Antioxidants are of interest in the CVD. Several observational studies have shown that higher consumption of different fruits and vegetables, the two main dietary sources of antioxidants( Reference Carlsen, Halvorsen and Holte 1 ), was significantly and inversely associated with risk of CVD including CHD( Reference Aune, Giovannucci and Boffetta 2 – Reference Liu, Lee and Ajani 4 ), stroke( Reference Gillman, Cupples and Gagnon 5 , Reference Oude Griep, Verschuren and Kromhout 6 ), myocardial infarction( Reference Martinez-Gonzalez, Fernandez-Jarne and Martinez-Losa 7 ) and heart failure( Reference Rautiainen, Levitan and Mittleman 8 ). Higher consumption of fruits and vegetables is accompanied by lower glycaemic load, higher fibre intake, higher dietary anti-inflammatory properties and higher electrolyte intake; which in turn are associated with a lower risk of CVD( Reference Alissa and Ferns 9 ). However, besides these beneficial features, a potentially cardioprotective role for dietary antioxidants has been proposed( Reference Nunez-Cordoba and Martinez-Gonzalez 10 , Reference Voutilainen, Nurmi and Mursu 11 ).
It was shown in two recent prospective cohort studies in US and European populations that adherence to a diet with high antioxidant capacity was associated with a lower CVD mortality risk( Reference Bastide, Dartois and Dyevre 12 , Reference Kim, Vance and Chen 13 ). Oxidative stress, vascular inflammation and low-grade systemic inflammation are some of the mediatory pathways through which a possible link has been proposed between dietary antioxidants and CVD risk( Reference Aviram, Kaplan and Rosenblat 14 – Reference Zhang, Xu and Li 17 ). In accordance with this evidence, several observational studies have suggested an inverse relationship of higher intakes of vitamin E and vitamin C with the risk of CHD and stroke( Reference Chen, Lu and Pang 18 – Reference Stampfer, Hennekens and Manson 21 ). A pooled analysis of nine prospective cohort studies showed a weak inverse association between higher intake of vitamin E and the risk of CHD, but failed to show such a protective effect for total and individual carotenoids( Reference Knekt, Ritz and Pereira 22 ). Another meta-analysis of prospective cohort studies suggested an inverse association of higher intakes of vitamin E and vitamin C with the risk of CHD, and like the previous review, found a non-significant association for β-carotene( Reference Ye and Song 23 ).
Although observational studies have suggested an association between a higher intake of antioxidants and a lower risk of CVD, interventional studies have failed to show that supplementation with vitamin C, vitamin E and β-carotene can decrease the risk of CVD( Reference Al-Khudairy, Flowers and Wheelhouse 24 – Reference Ye, Li and Yuan 26 ). By contrast, a recent meta-regression analysis of fifty-three randomized controlled trials indicated that high-dose supplementations with vitamin E and β-carotene might increase the risk of all-cause mortality( Reference Bjelakovic, Nikolova and Gluud 27 ). High-dose supplementations, short-term follow-up durations and prior history of CVD or other chronic diseases are some of the proposed explanations for the inconsistent findings in interventional studies( Reference Lawlor, Smith and Bruckdorfer 28 ). Thus, determining the shape of the dose–response relationships between the abovementioned antioxidants and the risk of CVD mortality may clarify whether higher dietary intakes of these antioxidants are associated with a higher CVD mortality risk. To our knowledge, no systematic review and meta-analysis has assessed the association of dietary vitamin E, vitamin C and β-carotene with the risk of CVD mortality. The extent to which these antioxidants are associated with the risk of CVD mortality is still unclear. Furthermore, a recent meta-analysis of prospective cohort studies has suggested that the circulating biomarkers of carotenoids were more strongly associated with risk of breast cancer than dietary intakes( Reference Aune, Chan and Vieira 29 ), but this hypothesis has not been examined in the context of CVD. Therefore, the objective of the present study was to summarize data about the associations of dietary intake and circulating concentration of vitamin C, vitamin E and β-carotene with the risk of total CVD mortality in the general population, with the use of prospective observational studies.
Methods
The present systematic review has been reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement( Reference Moher, Liberati and Tetzlaff 30 ).
Search strategy
A systematic literature search was done in PubMed and Scopus, with studies published from 1966 up to October 2017. The search was performed as part of a larger search on the association between antioxidants and mortality. The systematic search included combinations of keywords relevant to dietary antioxidants intake, circulating antioxidants concentration, mortality and study design (see online supplementary material, Supplemental Table 1). The reference lists of all related articles and reviews were also manually searched. The search was restricted to articles published in English.
Eligibility and study selection
Two authors (A.J., M.S.Z.) independently reviewed the title and abstract of all studies identified. Prospective cohort, nested case–control, case–cohort studies or prospective reports within randomized controlled trials were obtained and included in the current review if they: (i) were conducted among adults aged 18 years or older; (ii) measured and reported baseline dietary intake or serum/plasma concentration of vitamin C, vitamin E or β-carotene in at least three categories; (iii) reported the outcome of interest as total CVD mortality at follow-up; (iv) reported risk estimates (relative risk (RR) or hazard ratio or odds ratio) and the corresponding 95 % CI of CVD mortality for each category of the abovementioned dietary/circulating antioxidants; and (v) reported the number of cases and participants/non-cases or person-years in each category of the abovementioned exposures, or reported sufficient information to estimate those numbers. Studies that reported results per unit increment in any of the dietary/circulating antioxidants or per sd increment were also included. We excluded studies that were: (i) conducted in children and adolescents; and (ii) conducted among patients with specific diseases such as hypertension and type 2 diabetes, or in institutionalized elders.
Data extraction
Two independent authors (A.J., M.P.) reviewed the full text of selected eligible studies and extracted the following information: first author’s name, publication year, location, follow-up duration, number of participants/cases, mean age and/or age range, sex, exposures, exposure assessment method, covariates adjusted for in the multivariate analyses, exposure levels, number of cases/participants, and reported risk estimates and 95 % CI of CVD mortality across different categories of each dietary/circulating antioxidant. The models with the most comprehensive covariate adjustments were selected and included in the meta-analysis.
Quality of meta-evidence
A nine-point Newcastle–Ottawa Scale was used to assess the quality of included studies and studies with more than seven stars were considered high quality( Reference Stang 31 ). In the main analyses, both low- and high-quality studies were included. However, we conducted sensitivity analyses by restricting only to the high-quality studies (≥7 stars). Furthermore, to provide more reliable measures for judgement about the quality of meta-evidence, we applied the new NutriGrade scoring system (a maximum of 10 points) developed by Schwingshackl et al.( Reference Schwingshackl, Knüppel and Schwedhelm 32 ). Based on this scoring system, we recommended four categories to judge the meta-evidence: high (≥8 points), moderate (6 to 8 points), low (4 to 6 points) and very low (0 to 4 points).
