Replacing fishmeal (FM) with various protein sources has been investigated in aquafeeds due to the increasing demand, limited supply and high price of FM(Reference Gatlin, Barrows and Brown1). Soyabean meal (SBM) has been intensively studied as an alternative protein source because of its high protein content, relatively well-balanced amino acid profiles, availability and reasonable cost(Reference Lim, Kim and Ko2). However, the use of SBM in aquafeeds, especially for carnivorous fishes, is still limited because of its plant-origin characteristics, such as poor palatability, low nutrient digestibility, presence of certain anti-nutritional factors and the lack of several essential amino acids(Reference Peres, Lim and Klesius3). Thus, some essential amino acids, attractants and minerals are often supplemented when FM is replaced by SBM, but there are numerous essential nutrients that are often overlooked. For example, the increasing proportion of SBM in feed formulations will reduce the level of dietary cholesterol, which is rich in FM but deficient in SBM(Reference Bureau4, Reference Tocher, Bendiksen and Campbell5). Feeds traditionally formulated with FM will provide at least 1 g cholesterol per kg feed(Reference Tocher, Bendiksen and Campbell5). Thus, the replacement of FM with SBM will greatly reduce the dietary cholesterol level. On the other hand, soya proteins and non-protein compounds present in SBM (such as soya saponins and phytosterol) reportedly lower the blood cholesterol level in fish(Reference Hossain, Focken and Becker6, Reference Deng, Mai and Ai7). Thus, the cholesterol-lowering action has already been reported in many fish species fed diets with SBM instead of FM(Reference Lim, Kim and Ko2, Reference Sagstad, Sanden and Krogdahl8, Reference Kader, Koshio and Ishikawa9). Moreover, the expression of several genes involved in the cholesterol biosynthesis pathway, such as 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGR) and lanosterol 14-α demethylase, is up-regulated in fish fed diets with high SBM levels, which suggests stimulation of the cholesterol biosynthesis pathway(Reference Vilhelmsson, Martin and Médale10, Reference Geay, Ferraresso and Zambonino-Infante11). These findings strongly suggest that replacing FM with high levels of SBM may result in an inadequate supply of cholesterol in fish. Thus, the effects on cholesterol metabolism may be an area requiring consideration in the future as the proportion of SBM in dietary formulations increases(Reference Tocher, Bendiksen and Campbell5).
Cholesterol is a necessary constituent for eukaryotic cell growth and development. The biosynthesis of cholesterol provides crucial building blocks for cell membranogenesis and membrane fluid regulation(Reference Russell12). In addition, cholesterol serves as a precursor of many physiologically active compounds, such as sex hormones, adrenal corticoids, bile acids and vitamin D. Thus, cholesterol plays an important role in maintaining the physiological functions of fish. Previous studies have shown that lower blood cholesterol levels are associated with lower growth performance in fish fed diets containing SBM instead of FM(Reference Sagstad, Sanden and Krogdahl8, Reference Hansen, Karlsen and Rosenlund13) and higher mortality rates due to bacterial infections and occurrences of green liver(Reference Maita, Satoh and Fukuda14, Reference Maita, Maekawa and Satoh15), suggesting the possibility that inadequate cholesterol might be limiting normal growth of fish fed SBM-based diets(Reference Schoknecht, Ebner and Pond16). Cholesterol metabolism is affected by de novo cholesterol synthesis in the liver (endogenous cholesterol) and dietary cholesterol contents (exogenous cholesterol)(Reference Maita, Maekawa and Satoh15). Thus, we deem that dietary cholesterol supplementation may improve the growth performance of fish fed soya product-based diets, which has been demonstrated by previous studies(Reference Twibell and Wilson17–Reference Yun, Ai and Mai20). However, there is no report on the potential mechanism of the growth promotion of dietary cholesterol so far. The objective of the present study was to determine the effect of increasing levels of cholesterol supplementation on the growth and cholesterol metabolism of rainbow trout (Oncorhynchus mykiss) fed SBM-based diets, and thereby to investigate the preliminary mechanism of growth-promoting effects of dietary cholesterol.
Materials and methods
Experimental diets
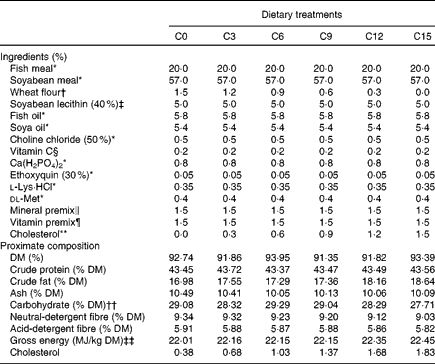
A total of six isonitrogenous (crude protein 43 %) and isoenergetic (gross energy 21 kJ/g) practical diets were formulated to contain increasing levels of cholesterol. A basal diet (C0) was formulated using a combination of FM (accounting for approximately 30 % of the dietary protein) and SBM (about 70 %) as the primary protein sources. The other five diets (C3, C6, C9, C12 and C15) were supplemented with 0·3, 0·6, 0·9, 1·2 and 1·5 % cholesterol (purity ≥ 99 %; Shanghai Shunbo Biotech Company, Limited) in place of wheat flour in the basal diet. The ingredients and the chemical composition of the diets are presented in Table 1. The actual cholesterol content in the C0, C3, C6, C9, C12 and C15 diets were 0·38, 0·68, 1·03, 1·37, 1·68 and 1·83 %, respectively.
