospitalization rate for pregnant women with the rate for women of childbearing age. In all cases the comparison group was those with no risk factors. The attributable fraction in the exposed and the population attributable fraction were estimated for each pre-existing condition.
RESULTS
In total, 2416 hospitalized cases were reported from 1 April 2009 to 6 January 2010. Reports were made by 77% of eligible hospital trusts (129/168). Many of the trusts which did not submit data were already reporting to a separate research project. Those trusts that did not make reports were no different from those that did in complexity of referrals (secondary vs. tertiary facility) or number of beds (mean 619 vs. 711, P=0·2).
The crude overall rate of hospitalizations for the 8-month period encompassing the two waves of the pandemic was 4·6/100 000 population (Table 1). The rate of hospitalization decreased markedly with increasing age. The median age of admitted patients was 20 years [interquartile range (IQR) 6-38] and 48% (1160/2411) were male. The median length of hospital stay was 2 days (IQR 1-5). Length of stay was greater for adults (median 3 days, IQR 1-6) than for children (median 1 day, IQR 1-3), P<0·001.
Table 1. Laboratory-confirmed hospitalization and critical care admission rates for pandemic influenza A(H1N1) by age group

CI, Confidence interval.
Estimated cases and hospitalizations occurred in two waves (Fig. 1). The first wave peaked in mid-July 2009, the second in late October 2009. The greatest number of admissions within the ten English NHS regions was reported from hospitals in the West Midlands (477, 8·8 cases/100 000 population) and in London (401, 5·3 cases/100 000 population).

Fig. 1. Estimated cases (——) of pandemic influenza A(H1N1) within the population and number of laboratory-confirmed hospitalizations (![]() ) by week.
) by week.
The estimated hospitalization rate for cases of pandemic influenza A(H1N1) fell over the course of the pandemic. In June 2009 it was 1580/100 000 cases and by October 2009 it had fallen to 260/100 000 cases (test for trend P<0·0001). Overall, 310 (range 120–680) of every 100 000 estimated cases were admitted to hospital. Those at the extremes of age had the highest rates of hospitalization (Fig. 2).

Fig. 2. Estimated hospitalization rates for cases of pandemic influenza A(H1N1) in different age groups within the population.
Risk factors for hospitalization
Information on pre-existing medical conditions was available for 91% (2209/2416) of hospitalized patients. Of these 58% (1296/2209) had one or more pre-existing conditions. This proportion increased with age (Fig. 3a). Patients with pre-existing conditions had a greater length of stay (median 3 days, IQR 1-6) than those without (median 1 day, IQR 1-3) (P<0·001).

Fig. 3. Distribution of pre-existing conditions by age group for (a) all pandemic influenza A(H1N1) hospitalizations and (b) critical care admissions. ▪, With underlying condition; □, without underlying condition.
The relative risk of hospitalization was ten times greater for those with a pre-existing medical condition compared to those without, in those aged 6 months to 64 years (Table 2). The pre-existing conditions conferring the highest relative risks of hospitalization in those aged 6 months to 64 years were immunosuppression, chronic renal disease, chronic neurological disease and chronic respiratory disease (Table 2).
Table 2. Hospitalization rates, relative risk and attributable fractions for different pre-existing conditions
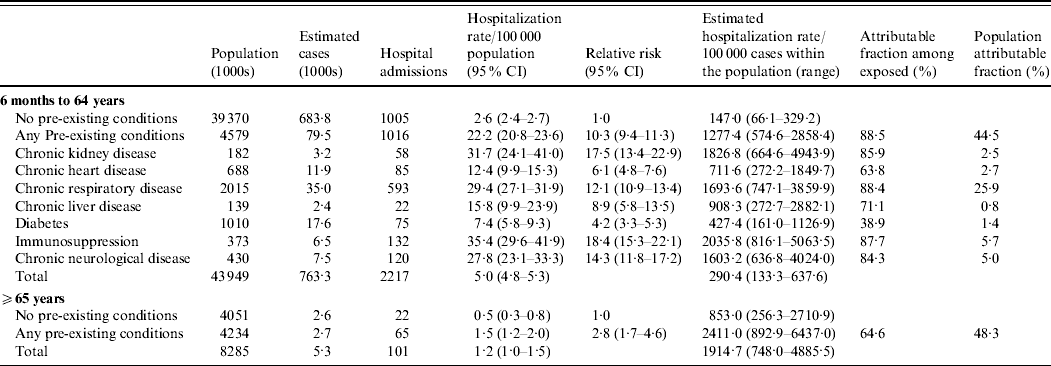
CI, Confidence interval.
Population estimates are mid-2008 estimates [Reference Gates18]. Cases may have more than one pre-existing condition. The relative risk is calculated relative to the group with no pre-existing conditions for that age group. For the age group 6 months to 64 years a pooled Mantel–Haenszel age-adjusted relative risk was calculated.
Twenty-one percent of all women aged 15–44 years were pregnant at the time of admission. Pregnancy conferred a 7·8-fold greater risk of admission than that for all females of childbearing age (Table 3). The risk of admission was greater during the second and third trimesters than the first trimester. The majority of pregnant women had no other pre-existing conditions (72%, 97/135). The most common pre-existing conditions in pregnancy were asthma (23/135), immunosuppression (5/125) and diabetes (4/133).
Table 3. Hospitalization rates and relative risk for pregnant women (aged 15–44 years)