Statistical analysis
The RR and 95 % CI were considered as the effect size of all studies. The reported odds ratios and hazard ratios were considered equal to the RR. For the highest v. lowest category meta-analysis, the reported risk estimates for the highest compared with the lowest category of dietary/circulating antioxidants were combined using the DerSimonian and Laird random-effects model( Reference DerSimonian and Laird 33 ). If studies reported results by sex or other subgroups separately, we combined subgroup-specific risk estimates using a fixed-effects model and used the combined effect size for meta-analysis. If studies reported risk estimates per sd increment of dietary/circulating antioxidants, we used the following method to translate the per sd increment risk estimate to the high v. low RR: first, we calculated the differences between the median points of the highest and lowest categories of that dietary/circulating antioxidant in other studies included in the relevant analysis. Then, mean difference between the medians of the highest and lowest categories was calculated. Finally, per sd increment risk estimate was translated to per ‘calculated mean difference’ and was included in the relevant analysis. If the exact amount of sd was not reported in the primary study, we assumed the difference between the highest and lowest categories as 2·18 times the sd ( Reference Danesh, Collins and Appleby 34 ). Potential small-study effects such as publication bias were explored by funnel plots and tested by Egger’s test( Reference Egger, Smith and Schneider 35 ) and Begg’s test( Reference Begg and Mazumdar 36 ) (P<0·10) when there were sufficient studies (n≥10)( Reference Higgins and Green 37 ). To test the potential effect of each study on pooled effect size, influence analysis was done by stepwise exclusion of each study at a time. Subgroup analyses were performed based on sex, geographical location, baseline age, follow-up duration, number of cases, dietary assessment method (in the analyses of dietary antioxidants) and adjustment for main confounders. We included all eligible studies in the main analyses. However, to provide more reliable results, we performed additional sensitivity analyses by restricting only to studies that controlled for main confounders including BMI, physical activity, smoking status and energy intake in their multivariate analyses.
We tested the linear dose–response relationship using generalized least-squares trend estimation, according to the methods developed by Greenland and Longnecker( Reference Berlin, Longnecker and Greenland 38 , Reference Orsini, Bellocco and Greenland 39 ). The method needs the numbers of cases and participants/non-cases or person-years and adjusted risk estimates and their 95 % CI in each category of dietary/circulating antioxidants. Study-specific results were combined using a random-effects model. The median point in each category of dietary/circulating antioxidants was assigned. If medians were not reported, we estimated approximate medians by using the midpoint of the lower and upper bounds. If the highest category was open-ended, we considered it to have the same widths as the closest category. If the lowest category was open-ended, we considered the lower bound as equal to zero. If only the mean of each category was reported, we considered it the same as the median. If the median point of each category was reported per specific amount of energy intake (e.g. per 4184 kJ/1000 kcal) or per specific amount of another variable (e.g. per serum cholesterol in the analysis of α-tocopherol), we recalculated the median point taking the reported mean or median energy intake or serum cholesterol of that category into account. If the numbers of participants/cases or person-years had not been reported in the primary studies, we estimated them by dividing the total number of participants/cases or person-years by the number of categories, if the exposures were defined as quantiles( Reference Schwingshackl, Schwedhelm and Hoffmann 40 ). For studies in which the reference category was not the lowest one, we recalculated risk estimates assuming the lowest category as reference, if the numbers of participants and cases across different categories were reported( Reference Hamling, Lee and Weitkunat 41 ). A potential non-linear association was examined by modelling dietary/circulatory antioxidant levels using restricted cubic splines with three knots at fixed percentiles (10, 50 and 90 %) of the distribution( Reference Orsini, Li and Wolk 42 ). A P value for non-linearity of the meta-analysis was calculated by testing the null hypothesis that the coefficient of the second spline was equal to zero. All analyses were conducted with the statistical software package Stata version 13. P<0·05 was considered statistically significant.
Results
As presented in the online supplementary material, Supplemental Fig. 1, the systematic search identified 17 296 articles, plus six articles through hand-searching. Of these, 2161 articles were duplicates and another 14 995 were not relevant, which were eliminated based on screening the title and abstract. Of the remaining 146 articles, another 129 articles were excluded by assessing their full text and respective reasons for study exclusion are detailed in Supplemental Fig. 1. Eventually, seventeen articles were considered eligible for inclusion in the present review( Reference Bates, Hamer and Mishra 43 – Reference Zhao, Shu and Li 59 ). One article reported the results of the two separate cohort studies and was regarded as two separate studies( Reference Zhao, Shu and Li 59 ). Thus, eighteen studies, comprising a total of 320 548 participants and 16 974 cases of CVD mortality, were included in the final analyses. Five studies were from the USA( Reference Genkinger, Platz and Hoffman 47 – Reference Greenberg, Baron and Karagas 49 , Reference Loria, Klag and Caulfield 54 , Reference Sahyoun, Jacques and Russell 56 ), three studies (two articles) were from Asia( Reference Kubota, Iso and Date 53 , Reference Zhao, Shu and Li 59 ) and ten studies were from Europe( Reference Bates, Hamer and Mishra 43 – Reference Fletcher, Breeze and Shetty 46 , Reference Karppi, Laukkanen and Mäkikallio 50 – Reference Kilander, Berglund and Boberg 52 , Reference Martin-Calvo and Martinez-Gonzalez 55 , Reference Stepaniak, Micek and Grosso 57 , Reference Wright, Lawson and Weinstein 58 ). Five studies included only men( Reference Buijsse, Feskens and Kwape 44 , Reference Karppi, Laukkanen and Mäkikallio 50 , Reference Kilander, Berglund and Boberg 52 , Reference Wright, Lawson and Weinstein 58 , Reference Zhao, Shu and Li 59 ), one study included only women( Reference Zhao, Shu and Li 59 ), and the remainder included both sexes. Seven studies (six articles) assessed dietary intakes of antioxidants( Reference Martinez-Gonzalez, Fernandez-Jarne and Martinez-Losa 7 , Reference Buijsse, Feskens and Kwape 44 , Reference Genkinger, Platz and Hoffman 47 , Reference Kubota, Iso and Date 53 , Reference Stepaniak, Micek and Grosso 57 , Reference Zhao, Shu and Li 59 ), three studies measured plasma concentrations( Reference Buijsse, Feskens and Schlettwein-Gsell 45 , Reference Greenberg, Baron and Karagas 49 , Reference Khaw, Bingham and Welch 51 ), five studies measured serum concentrations( Reference Goyal, Terry and Siegel 48 , Reference Karppi, Laukkanen and Mäkikallio 50 , Reference Kilander, Berglund and Boberg 52 , Reference Loria, Klag and Caulfield 54 , Reference Wright, Lawson and Weinstein 58 ), and three studies reported both dietary and circulating antioxidants as exposure( Reference Bates, Hamer and Mishra 43 , Reference Fletcher, Breeze and Shetty 46 , Reference Sahyoun, Jacques and Russell 56 ). Three studies were prospective evaluations within interventional studies( Reference Fletcher, Breeze and Shetty 46 , Reference Greenberg, Baron and Karagas 49 , Reference Wright, Lawson and Weinstein 58 ), with the remainder being prospective cohort studies. Follow-up duration ranged from 4 to 22 years. The general characteristics of the studies are presented in Table 1.