Table 1 Ingredients and proximate composition of the experimental diets for rainbow trout (Oncorhynchus mykiss)

C0, basal diet; C3, 0·3 % cholesterol; C6, 0·6 % cholesterol; C9, 0·9 % cholesterol; C12, 1·2 % cholesterol; C15, 1·5 % cholesterol.
* Supplied by Kunming Tianyuan Feed Company, Limited; fishmeal, 67·0 % crude protein, 11·5 % crude lipid; soyabean meal, 49·3 % crude protein, 2·8 % crude lipid.
† Supplied by Zhaoqing Four Gardener Flour Company, Limited, 12·9 % crude protein, 2·8 % crude lipid.
‡ Supplied by Shanghai Hanhong Chemical Company, Limited.
§ l-Ascorbate-2-polyphosphate (35 %), supplied by Galaxy Chemicals Company, Limited.
∥ Mineral premix (g/kg of mixture): MgSO4·7H2O, 180·0; KI, 1·0; FeSO4·H2O, 260; ZnSO4·H2O, 180; CuSO4·5H2O, 25; Na2Se2O3, 0·01; MnSO4·H2O, 180; CoCl2·6H2O, 0·75.
¶ Vitamin premix (g/kg of mixture): retinyl acetate, 2; cholecalciferol, 0·03; dl-α-tocopheryl acetate, 30; menadione, 3; thiamine hydrochloride, 8; riboflavin, 11; pyridoxine hydrochloride, 8; vitamin B12, 0·02; ascorbic acid, 50; folic acid, 1; biotin 0·1; niacin, 30; calcium d-pantothenate, 32; inositol, 25.
** Supplied by Shanghai Shunbo Biotech Company, Limited.
†† Carbohydrate = 100 − (crude protein+crude fat+ash).
‡‡ Gross energy was calculated using the factors 39·5 kJ/g for fat, 23·7 kJ/g for protein and 17·2 kJ/g for carbohydrate.
Experimental ingredients were ground into fine powder by passing through a 320-μm mesh. Cholesterol was pre-blended into fish oil. All the ingredients were thoroughly mixed with soyabean oil and fish oil, and water was added to produce a stiff dough, which was then extruded by a pellet feed maker (KS-180, Jiangsu Jingu Rice Mill Company, Limited) through a 3 mm die. The moist pellets were dried in a forced air oven at room temperature for about 12 h and then stored at − 20°C until use.
Experimental animals and conditions
The present study was conducted according to the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of the People's Republic of China on 31 October 1988. Rainbow trout (O. mykiss) were transported from a local commercial farm (Fenghong Fisheries Company, Limited) to the aquaculture laboratory at Yunnan Agricultural University (Kunming, China). Prior to the start of the experiment, the fish were acclimatised to the experimental tanks and fed a commercial diet (TR-2242, Salmofood S.A.) for 2 weeks. A total of 450 fish with an initial mean weight of 57·81 g were randomly distributed in eighteen tanks with twenty-five juveniles per tank (triplicate groups per dietary treatment). The rectangular fibreglass aquaria (1·0 m × 0·7 m × 0·8 m) were supplied with continuous water flow and aeration. The water was recirculated through a 4000 litre biological and mechanical filtration system containing a vertical quartz sand filter and an activated carbon purifier to remove solid and nitrogenous wastes. During the experimental period, the temperature ranged from 14 to 18°C, dissolved oxygen concentrations were 7·3–8·0 mg/l and pH was maintained at 6·7–6·9. The fish were subjected to a natural photoperiod and were hand fed to apparent satiation two times per d (08.00 and 16.00 hours) with one of the six experimental diets over a period of 9 weeks. Any uneaten feed was collected 1 h after every feeding, and the DM content was determined for both supplied and uneaten diets(Reference Deng, Zhang and Bi21). Leaching loss in the uneaten diets was estimated by leaving four samples of each diet in tanks without fish for 1 h, followed by recovering, drying and reweighing them. Feed consumption was recorded by subtracting the amount of uneaten diet from total amount of diet fed on a DM basis. Fish were not pair-fed in the present study, as intakes were not expected to be different among the dietary treatments. Previous studies reported that dietary cholesterol content did not have any impact on feed intake using a two-choice preference test(Reference Ueda and Shigemizu22, Reference Ueda and Suehiro23).
Sampling
At the end of the feeding trial, the fish were fasted for 24 h before harvesting. All experimental fish were anaesthetised with eugenol (1:12 000) (Shanghai Chemical Reagent Company, Limited) before sampling. The total number of fish was counted and the mean body weight of fish in each tank was measured to calculate growth performance and feed utilisation. A total of seven fish per tank at termination were randomly collected and stored frozen ( − 20°C) for proximate composition analysis. Another seven fish per tank were sampled to analyse the plasma lipoprotein levels. Blood was collected from the caudal vein using a heparinised syringe and transferred into a heparinised tube. Plasma was recovered after centrifugation (4000 g, 10 min) at 4°C and immediately stored at − 80°C until analysis. The intestines were removed from the abdominal cavity, and the contents were squeezed out and mixed for lipid analysis. Liver and muscle samples were frozen in liquid N2 and then stored at − 80°C for subsequent determination. All samples were pooled by tank for analysis.