CI, Confidence interval.
The number of women of childbearing age (15–44 years) with no pre-existing conditions is an estimate based on the proportion of the whole population aged 6 months to 64 years who do not have any pre-existing conditions.
Critical care admission
Overall, 33 of every 100 000 estimated cases within the population were admitted to critical care. Of the patients admitted to hospital, 10·5% were admitted to critical care. The proportion of hospital patients admitted to critical care increased with age (Table 1). The median length of stay in critical care was 5 days (IQR 2–12 days, n=136). The length of stay in critical care was not affected by the presence of pre-existing conditions (median 5 days vs. 4·5 days for no pre-existing condition, P=0·55).
Pre-existing conditions were significantly more common in patients admitted to critical care than in hospitalized cases as a whole (79% vs. 54%, P<0·001; Fig. 3). Of patients admitted to critical care, the proportion with a pre-existing condition was similar across the age groups for those aged <65 years (test for trend P=0·59; Fig. 3b). A lower proportion of those aged ⩾65 years had a pre-existing conditions than those aged ⩽64 years (60% vs. 82%, P=0·01). By contrast, for hospitalized patients as a whole, the proportion with a pre-existing condition increased with age (Fig. 3a).
Use of antiviral medication
Data on antiviral medication was available for 81% (1959/2416) of cases. Antiviral medication was administered during the hospital stay for 67% (1299/1927) of patients. Antiviral medication had been started within the recommended 48-h window after symptom onset in 44% (617/1416) and prior to admission in 12% (213/1826) of cases. Patients who received antiviral medication within the recommended 48 h after onset of symptoms were less likely to be admitted to critical care than those who received them after 48 h, after adjusting for age, sex and underlying risk factors [odds ratio (OR) 0·68, 95% CI 0·47–0·99, P=0·047]. No effect on mortality was observed (OR 0·77, 95% CI 0·42–1·43, P=0·41).
Complications
Complications were reported in 349 cases. The most commonly reported complications were pneumonia (321/2416, 13·3%), acute respiratory distress syndrome (44/2416, 1·8%), renal failure (31/2416, 1·3%), shock (25/2416, 1·0%) and encephalopathy (10/2416 0·4%).
Information on the conclusion of the hospital admission was available in 93% of cases (2242/2416). Of these, 79 deaths were reported. This gives a hospital case-fatality rate of 3·5%. The hospital case-fatality rate was highest in those aged >64 years (20%) and lowest in those aged <5 years (0·4%). The hospital case-fatality rate was significantly higher for those with pre-existing conditions compared to those without (5·1% vs. 1·4%, P<0·001).
DISCUSSION
Over two waves, pandemic influenza A(H1N1) in England caused 4·7/100 000 people to be hospitalized. This rate is lower than that reported in other countries, including Australia (22·8/100 000 population), The Netherlands (13·1) and Argentina (27·5) [Reference Baker, Kelly and Wilson16, Reference ‘t Klooster22]. England has taken an aggressive approach to the pandemic, including a media campaign promoting hand-washing and early widespread access to antiviral medication for cases within the community. This may have contributed to the low hospitalization rate. Alternatively, the lower rate of hospitalization in England may be explained by international differences in case definition, reporting systems or thresholds for admission.
We have calculated the relative risk of hospitalization associated with different pre-existing conditions. Chronic kidney disease, chronic neurological disease, chronic respiratory disease and immunosuppression are associated with a 10- to 20-fold increased risk of hospitalization. Reports of pregnancy as a risk factor for pandemic influenza were also substantiated by this study with the greatest risk for those in the third trimester. Other studies have identified chronic respiratory disease and chronic neurological disease as the most common pre-existing conditions in those hospitalized or dying [Reference Cullen23–Reference Oliveira25]. These studies have not quantified the risk of hospitalization for people with these conditions. By contrast, our study takes account of chronic disease prevalence in the population. In so doing, we show that other, less common, pre-existing conditions, such as chronic kidney disease and immunosuppression, are associated with a similar, or even greater, risk of hospitalization than chronic respiratory disease and chronic neurological disease. It is possible that different admission criteria applied to those with pre-existing conditions could contribute to the higher hospitalization rates. However, the high risk of severe disease in this group suggests this contribution is likely to be small. Quantifying these risks is important. It can guide advice to the public, particularly for those with existing illnesses. Quantifying the risks can also help clinicians in decisions about the timing of treatment of high-risk patients. It can also guide policy decisions about vaccination.