Table 1 General characteristics of studies included in the present meta-analysis of dietary/circulating antioxidants and risk of cardiovascular mortality

SENECA, Survey in Europe on Nutrition and the Elderly; NHANES, National Health and Nutrition Examination Survey; EPIC, European Prospective Investigation into Cancer and Nutrition; SUN, Seguimiento University of Navarra; HAPIEE, Health, Alcohol and Psychosocial factors In Eastern Europe; W, women; M, men; HDL-C, TC, total cholesterol; HDL-cholesterol; SBP, systolic blood pressure; DM, diabetes mellitus; HTN, hypertension; LDL-C, LDL-cholesterol; hs-CRP, high-sensitivity C-reactive protein; MDS, Mediterranean diet score; WHR, waist-to-hip ratio.
Vitamin C and CVD mortality
Ten studies (nine articles) with a total of 242 677 participants and 7933 cases were included in the analysis of dietary vitamin C and CVD mortality risk( Reference Bates, Hamer and Mishra 43 , Reference Buijsse, Feskens and Kwape 44 , Reference Fletcher, Breeze and Shetty 46 , Reference Genkinger, Platz and Hoffman 47 , Reference Kubota, Iso and Date 53 , Reference Martin-Calvo and Martinez-Gonzalez 55 – Reference Stepaniak, Micek and Grosso 57 , Reference Zhao, Shu and Li 59 ). Participants belonging to the highest category of dietary vitamin C had a 21 % lower risk of CVD mortality compared with those in the lowest category (RR=0·79; 95 % CI 0·68, 0·89; Table 2), with moderate evidence of heterogeneity in the data (I 2=45·7 %, P heterogeneity=0·06; see online supplementary material, Supplemental Fig. 2). In the sensitivity analysis removing each study at a time, none of the excluded studies altered the summary result materially (RR ranged between 0·77 and 0·82). In a sensitivity analysis by restricting only to studies with high quality score (≥7 points), the RR altered to 0·82 (0·95 % CI 0·76, 0·89; I 2=0 %, n studies 9). Additionally, we performed a sensitivity analysis by restricting only to studies that controlled for BMI, physical activity, energy intake and smoking status in their multivariate analyses; the summary result remained significant (RR=0·80; 95 % CI 0·72, 0·87; I 2=0 %, n studies 6).
Table 2 Meta-analysis of dietary/circulatory antioxidants (highest v. lowest category analysis) and risk of total cardiovascular mortality

RR, relative risk.
In the subgroup analyses, a significant inverse association persisted across most of the subgroups apart from studies conducted in the USA, studies with older participants (mean age >60 years v. <60 years), studies without adjustment for BMI and physical activity, and studies that used methods other than FFQ to assess dietary intake (see online supplementary material, Supplemental Table 2). Stratifying by sources of vitamin C, the relative risk of CVD mortality was 0·83 (95 % CI 0·76, 0·90; I 2=0 %, n studies 7) when only dietary intake was taken into consideration and was 0·55 (95 % CI 0·26, 0·85; I 2=54·5 %, n studies 3) when intake from both food and supplements was taken into account. The subgroup analyses suggested that number of cases, geographical region, follow-up duration, mean age, prior exclusion of participants with a history of CVD and adjustment for main confounders were potential sources of the heterogeneity. No evidence of publication bias was found with Egger’s test (P=0·33) and with Begg’s test (P=0·86), but the funnel plot showed some evidence of asymmetry (Supplemental Fig. 3).
In the linear dose–response meta-analysis, a 50mg/d increment in dietary vitamin C intake was associated with an 8 % lower risk (RR=0·92; 95 % CI 0·88, 0·96), with moderate evidence of heterogeneity (I 2=49·7 %, P heterogeneity=0·04; Fig. 1(a)). Sequential exclusion of each study from the pooled analysis minimally altered the association (RR changed between 0·91 and 0·93). A non-linear dose–response meta-analysis demonstrated a significant dose–dependent association, in which the risk decreased linearly from a baseline of 40mg/d up to an intake of ~200mg/d, and then reached a plateau (P non-linearity<0·001; Fig. 1(b)).
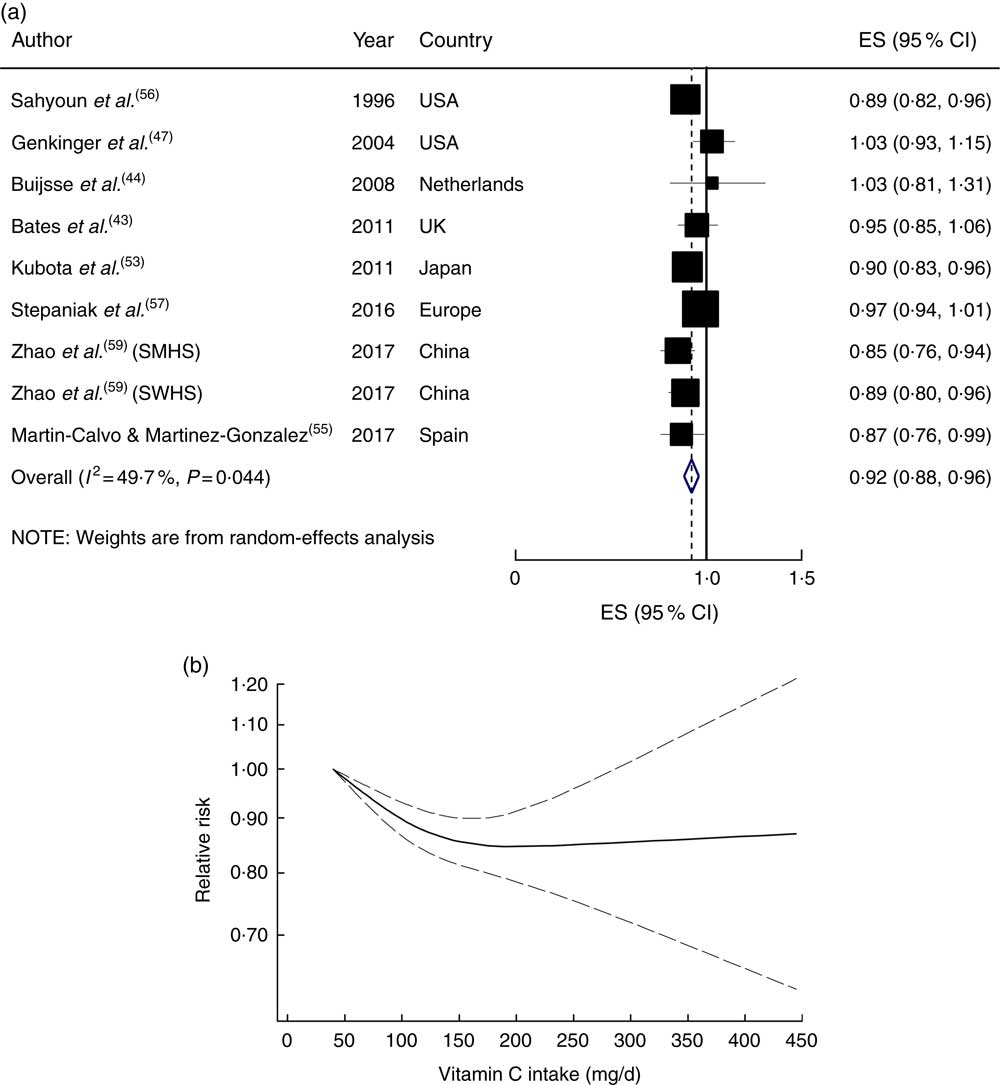