Analyses
Chemical analyses
Analysis of DM (105°C, 24 h), crude protein (Kjeldahl N × 6·25), crude lipid (diethyl ether extraction via the Soxhlet method) and ash (550°C, 18 h) in feed ingredients, experimental diets and whole-body samples was performed using standard laboratory procedures(24). Carbohydrate was estimated as the weight difference using crude protein, crude lipid and ash content data. Neutral-detergent fibre and acid-detergent fibre were determined using the Association of Official Analytical Chemists official method(24). Gross energy content was calculated using 23·7 kJ/g of protein, 39·5 kJ/g of lipid and 17·2 kJ/g of carbohydrate(Reference Brett, Groves, Hoar, Randall and Brett25).
Cholesterol and total bile acids analyses
The total cholesterol (TC), HDL-cholesterol (HDL-C) and TAG levels in plasma were determined without extraction using commercial enzymatic kits (Shanghai Jiemen Bio-Tech Company). Plasma LDL-cholesterol (LDL-C) level was calculated using the method described by Friedewald et al. (Reference Friedewald, Levy and Fredrickson26). The TC content in the diets, liver, muscle and intestinal contents, and the TAG content in the liver and muscle were determined using the same enzymatic kits used for plasma, except these samples were extracted with chloroform–methanol (2:1, v/v), evaporated under N2 and then resuspended in chloroform according to the method of Folch et al. (Reference Folch, Lees and Sloane Stanley27). The total lipid content in fish liver and muscles were determined gravimetrically(Reference Folch, Lees and Sloane Stanley27). The free cholesterol (FC) and total bile acid (TBA) levels in the intestinal contents were measured using commercial ELISA kits for fish (R&D Systems, Inc.), according to the manufacturer's protocols. The cholesteryl ester (CE) level in the intestinal contents was calculated by subtracting the FC value from the TC value.
Plasma triiodothyronine, thyroxine, cortisol, calcium and phosphorus contents
Plasma thyroid hormone (thyroxine and triiodothyronine (T3)) levels were measured using RIA, as described by Martinez et al. (Reference Martinez, Dreyer and Argersborg28). Plasma cortisol was measured via RIA, as described by Metz et al. (Reference Metz, Geven and van den Burg29). Plasma Ca and P levels were determined using commercial kits (Nanjing Jiancheng Biological Engineering Research Institute).
Hepatic acyl-CoA:cholesterol acyl transferase activity, 3-hydroxy-3-methyl-glutaryl-CoA reductase and cholesterol 7α-hydroxylase activities
Microsomes were prepared according to the method described by Rasmussen et al. (Reference Rasmussen, Ekstrand and Zamaratskaia30) with a slight modification. Briefly, 1 g of liver tissue was homogenised in 4 ml of ice-cold Tris–HCl buffer (0·1 m-Tris–HCl with 0·5 m-KCl, 1 mm-EDTA and 0·1 mm-phenylmethanesulfonyl fluoride; pH 7·4). The homogenates were centrifuged twice at 10 000 g and at 12 000 g for 10 min at 4°C. The supernatant liquids were then centrifuged twice in an ultracentrifuge at 100 000 g for 60 min at 4°C. The resulting microsomal pellets were then redissolved in 1 ml of homogenation buffer to analyse the protein content, as well as acyl-CoA:cholesterol acyl transferase (ACAT), HMGR and cholesterol 7α-hydroxylase (CYP7A1) activities. The ACAT, HMGR and CYP7A1 activities were measured using commercial ELISA kits for fish (R&D Systems, Inc.), according to the manufacturer's protocols. The protein content in the microsomes was assayed according to the method described by Bradford(Reference Bradford31).
Calculations
The following formulae were applied to the data:
where W i and W f are the initial and final fish mean weights (g), d is feeding days and DI is the dry diet intake per fish (g DM/fish) during the experimental period.
Statistical analyses
Percentage data were subjected to arcsine transformation before statistical analysis. All data were analysed using one-way ANOVA, followed by a Tukey's multiple comparisons test. The level of significance was set to P< 0·05. Statistical analysis was performed using SPSS 16.0 for Windows (SPSS, Inc.).
Results
Survival and growth
Survival rate of fish ranged from 88·9 to 100 % and was non-significantly different among the dietary treatments (Table 2). Similarly, no significant differences in the first week feed intake (g/fish) and total feed intake (g/kg MBW per d) were observed among the dietary treatments. The DGC increased steadily, as the levels of supplemental cholesterol were increased by up to 1·2 % and then declined with further addition. Fish fed the C12 diet showed significantly higher feed efficiency compared with those fed the other diets. The final body compositions of fish were non-significantly affected by dietary cholesterol levels (Table 3).
Table 2 Feed intake, growth and feed efficiency of rainbow trout (Oncorhynchus mykiss) during the feeding period (Mean values with their standard errors, n 3)

C0, basal diet; C3, 0·3 % cholesterol; C6, 0·6 % cholesterol; C9, 0·9 % cholesterol; C12, 1·2 % cholesterol; C15, 1·5 % cholesterol; DGC, daily growth coefficient; MBW, metabolic body weight.
a,b,c,dMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Table 3 Proximate composition (g/kg wet weight) of the whole body of rainbow trout (Oncorhynchus mykiss) fed with diets containing different cholesterol levels (Mean values with their standard errors, n 3)

C0, basal diet; C3, 0·3 % cholesterol; C6, 0·6 % cholesterol; C9, 0·9 % cholesterol; C12, 1·2 % cholesterol; C15, 1·5 % cholesterol.