A small number of studies have estimated the total number of cases of pandemic influenza A(H1N1) within the population so that it could be used as a denominator for calculating hospitalization rates for affected people. Our study found that an estimated 0·31% of cases of pandemic influenza A(H1N1) were hospitalized. This is similar to New Zealand (0·30%) and the USA (0·44%) [Reference Baker, Kelly and Wilson16, 26]. Our age-specific estimated hospitalization rates for cases of pandemic influenza A(H1N1) show a U-shaped distribution by age. Both the elderly and the young have high rates of admission when infected with pandemic influenza. This distribution is similar to that observed for deaths in the current and previous pandemics [Reference Donaldson1, Reference Echevarria-Zuno24, Reference Luk, Gross and Thompson27]. In contrast, the hospitalization rate within the overall population declines as age increases. This reflects the very low clinical incidence of infection in older people, probably due to pre-existing immunity [28].
Overall, 10·5% of hospitalized cases were admitted to critical care, with the proportion requiring critical care increasing with age. Those who received antiviral medication within the recommended 48-h window after symptom onset were less likely to be admitted to critical care than those receiving antiviral medication after this window. It is of concern, therefore, that early antiviral use in this study was low. Use of antiviral medication at any point during the hospital stay (67%) was lower than observed in France (81%) and the USA (75%) [Reference Jain5, Reference Fuhrman29]. This may reflect lack of familiarity with antiviral medication, a perception of low efficacy among clinicians, or a generally negative attitude fuelled by media coverage of ‘side-effects’. Alternatively, it may reflect poor documentation in clinical notes.
England had a targeted vaccination campaign. In the first phase, vaccination was offered to those with pre-existing medical conditions including pregnancy. In the second phase, vaccination was offered to all children aged <5 years. This paper supports this prioritization. Those with pre-existing medical conditions had the highest rates of hospital admission. By age group, hospitalization rates were highest for those aged <5 years.
Our study has a number of strengths. We have collected data on a whole country, capturing key epidemiological characteristics for a large number of hospital admissions. By using an estimate of the prevalence in the community, we have been able to quantify the relative risks associated with pre-existing conditions. As with similar surveillance systems operating during a pandemic, under-ascertainment of cases may have occurred. This is likely to have occurred equally across age and pre-existing conditions. While the absolute rate of hospitalization may be an under-estimate, the relative risk estimates are likely to be valid. The case estimates used to calculate the estimated hospitalization rates for cases of pandemic influenza A(H1N1) are uncertain. This is reflected in the large confidence intervals.
Setting up and maintaining a national surveillance system for hospitalizations due to pandemic influenza A(H1N1) requires high level political support. By using a secure electronic portal for reporting an additional burden was placed on clinicians in acute hospitals who were busy responding to the pandemic. In including so many hospitals it was difficult to quality-assure the system. While the system was able to capture key data, a sentinel hospital surveillance network is being piloted to capture data for the forthcoming influenza season.
CONCLUSIONS
In England the risk of hospitalization with pandemic influenza A(H1N1) has been concentrated in the young and those with pre-existing conditions. Establishing a national hospital surveillance system has allowed us to quantify the risk factors for hospitalization. This is valuable for patients, clinicians and policy makers, informing decision-making. This information will be essential when planning for the next winter, both in the southern and northern hemispheres, and for future pandemics.
ACKNOWLEDGEMENTS
We are grateful to the many clinicians in England who supplied clinical information on their patients and to Esther Adeyemi for coordinating contacts with hospital clinicians. We are also indebted to colleagues at the HPA Centre for Infections; Liz Miller, Nick Phin, John Watson, Bev Paterson, Maria Zambon, Pia Hardelid and the CfI Statistics team; Christine McCartney and colleagues at the Regional Microbiology Network for invaluable contributions; Sam Bracebridge and colleagues in HPA Local and Regional Services; Asaf Niaz and the development team for building the data collection tool.
DECLARATION OF INTEREST
All authors have support from their respective institutions for the submitted work. L. J. D., as Chief Medical Officer advised government on public health policy, P. J. W. is a member of UK Government's Scientific Advisory Group for Emergencies (SAGE) and Scientific Pandemic Influenza Advisory Committee modelling sub-group (SPI-M); O. J. M., P. D. R., and N. S. advised the Chief Medical Officer.