Fig. 1 (a) Forest plot showing relative risk of cardiovascular mortality for a 50 mg/d increment in vitamin C intake. The study-specific effect size (ES) and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the blue open diamond and the vertical dashed line represent the pooled ES, and the width of the blue open diamond represents the pooled 95 % CI (SMHS, Shanghai Men’s Health Study; SWHS, Shanghai Women’s Health Study). (b) Dose–response association of vitamin C intake and risk of total cardiovascular mortality (———), with 95 % CI (— — —)
Six studies with 45 040 participants and 2992 cases were analysed for the association between circulating vitamin C concentration and risk of CVD mortality( Reference Bates, Hamer and Mishra 43 , Reference Fletcher, Breeze and Shetty 46 , Reference Goyal, Terry and Siegel 48 , Reference Khaw, Bingham and Welch 51 , Reference Loria, Klag and Caulfield 54 , Reference Sahyoun, Jacques and Russell 56 ). The relative risk of CVD mortality for the highest compared with the lowest category of circulating vitamin C was 0·60 (0·95 % CI 0·42, 0·78), with high heterogeneity (I 2=64·7 %, P heterogeneity=0·01; see online supplementary material, Supplemental Fig. 4). In the sensitivity analysis excluding each study sequentially from the pooled analysis, the association ranged from 0·55 (95 % CI 0·36, 0·74) with the exclusion of the NHANES III study( Reference Goyal, Terry and Siegel 48 ) to 0·71 (95 % CI 0·59, 0·82) with the exclusion of the EPIC-Norfolk study( Reference Khaw, Bingham and Welch 51 ); and the latter study explained all of the observed heterogeneity (I 2=0 %). In the sensitivity analyses, when the analysis was restricted only to the high-quality studies and studies that controlled for main confounders, the relative risk changed to 0·72 (95 % CI 0·60, 0·85; I 2=0 %, n studies 4) and 0·74 (0·95 % CI 0·58, 0·90; I 2=0 %, n studies 2), respectively. In the subgroup analyses, a significant inverse association persisted across all subgroups, and appeared stronger among European studies compared with US studies (RR=0·46 v. 0·75), studies with <500 cases v. >500 cases (RR=0·46 v. 0·78), follow-up durations <10 years v. >10 years (RR=0·37 v. 0·72), studies that controlled for vitamin supplementation v. studies without adjustment (RR=0·55 v. 0·66), as well as among older participants with a mean age of >60 years v. <60 years (RR=0·58 v. 0·62; Supplemental Table 3). The subgroup analyses suggested that baseline mean age and adjustment for BMI and vitamin supplementation were potential sources of the heterogeneity. Publication bias tests were not performed (n<10).
All studies were eligible for inclusion in the dose–response meta-analysis, with the results showing that a 20µmol/l increment in circulating vitamin C concentration was associated with a 13 % lower risk (RR=0·87; 95 % CI 0·80, 0·94), with high heterogeneity (I 2=70·7 %, P heterogeneity=0·004; Fig. 2(a)). A significant inverse association persisted when each study was sequentially excluded from the pooled analysis (RR ranged between 0·84 and 0·90). In the stepwise exclusion of each study, most of the heterogeneity was explained by the NHANES II study( Reference Loria, Klag and Caulfield 54 ); when this study was excluded, the heterogeneity was reduced and the association changed to 0·84 (95 % CI 0·79, 0·90; I 2=36·6 %, P heterogeneity=0·18). A non-linear dose–response meta-analysis demonstrated that the risk of CVD mortality decreased linearly with increasing circulatory vitamin C concentration from baseline up to about 60µmol/l, without further changes in effect estimate (P non-linearity<0·001; Fig. 2(b)).
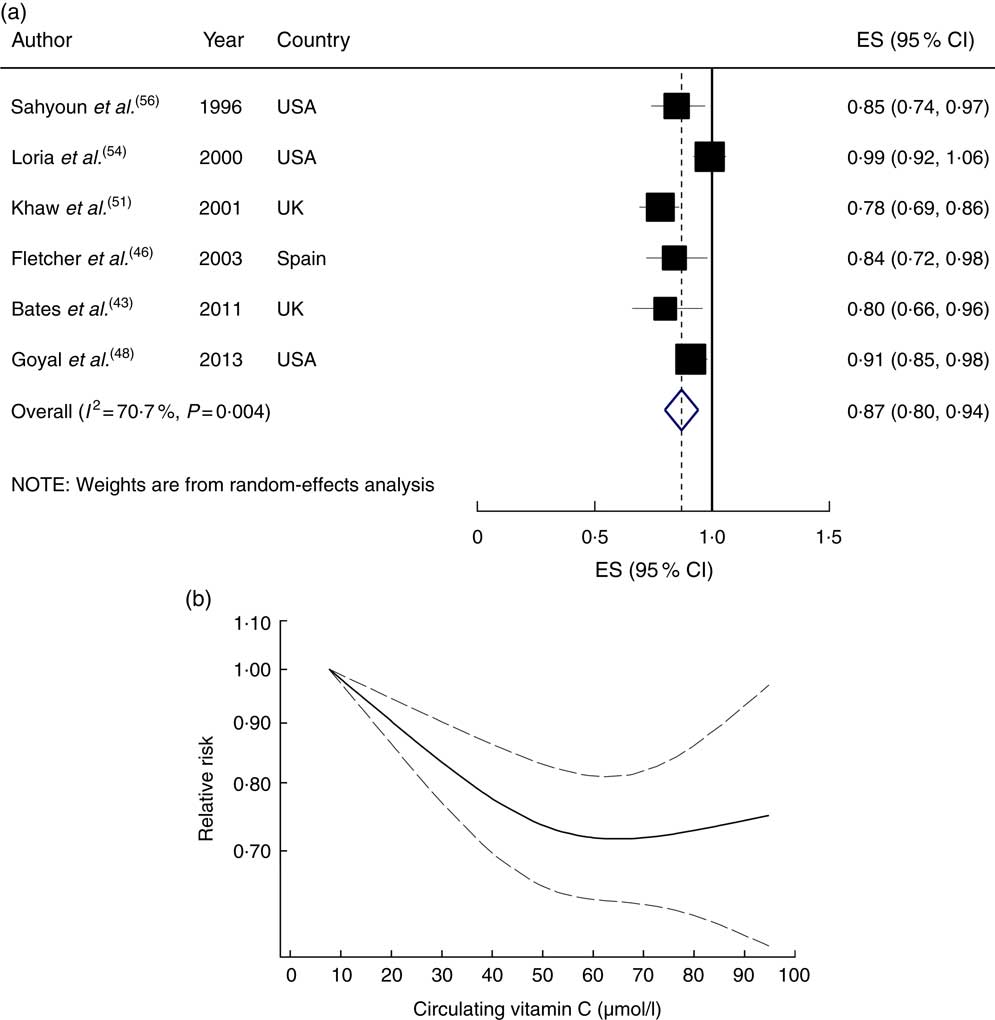
Fig. 2 (a) Forest plot showing relative risk of cardiovascular mortality for a 20 µmol/l increment in circulating vitamin C concentration. The study-specific effect size (ES) and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the blue open diamond and the vertical dashed line represent the pooled ES, and the width of the blue open diamond represents the pooled 95 % CI. (b) Dose–response association of circulating vitamin C concentration and risk of total cardiovascular mortality (———), with 95 % CI (— — —)