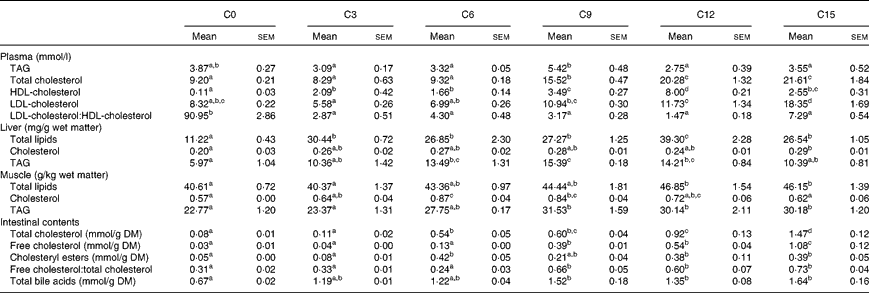
Cholesterol levels in plasma, liver, muscle and intestinal contents
Dietary cholesterol content significantly affected plasma lipoprotein levels (Table 4). Fish fed the C9 diet showed a significantly higher plasma TAG level compared with those fed the C3, C6, C12 and C15 diets. The plasma TC level in fish fed the C9 diet was significantly higher than that in fish fed the C0, C3 and C6 diets, but lower than that in fish fed the C12 and C15 diets. The plasma HDL-C level was highest in fish fed the C12 diet and lowest in fish fed the C0 diet. However, the highest plasma LDL-C level was observed in fish fed the C15 diet. The plasma LDL-C:HDL-C ratio was significantly decreased by dietary cholesterol supplementation, regardless of inclusion level.
Table 4 Lipid profiles in plasma, liver, muscle and intestinal contents in rainbow trout (Oncorhynchus mykiss) fed diets with different cholesterol levels (Mean values with their standard errors, n 3)

C0, basal diet; C3, 0·3 % cholesterol; C6, 0·6 % cholesterol; C9, 0·9 % cholesterol; C12, 1·2 % cholesterol; C15, 1·5 % cholesterol.
a,b,c,dMean values within a row with unlike superscript letters are significantly different (P< 0·05).
Dietary cholesterol supplementation significantly increased hepatic total lipid content, regardless of inclusion level, and the highest value was observed in fish fed the C12 diet (Table 4). Fish fed the C15 diet showed a significantly higher hepatic cholesterol content compared with those fed the C0 diet. The hepatic TAG level increased steadily when the supplemental cholesterol was increased by up to 0·9 % and then declined with further addition.
Fish fed the C12 and C15 diets showed significantly higher muscle total lipid content compared with those fed the C0 and C3 diets (Table 4). However, muscle cholesterol content was significantly higher in fish fed the C6 and C9 diets compared with those fed the C0 and C15 diets. Muscle TAG content increased steadily when the supplemental cholesterol was increased by up to 0·9 % and then reached a plateau.
Dietary cholesterol supplementation generally increased TC, FC, CE and TBA levels in the intestinal contents (Table 4). FC and TC levels in the intestinal contents increased steadily with increasing supplemental cholesterol. The CE level in the intestinal contents was significantly higher in fish fed the C6, C12 and C15 diets compared with those fed the C0 and C3 diets. Similarly, the FC:TC ratio in the intestinal contents were significantly higher in fish fed the high-cholesterol diets (C9, C12 and C15) compared with those fed the low-cholesterol diets (C0, C3 and C6). The TBA level in the intestinal contents increased steadily when the supplemental cholesterol was increased by up to 0·9 % and then reached a plateau.
Plasma triiodothyronine, thyroxine, cortisol, calcium and phosphorus levels
Fish fed the C6, C9, C12 and C15 diets showed a significantly higher plasma T3 level compared with those fed the C0 diet (Table 5). The plasma Ca level was significantly higher in fish fed the C15 diet compared with those fed the C0, C3 and C9 diets. However, the plasma P level was significantly higher in fish fed the C0 diet compared with those fed the C3, C6 and C15 diets. No significant differences were observed in the plasma thyroxine and cortisol levels among the dietary treatments.