Vitamin E and CVD mortality
Eight studies (seven articles) involving a total of 228 531 participants with 7777 cases were included in the analysis of dietary vitamin E and CVD mortality risk( Reference Bates, Hamer and Mishra 43 , Reference Buijsse, Feskens and Kwape 44 , Reference Fletcher, Breeze and Shetty 46 , Reference Genkinger, Platz and Hoffman 47 , Reference Kubota, Iso and Date 53 , Reference Stepaniak, Micek and Grosso 57 , Reference Zhao, Shu and Li 59 ). All studies were at high quality. We did not include the Massachusetts Nutrition Status Survey in the analysis of dietary vitamin E because of the very high vitamin E consumption in comparison with other studies (almost twofold)( Reference Sahyoun, Jacques and Russell 56 ). The relative risk of CVD mortality for the highest compared with the lowest category of dietary vitamin E intake was 0·91 (95 % CI 0·79, 1·03), with moderate heterogeneity (I 2=51·3 %, P heterogeneity=0·04; see online supplementary material, Supplemental Fig. 5). The association did not reach statistical significance when each study was sequentially excluded from the pooled analysis. We performed an additional sensitivity analysis by restricting only to studies that controlled for main confounders, but the risk did not reach statistical significance (RR=0·86; 95 % CI 0·69, 1·03). A non-significant association persisted across all subgroups and between sexes. The subgroup analyses suggested that number of cases, geographical region, follow-up duration, baseline age, dietary assessment method and adjustment for main confounders were potential sources of the heterogeneity in the data (Supplemental Table 4).
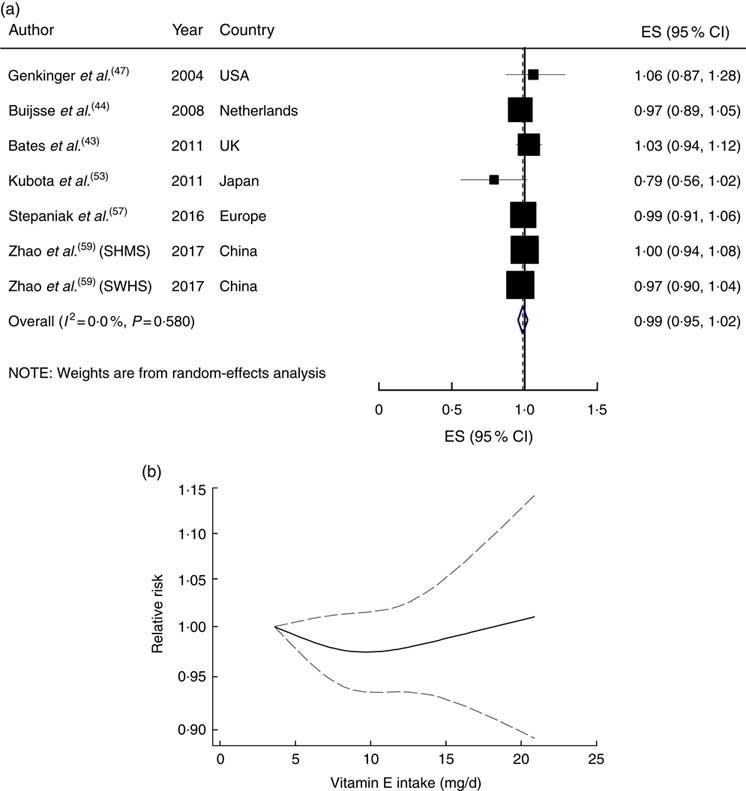
The linear trend estimation indicated that a 5mg/d increment in dietary vitamin E intake was not associated with the risk of CVD mortality (RR=0·99; 95 % CI 0·95, 1·02), with no evidence of heterogeneity (I 2=0 %, P heterogeneity=0·58; Fig. 3(a)). The risk of CVD mortality did not change materially with the increase in dietary vitamin E intake (P non-linearity=0·44; Fig. 3(b)).

Fig. 3 (a) Forest plot showing relative risk of cardiovascular mortality for a 5 mg/d increment in vitamin E intake. The study-specific effect size (ES) and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the blue open diamond and the vertical dashed line represent the pooled ES, and the width of the blue open diamond represents the pooled 95 % CI (SMHS, Shanghai Men’s Health Study; SWHS, Shanghai Women’s Health Study). (b) Dose–response association of vitamin E intake and risk of total cardiovascular mortality (———), with 95 % CI (— — —)
Five studies with 34 285 participants and 6542 cases were included in the analysis of circulating α-tocopherol( Reference Bates, Hamer and Mishra 43 , Reference Buijsse, Feskens and Schlettwein-Gsell 45 , Reference Fletcher, Breeze and Shetty 46 , Reference Kilander, Berglund and Boberg 52 , Reference Wright, Lawson and Weinstein 58 ). Four studies reported an inverse association, which was statistically significant in one study, and another study showed a non-significant positive relationship. A significant inverse association was found for the highest compared with the lowest category of circulating α-tocopherol concentration (RR=0·82; 95 % CI 0·76, 0·88; I 2=0 %, P heterogeneity=0·55, n studies 5; see online supplementary material, Supplemental Fig. 6) and for a 10µmol/l increment in circulating concentration (RR=0·93; 95 % CI 0·91, 0·95; I 2=0·20 %, P heterogeneity=0·39, n 4 studies; Fig. 4). However, the weight of the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study( Reference Wright, Lawson and Weinstein 58 ) was much bigger than other studies and when this study was excluded from the pooled analyses, the association became non-significant in both high v. low (RR=0·86; 95 % CI 0·69, 1·03) and in the linear dose–response analyses (RR=0·94; 95 % CI 0·80, 1·07). Only two studies reported sufficient information( Reference Fletcher, Breeze and Shetty 46 , Reference Wright, Lawson and Weinstein 58 ), so we were unable to test the potential non-linear dose–response relationship.