Table 5 Plasma triiodothyronine, thyroxine, cortisol, calcium and phosphorus contents in the plasma of rainbow trout (Oncorhynchus mykiss) fed diets with different cholesterol levels (Mean values with their standard errors, n 3)

C0, basal diet; C3, 0·3 % cholesterol; C6, 0·6 % cholesterol; C9, 0·9 % cholesterol; C12, 1·2 % cholesterol; C15, 1·5 % cholesterol.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Hepatic acyl-CoA:cholesterol acyl transferase, 3-hydroxy-3-methyl-glutaryl-CoA reductase and cholesterol 7α-hydroxylase activities
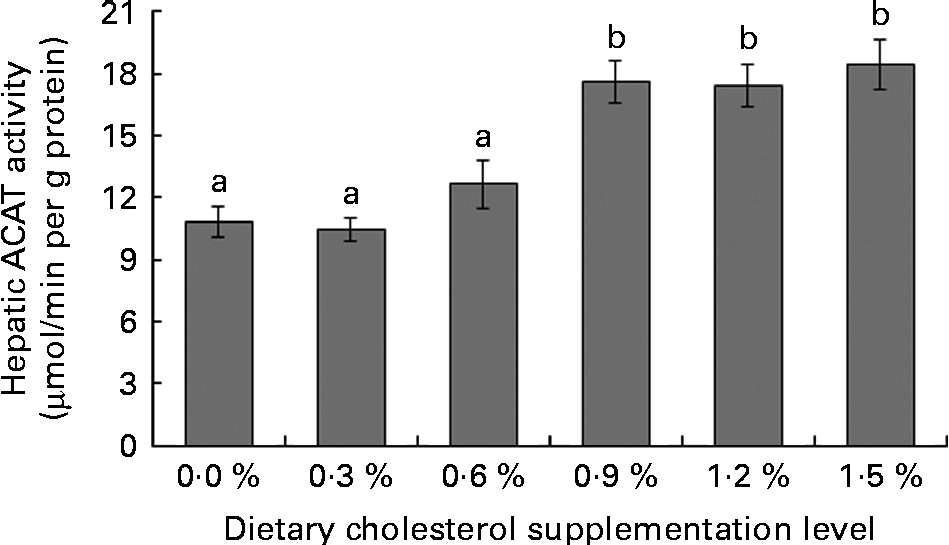
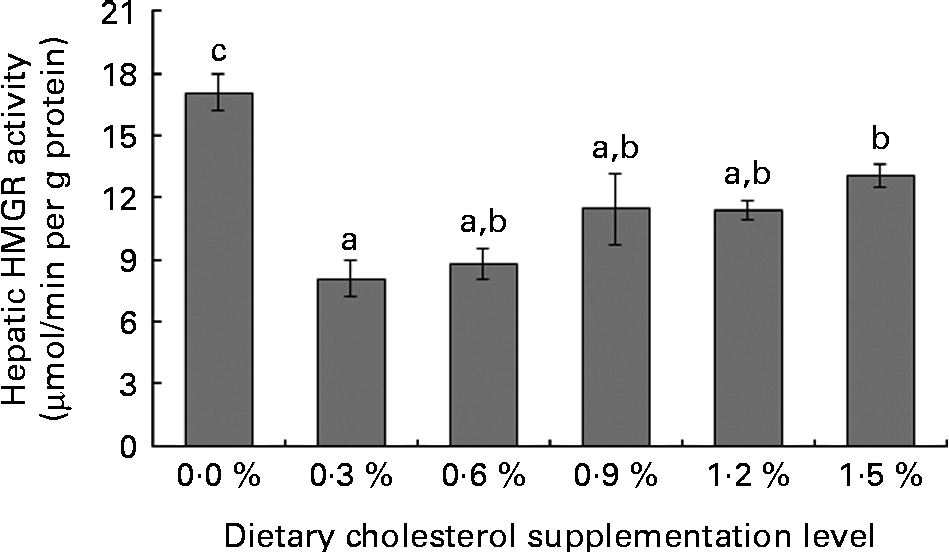
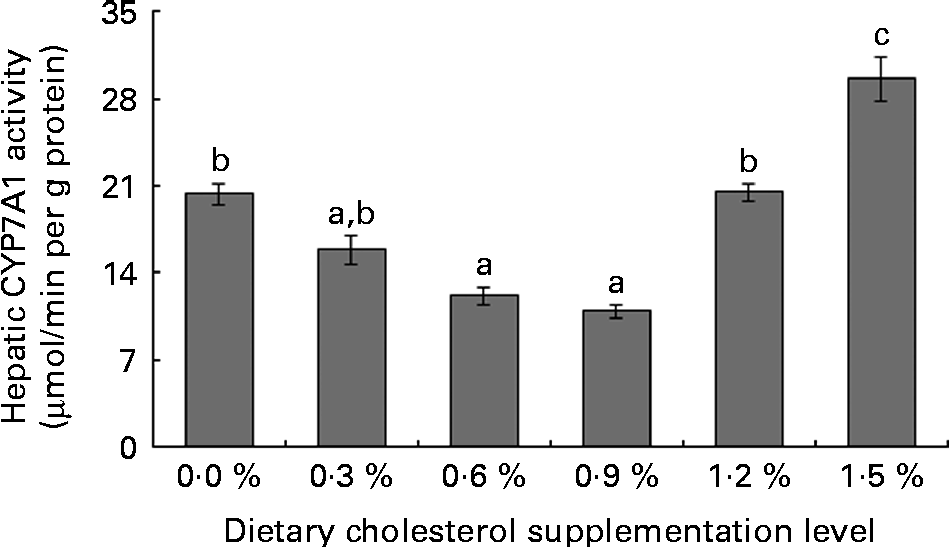
Low levels of dietary cholesterol supplementation (0·3–0·6 %) did not affect hepatic ACAT activity, but high levels of dietary cholesterol supplementation (0·9–1·5 %) significantly increased hepatic ACAT activity (Fig. 1). Dietary cholesterol supplementation significantly decreased the hepatic HMGR activity, regardless of inclusion level, and the decrease was most significant at 0·3 % supplemental cholesterol (Fig. 2). Similarly, low levels of dietary cholesterol supplementation (0·6–0·9 %) significantly decreased the hepatic CYP7A1 activity, but a high level of dietary cholesterol supplementation (1·5 %) significantly increased the hepatic CYP7A1 activity (Fig. 3).

Fig. 1 Hepatic acyl-CoA:cholesterol acyl transferase (ACAT) activity in rainbow trout (Oncorhynchus mykiss) fed with diets containing different cholesterol levels. Values are means, with their standard errors represented by vertical bars (n 3). a,bMean values with unlike letters were significantly different (P< 0·05).

Fig. 2 Hepatic 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGR) activity in rainbow trout (Oncorhynchus mykiss) fed with diets containing different cholesterol levels. Values are means, with their standard errors represented by vertical bars (n 3). a,b,cMean values with unlike letters were significantly different (P< 0·05).

Fig. 3 Hepatic cholesterol 7α-hydroxylase (CYP7A1) activity in rainbow trout (Oncorhynchus mykiss) fed with diets containing different cholesterol levels. Values are means, with their standard errors represented by vertical bars (n 3). a,b,cMean values with unlike letters were significantly different (P< 0·05).
Discussion
Growth
Replacing FM with SBM decreases the blood cholesterol level of fish, and the cholesterol-lowering effect of SBM is positively associated with its growth-depressing action(Reference Lim, Kim and Ko2, Reference Sagstad, Sanden and Krogdahl8, Reference Kader, Koshio and Ishikawa9). Cholesterol, a vital component of cell membranes and the precursor of bile acids, steroid and hormones, is essential for diverse cellular functions(Reference Thomas, Shentu, Singh and Ekinci32). Thus, cholesterol supplementation may be required as plant-derived protein sources (such as SBM, cottonseed meal and rapeseed meal) become the main protein source in fish feeds(Reference Bureau4, Reference Tocher, Bendiksen and Campbell5, Reference Yun, Mai and Zhang19). In the present study, dietary cholesterol supplementation (0·6–1·5 %) significantly increased the DGC of rainbow trout fed a SBM-based diet containing 0·38 % cholesterol, and the maximum DGC was observed in fish fed a diet supplemented with 1·2 % cholesterol. Similar results were reported in studies on channel catfish (Ictalurus punctatus)(Reference Twibell and Wilson17), Japanese flounder (Paralichthys olivaceus)(Reference Deng, Mai and Ai18) and turbot (Scophthalmus maximus)(Reference Deng, Mai and Ai18, Reference Yun, Ai and Mai20) fed soya-based diets. However, the growth-promoting effect of dietary cholesterol was not observed in Atlantic salmon (Salmo salar)(Reference Bjerkeng, Storebakken and Wathne33), hybrid striped bass (Morone chrysops× M. saxatilis)(Reference Sealey, Craig and Gatlin34) and Japanese flounder(Reference Deng, Mai and Ai18) fed FM-based diets and in channel catfish(Reference Twibell and Wilson17) fed casein-based diets. These results indicate that dietary cholesterol supplementation may alleviate the negative effects of SBM; the underlying mechanism will be discussed later.
In the present study, fish were not pair-fed, as feed intakes were not expected to be different among the dietary treatments. Previous studies reported that dietary cholesterol level did not make an impact on feed consumption of chickens using a two-choice preference test(Reference Ueda and Shigemizu22, Reference Ueda and Suehiro23) and feed intake (expressed as g/kg MBW per d) of Japanese flounder(Reference Deng, Mai and Ai18). Similar results were also observed in the present study. Dietary cholesterol supplementation did not affect the first week feed intake (g/fish) and total feed intake (g/kg MBW per d), regardless of inclusion level (Table 2), which indicates that dietary cholesterol does not affect the feed palatability, and the apparent increase in feed consumption may be primarily attributed to the large fish size at the later stage. Thus, we deem that the effects on growth rate are primarily due to the diet composition (i.e. the incremental level of cholesterol) other than the feed intake in the present study.
Cholesterol metabolism and homeostasis
The hypocholesterolaemic effect of SBM compared with FM is well documented in fish(Reference Lim, Kim and Ko2, Reference Kader, Koshio and Ishikawa9). At present, information on cholesterol metabolism in fish is limited(Reference Estévez, Delgado and Hortelano35). Blood cholesterol level is the result of dietary cholesterol (exogenous cholesterol) and de novo cholesterol synthesis in the liver (endogenous cholesterol). In the present study, low levels of supplemental cholesterol (0·3–0·6 %) do not affect the plasma TC level, which indicates that SBM partially alleviates the negative effects of supplemental cholesterol on plasma TC. This observation is in accordance with that by Chen(36), who reported that 0·5 % cholesterol supplementation did not affect the plasma TC level in Japanese flounder fed SBM-based diets. Our previous studies also showed that the plasma TC level of Japanese flounder is unaffected by supplementation with 1 % cholesterol in soya protein concentrate-based or soya protein isolate-based diets(Reference Deng, Mai and Ai18). Furthermore, several studies with fish revealed that soya protein concentrate has cholesterol-lowering effects compared with soya protein isolate, and raw SBM compared with purified SBM or soya protein concentrate(Reference Hossain, Focken and Becker6, Reference Deng, Mai and Ai18). Thus, several studies insisted that the hypocholesterolaemic effect of SBM might be related to non-protein compounds, such as the wide range of phytochemicals present in SBM, but absent in FM(Reference Hossain, Focken and Becker37, Reference Afuang, Siddhuraju and Becker38). On the other hand, the present study also shows that high levels of supplemental cholesterol (0·9–1·5 %) significantly increase the plasma TC levels of rainbow trout. Similar results were observed in studies on Japanese flounder(36), yellowtail (Seriola quinqueradiata)(Reference Maita, Maekawa and Satoh15) and turbot(Reference Yun, Mai and Zhang19). The blood TC levels of these fish were increased to levels comparable with fish fed FM-based diets, but the actual cholesterol content was apparently higher in SBM-based diets with supplemental cholesterol (1·07–1·78 %) compared with FM-based diets (0·29–0·63 %). Thus, we suggest that the hypocholesterolaemia in fish induced by replacing FM with high levels of SBM may be related to both insufficient dietary cholesterol and a decrease in endogenous cholesterol.