Fig. 4 Forest plot showing relative risk of cardiovascular mortality for a 10 µmol/l increment in circulating α-tocopherol concentration. The study-specific effect size (ES) and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the blue open diamond and the vertical dashed line represent the pooled ES, and the width of the blue open diamond represents the pooled 95 % CI
β-Carotene and CVD mortality
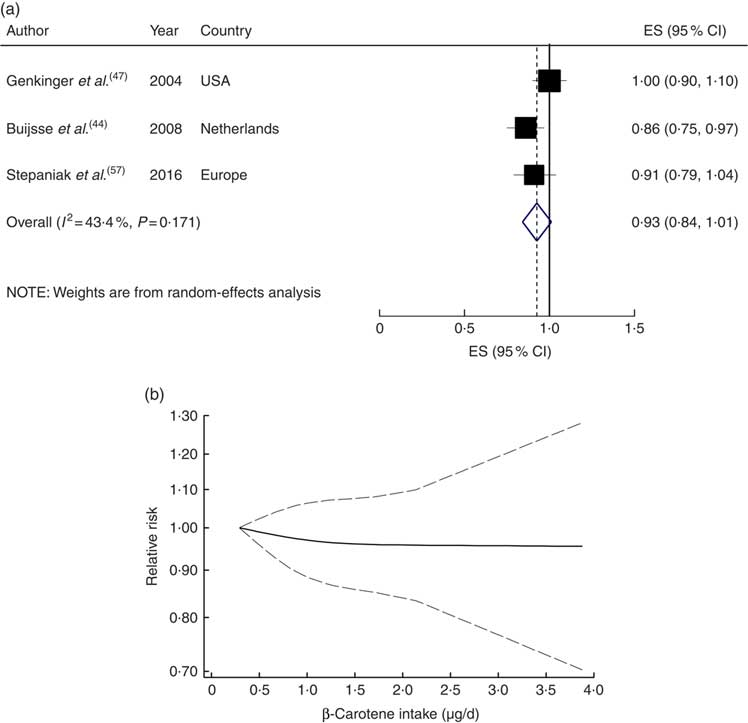
Four studies with 34 917 participants and 1700 cases were included in the analysis of dietary β-carotene with risk of CVD mortality( Reference Bates, Hamer and Mishra 43 , Reference Fletcher, Breeze and Shetty 46 , Reference Genkinger, Platz and Hoffman 47 , Reference Stepaniak, Micek and Grosso 57 ). A non-significant inverse association was found for the highest compared with the lowest category of dietary β-carotene intake (RR=0·89; 95 % CI 0·73, 1·05; I 2=34·3 %, P heterogeneity=0·21; see online supplementary material, Supplemental Fig. 7) and for a 1µg/d increment in dietary β-carotene intake (RR=0·93; 95 % CI 0·84, 1·01; I 2=43·4 %, P heterogeneity=0·17; n studies 3; Fig. 5(a)). A non-linear dose–response meta-analysis demonstrated that the risk of CVD mortality did not change with increasing dietary β-carotene intake (P non-linearity=0·78; Fig. 5(b)).
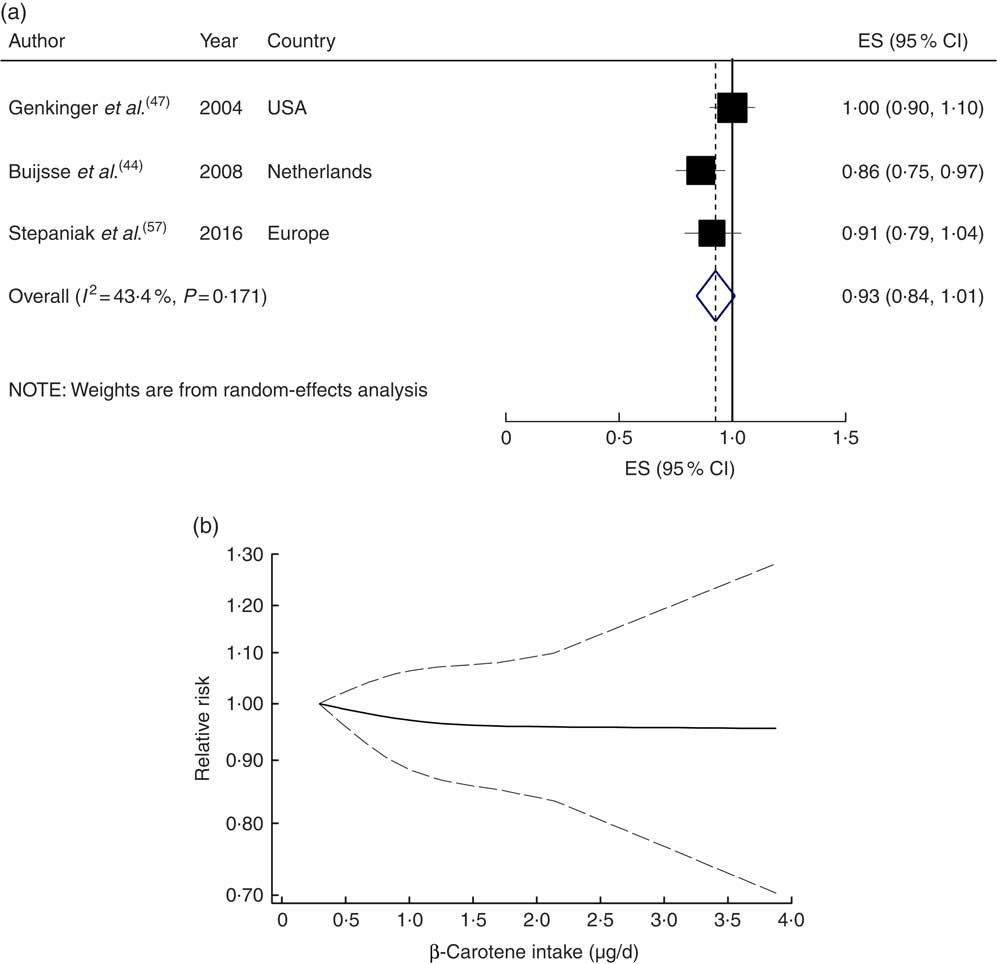
Fig. 5 (a) Forest plot showing relative risk of cardiovascular mortality for a 1 µg/d increment in β-carotene intake. The study-specific effect size (ES) and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the blue open diamond and the vertical dashed line represent the pooled ES, and the width of the blue open diamond represents the pooled 95 % CI. (b) Dose–response association of β-carotene intake and risk of total cardiovascular mortality (———), with 95 % CI (— — —)
Six studies with 22 784 participants and 2758 cases were included in the analysis of circulating β-carotene( Reference Bates, Hamer and Mishra 43 , Reference Fletcher, Breeze and Shetty 46 , Reference Goyal, Terry and Siegel 48 , Reference Greenberg, Baron and Karagas 49 , Reference Karppi, Laukkanen and Mäkikallio 50 , Reference Kilander, Berglund and Boberg 52 ). A higher circulating β-carotene concentration was associated with a 32 % lower risk of CVD mortality (RR=0·68; 95 % CI 0·52, 0·83), with moderate heterogeneity (I 2=50·1 %, P heterogeneity=0·08; see online supplementary material, Supplemental Fig. 8). In the sensitivity analysis by removing each study in turn, none of the excluded studies changed the summary result materially (RR ranged between 0·63 and 0·72). A significant inverse association persisted among high-quality studies (RR=0·73; 95 % CI 0·58, 0·87; I 2=36·7 %, n studies 5), but not among studies that controlled for main confounders (RR=0·67; 95 % CI 0·33, 1·01; I 2=65·0 %, n studies 3). The subgroup analyses suggested follow-up duration, baseline age and adjustment for main confounders as the potential sources of the heterogeneity (Supplemental Table 5).