Cholesterol homeostasis is achieved by regulating cholesterol uptake, cholesterol biosynthesis, cholesterol conversion to bile acids and excretion of bile acids. The absorption of dietary cholesterol from the intestines is an important part of cholesterol homeostasis and represents the first step that allows dietary cholesterol to exert its metabolic effects(Reference Lu, Lee and Patel39). The major form of dietary cholesterol is FC (unesterified form of cholesterol), and CE is not absorbed as is but must be hydrolysed into FC by pancreatic enzymes before uptake(Reference Park, Jeon and Byun40). In the present study, dietary cholesterol supplementation causes a linear increase in the FC and TC levels in the intestinal contents. However, the FC:TC ratio is markedly lower in the fish fed diets containing lower cholesterol levels (C0, C3 and C6) compared with those fed diets containing higher cholesterol levels (C9, C12 and C15). This result indicates that the non-protein compounds present in SBM inhibited cholesterol absorption by interfering with the hydrolysis of CE in the intestines. Previous studies involving fish also showed that non-protein compounds (such as saponins, fibre and NSP) with particularly defined structural characteristics form insoluble complexes with cholesterol, thereby decreasing the absorption of both endogenous and exogenous cholesterol(Reference Krogdahl, Hemre and Mommsen41–Reference Kumar, Makkar and Becker43). After absorption, FC and fatty acids are re-esterified in enterocytes by ACAT(Reference Lu, Lee and Patel39). High levels of cholesterol supplementation significantly improved the hepatic microsomal ACAT activity in the present study, which is consistent with in vivo studies on chicks(Reference Garcia-Gonzalez, Segovia and Alejandre44), pigs(Reference Sun, Fernandez and Lin45) and rats(Reference Wang, Zhang and Xue46). The in vitro addition of cholesterol to microsomal fractions significantly increases ACAT activity(Reference Erickson, Shrewsbury and Brooks47, Reference Mitropoulos, Venkatesan and Synouri-Vrettakou48). These results indicate that non-protein compounds present in SBM may interfere with the intestinal absorption of cholesterol, while redundant cholesterol from exogenous supplementation (i.e. unbound cholesterol in the intestinal contents) may be absorbed if available.
Cholesterol biosynthesis is controlled by a feedback mechanism with HMGR as the key enzyme. HMGR, an endoplasmic reticulum-bound enzyme, catalyses the rate-limiting step in cholesterol synthesis(Reference Bucher, Overath and Lynen49). Although HMGR is found in all tissues, it is highly expressed in the liver(Reference Ness and Chambers50), wherein the cholesterol feedback regulation of HMGR mainly occurs(Reference Andersen, Turley and Dietschy51). Thus, the feedback regulation of hepatic HMGR activity or mRNA level contributes to the overall cholesterol homeostasis(Reference Ness and Chambers50, Reference Chambers and Ness52). The present study shows that low levels of supplemental cholesterol (0·3–0·6 %) markedly decreases hepatic HMGR activity, but does not affect the hepatic and plasma cholesterol contents. This result indicates that the decreased hepatic HMGR activity in response to the increased cholesterol that reaches the liver via chylomicron remnants provides an effective means of maintaining desirable cholesterol levels(Reference Ness and Chambers50). However, the hepatic HMGR activity is not further decreased by higher levels of supplemental cholesterol (0·9–1·5 %), thereby increasing plasma cholesterol levels. These results indicate that rainbow trout has a certain ‘cholesterol-buffering capacity’, which protects the body against increased serum cholesterol levels caused by dietary cholesterol. The ‘cholesterol-buffering capacity’ implies the possibility of the inhibition of exogenous cholesterol absorption and/or inadequate endogenous production of cholesterol in rainbow trout fed SBM-based diets.
Cholesterol metabolism into bile acids in the liver represents the major route for cholesterol clearance(Reference Turley, Spady and Dietschy53). Bile acids synthesised in the liver are immediately secreted into the bile, reabsorbed in the intestines and transported back to the liver. CYP7A1 is the first and the rate-limiting enzyme of the classical neutral pathway for bile acid synthesis(54), and its activity is regulated by the flux of cholesterol through the liver and the return of bile acids via the enterohepatic circulation(Reference Jelinek, Andersson and Slaughter55). The present study shows that low levels of supplemental cholesterol (0·3–0·9 %) generally increase bile acid concentration in the intestinal contents, but decrease hepatic CYP7A1 activity. This result indicates that low levels of supplemental cholesterol inhibit cholesterol catabolism. However, hepatic CYP7A1 activity was markedly increased by high levels of cholesterol supplementation (1·5 %), which is consistent with studies involving rats(Reference Wang, Zhang and Xue46). These results indicate that high levels of dietary cholesterol promote the cholesterol catabolic pathway(Reference Wang, Zhang and Xue46, Reference Jelinek, Andersson and Slaughter55). Unfortunately, the faecal rate of cholesterol excretion was not determined because faecal samples were not collected.