A 0·10µmol/l increment in circulating β-carotene concentration was associated with a 5 % lower risk (RR=0·95; 95 % CI 0·91, 0·99; I 2=55·4 %, P heterogeneity=0·06; Fig. 6(a)). In the sensitivity analysis by sequential exclusion of each study, none of the excluded studies negated the significance. There was evidence of a non-linear association between circulatory β-carotene concentration and risk of CVD mortality (P non=linearity=0·007; Fig. 6(b)).
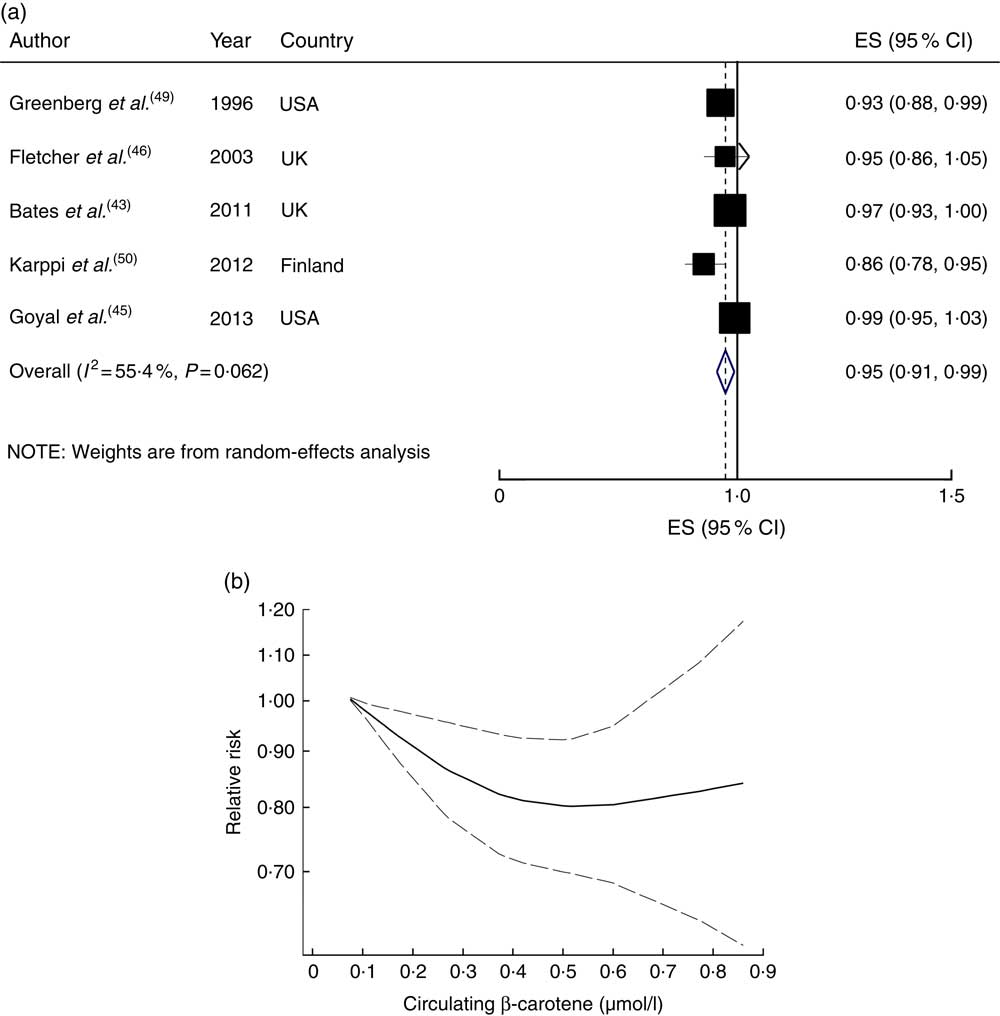
Fig. 6 (a) Forest plot showing relative risk of cardiovascular mortality for a 0·10 µmol/l increment in circulating β-carotene concentration. The study-specific effect size (ES) and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the blue open diamond and the vertical dashed line represent the pooled ES, and the width of the blue open diamond represents the pooled 95 % CI. (b) Dose–response association of circulating β-carotene concentration and risk of total cardiovascular mortality (———), with 95 % CI (— — —)
Quality of meta-evidence
The NutriGrade meta-evidence rating was ‘high’ for dietary vitamin C; ‘moderate’ for circulating vitamin C; and ‘low’ for dietary vitamin E, circulating α-tocopherol, and dietary and circulating β-carotene (Table 2).
Discussion
The present study summarized data about the association of dietary intake and circulating concentration of major antioxidants with the risk of total CVD mortality. The results of the present review indicate that higher dietary/circulating vitamin C and higher circulating β-carotene and α-tocopherol concentrations were significantly and inversely associated with the risk of CVD mortality, whereas higher dietary intake of β-carotene and vitamin E did not show such protective effects. The study suggests that the circulating biomarkers of antioxidants may have better predictive value in relation to the risk of total CVD mortality.
Vitamin C has powerful antioxidant, anti-inflammatory and immune-modulatory properties( Reference Ellulu 60 – Reference Padayatty, Katz and Wang 62 ). Also, by increasing nitric oxide synthesis and release, it is associated with arterial dilation, lower blood pressure and better endothelial function( Reference Ellulu 60 , Reference Padayatty, Katz and Wang 62 ). The non-linear dose–response meta-analysis suggested a possible threshold at an intake of about 200mg/d. This finding is in agreement with some evidence suggesting an intake of about 200 mg/d as the optimal vitamin C intake; the dose at which the plasma is relatively saturated and the blood cells are completely saturated with vitamin C( Reference Frei, Birlouez-Aragon and Lykkesfeldt 63 ). However, only two studies reported risk estimates of CVD mortality for an intake of ≥300mg/d( Reference Martin-Calvo and Martinez-Gonzalez 55 , Reference Sahyoun, Jacques and Russell 56 ). Hence, there was insufficient data for judgement about the cardiovascular outcomes of high vitamin C consumption. A previous meta-analysis of prospective cohort studies suggested a U-shaped association between vitamin C intake and risk of stroke( Reference Chen, Lu and Pang 18 ). Thus, our results regarding the cardiovascular outcomes of vitamin C intake ≥300mg/d should be interpreted cautiously.
The analyses of vitamin C, vitamin E and β-carotene suggested that the circulating biomarkers of antioxidants may be more strongly associated with the risk of CVD mortality compared with dietary intakes. These findings are similar to those of a recent meta-analysis of prospective cohort studies on dietary and circulating carotenoids and the risk of breast cancer( Reference Aune, Chan and Vieira 29 ). However, it should be noted that the circulating biomarkers of antioxidants do not necessarily reflect dietary intakes because several dietary and non-dietary factors such as age, method of storage and food preparation, alcohol consumption, cigarette smoking, physical activity, socio-economic status, co-morbidities and even genetic factors may effectively affect their circulatory concentrations( Reference Lawlor, Smith and Bruckdorfer 28 , Reference Dehghan, Akhtar-Danesh and McMillan 64 – Reference Giovannucci 66 ). In fact, higher circulating concentration of antioxidants may be a consequence of several dietary and non-dietary factors such as higher diet quality, healthier lifestyle-related behaviours, better health status and lower prevalence of co-morbidities; which in turn are associated with better survival.
The analyses of vitamin E demonstrated that higher circulating α-tocopherol concentration, but not higher dietary vitamin E intake, was associated with a lower risk of CVD mortality. The finding of the current review regarding the null association in the analysis of dietary vitamin E is consistent with those of previous investigations which have indicated a non-significant inverse association between higher dietary vitamin E intake and the risk of all-cause mortality and CVD events( Reference Agudo, Cabrera and Amiano 67 – Reference Todd, Woodward and Tunstall-Pedoe 69 ). A pooled analysis of nine relatively large-scale prospective cohort studies involving 4647 incident cases of CHD among ~300 000 participants in the USA and Europe showed similar results, in which only a weak marginally significant inverse association was found between higher dietary vitamin E intake and the risk of incident CHD (RR=0·84; 95 % CI 0·71, 1·00; P=0·17)( Reference Knekt, Ritz and Pereira 22 ). Vitamin E is a fat-soluble antioxidant and anti-inflammatory vitamin, and by inhibition of LDL oxidation has direct anti-atherogenic properties( Reference Badiou, Cristol and Morena 70 – Reference Rahmani, Samimi and Ebrahimi 72 ). Thus, it is reasonable to expect the observational studies to show a significant inverse association between higher vitamin E intake and the risk of heart disease.
Also, such inconsistent findings were observed in the analyses of dietary and circulating β-carotene. β-Carotene has strong antioxidant activity( Reference Paiva and Russell 73 ) and therefore, by decreasing the oxidation of LDL and subsequent risk of developing and progression of atherosclerosis( Reference Dugas, Morel and Harrison 74 – Reference Levy, Zaltsberg and Ben-Amotz 76 ), may be associated with a lower risk of CVD. In addition, β-carotene and other dietary carotenoids, through their functions against oxidative stress, are associated with a lower risk of developing CVD( Reference Willcox, Ash and Catignani 77 ). A possible explanation may be the measurement errors in the self-reported assessment of dietary intake, which might result in a weaker effect size. These errors do not exist in the measurement of circulating biomarkers; therefore, studies that evaluated the circulating biomarkers generally showed stronger inverse associations.
In the present meta-analysis of prospective observational studies, we found significant inverse associations of dietary vitamin C and circulating vitamin C, α-tocopherol and β-carotene concentrations with risk of total CVD mortality. However, our results are inconsistent with those of interventional studies which have indicated null findings in this regard( Reference Al-Khudairy, Flowers and Wheelhouse 24 – Reference Ye, Li and Yuan 26 ). Several explanations have been proposed to explain such inconsistent findings in observational and interventional studies. In general, interventional studies use high-dose supplementations, which may be associated with adverse health effects( Reference Verkaik-Kloosterman, McCann and Hoekstra 78 ). In addition, the follow-up duration of most interventional studies was inadequate to show benefits of antioxidants supplementation( Reference Lawlor, Smith and Bruckdorfer 28 ). Also, trials generally include participants with a prior history of CVD or other chronic diseases and these inconsistent findings may show that antioxidants may be useful for primary prevention of CVD, not for secondary prevention.
In the context of public health implications, the results of the present review support current dietary recommendations that promote an intake of at least five servings of fruits and vegetables per day( 79 ). Fruits and vegetables are the two main dietary sources of antioxidants( Reference Landete 80 , Reference Reboul, Richelle and Perrot 81 ). However, despite all efforts to increase the consumption of fruits and vegetables, current reports indicate that the prevalence of low fruit and vegetable consumption is high in both low- and middle-income and high-income countries. The World Health Survey (2002–2003) in fifty-two mainly low- and middle-income countries reported that the prevalence of low fruit and vegetable consumption, defined as less than a minimum of five servings of fruits and/or vegetables daily, was about 78 %( Reference Hall, Moore and Harper 82 ). This prevalence is similar to that of high-income countries, in which the prevalence of low fruit and vegetable consumption was about 75 and 76 % in the USA( Reference Blanck, Gillespie and Kimmons 83 ) and England( Reference Blake, Chaudhury and Deverill 84 ), respectively. Therefore, increasing the consumption of fruits and vegetables may be good and simple advice in order to reduce the risk of CVD deaths.
The present study has several strengths. We assessed the association of both dietary intakes and circulating concentrations of major antioxidants with the risk of total CVD mortality. Previous reviews have shown a null or weak inverse association between higher vitamin E and β-carotene consumption and the risk of CHD. However, we showed a significant inverse association between circulating α-tocopherol and β-carotene concentrations and risk of CVD mortality, which suggests that the null or weak inverse associations found in the analyses of dietary vitamin E and β-carotene may in part be due to measurement errors.
Some important limitations were experienced in the current study. First, the results were accompanied by some evidence of heterogeneity in the analyses of dietary and circulating vitamin C, dietary vitamin E and dietary β-carotene. However, the subgroup analyses suggested that some of the study and participant characteristics were potential sources of the heterogeneity in the data. Second, publication bias tests were performed only in the analysis of dietary vitamin C (n≥10). Therefore, our results may have been affected by publication bias. Third, higher antioxidant intakes are related to higher diet quality and better compliance with dietary guidelines, which leads to higher intakes of cardioprotective nutrients such as fibres, K, Mg and flavonoids; as well as lower intakes of unhealthy foods. Thus, owing to the inadequate adjustments for these dietary variables in the primary studies, we may have reached a biased conclusion. Finally, we were unable to test the associations across sexes.
Conclusion
In conclusion, the current meta-analysis suggests that a higher dietary intake and a higher circulating concentration of vitamin C are each associated with a lower CVD mortality risk. The analyses of vitamin E and β-carotene showed that higher circulating concentrations of these antioxidants are significantly and inversely associated with a lower CVD mortality risk, whereas higher dietary intakes did not show such protective effects. Considering that the rating of the meta-evidence was low in the analyses of vitamin E and β-carotene, there is a low confidence in effect estimates and future well-designed prospective cohort studies may be required to provide more reliable data for a more confident judgement about the degree and the direction of the associations.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: A.J. and S.S.-B. contributed to the conception and design of the work. A.J., M.S.Z. and M.P. contributed to the acquisition of data for the work. A.J., A.R.P. and S.S.-B. contributed to analysis and interpretation of the data. A.J., A.R.P., M.P., M.S.Z. and S.S.-B. drafted the manuscript. A.R.P. and S.S.-B. critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Ethics of human subject participation: Not applicable.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980018003725