Similar to that in mammals, fish lipoproteins are classified as VLDL, intermediate-density lipoprotein, LDL and HDL(Reference Chapman56). LDL carries cholesterol from the liver to the peripheral tissues, whereas HDL carries cholesterol from the peripheral tissues to the liver. Thus, the LDL-C:HDL-C ratio could be used as a marker of cholesterol transport(Reference Deng, Mai and Ai18, Reference Yun, Mai and Zhang19). In the present study, dietary cholesterol supplementation markedly depressed the plasma LDL-C:HDL-C ratio in fish fed SBM-based diets, regardless of inclusion levels. Similarly, previous studies also showed that dietary cholesterol supplementation (1·0–1·5 %) decreased the plasma LDL-C:HDL-C ratio in fish fed soya protein-based diets(Reference Deng, Mai and Ai18–Reference Yun, Ai and Mai20). These results indicate that supplemental cholesterol may increase the ability of carrying cholesterol from peripheral tissues to the liver in fish fed soya product-based diets. By contrast, dietary cholesterol supplementation markedly increases the plasma LDL-C:HDL-C ratio in fish fed FM-based diets(Reference Bjerkeng, Storebakken and Wathne33). These results indicate that dietary cholesterol and protein sources affect the distribution of cholesterol among plasma lipoproteins(Reference Deng, Mai and Ai18).
Thyroid hormones and their activities are deemed as good biological markers of growth capacity in fish(Reference Higgs, Fagerlund and Eales57). A low plasma concentration of T3, the biologically active hormone, is indicative of metabolic disruption expressed by poor growth(Reference Sumpter58). In the present study, dietary cholesterol content markedly influenced the plasma T3 level, and a good correlation (r 0·670, P< 0·01) between plasma T3 level and DGC in fish was observed. Similarly, many studies have shown a close relationship between growth rate and plasma T3 level in fish(Reference Regost, Arzel and Kaushik59, Reference Glencross, Evans and Rutherford60). Cortisol, a potent gluco- and mineralocorticoid in teleosts, is involved in the regulation of metabolism and growth in fish(Reference Reddy and Leatherland61). Plasma cortisol, the most commonly measured indicator of stress, has a negative-feedback effect on growth of fish(Reference Fevolden, Røed and Fjalestad62). The present study shows that there is a negative relationship (r= –0·554, P< 0·05) between plasma cortisol level and DGC of fish. Vitamin D, the chief nutrient and hormone regulator of body Ca and P metabolism, originates from 7-dehydrocholesterol of vertebrates. Thus, cholesterol is necessary for Ca and P metabolism(Reference Zhou, Jimi and Smith63). In the present study, dietary cholesterol levels significantly affect the plasma Ca level (r 0·743, P< 0·01), and serum cholesterol is positively related to serum Ca levels (r 0·627, P< 0·01). Similar results have been reported in fish(Reference Newcomb64), rats(Reference Hirai, Hasegawa and Takezoe65) and chickens(Reference Sloan, Harms and Russeld66). These results indicate that one of the hypercholesterolaemic actions of dietary cholesterol is to induce an increase in plasma Ca concentrations(Reference Zhou, Jimi and Smith63, Reference Hirai, Hasegawa and Takezoe65), which may be related to (1) the alteration of the membrane fluidity, (2) the increase in surface area resulting from the insertion of cholesterol into the membrane and (3) the interaction between cholesterol and the proteins of the Ca2+ channel and of the Ca2+ pump(Reference Zhou, Jimi and Smith63).
Blood cholesterol is generally expected to increase in response to dietary cholesterol(Reference Schoknecht, Ebner and Pond16). However, the plasma TC level was not different among fish fed the C0, C3 and C6 diets in the present study. The lack of response may have occurred because the animals do not absorb the available dietary cholesterol, catabolise or excrete the absorbed exogenous cholesterol or clear the absorbed exogenous cholesterol from the circulation by depositing it in cellular membranes and tissues associated with the increased body tissue accretion(Reference Schoknecht, Ebner and Pond16). Cholesterol is found to interact with saponins to form insoluble sterol–saponin compounds in the intestine, which no longer possess toxic and haemolytic properties(Reference Ueda and Shigemizu22, Reference Ueda, Matsumoto and Tanoue67), and to reduce the phytosterol (e.g. β-sitosterol, stigmasterol and campesterol) concentration in the intestinal mixed micelles via a dynamic competition mechanism, resulting in increased excretion of phytosterol(Reference Mel'nikov, Seijen ten Hoorn and Eijkelenboom68). In addition, our previous study showed that cholesterol might interact in vitro with tannins, pectin and β-glucan to form insoluble compounds (J Deng, X Long and X Zhang, unpublished results). Thus, cholesterol supplementation has been found to alleviate the negative effects of dietary soya saponins and soya isoflavones(Reference Ueda and Shigemizu22, Reference Ueda, Matsumoto and Tanoue67). These results imply that dietary cholesterol supplementation could partially alleviate the deleterious effects of non-protein components present in soya products, but the exact mechanism by which this occurs needs further elucidation.
The increased dietary cholesterol level (from 0·3 to 0·9 %) appears to have compensated for the impaired hepatic CYP7A1 activity, as the TBA level in the intestinal contents continues to increase with increasing supplemental cholesterol level up to 0·9 %. The increased TBA level in the intestinal contents will contribute to improvement in lipase activity and lipid digestibility(36), and thereby promote the total lipid depositions in the muscle and liver of rainbow trout(Reference Deng, Mai and Ai18, Reference Yun, Mai and Zhang19). The elevated muscle and hepatic total lipid depositions indicate increased energy reserves that could potentially result in improved growth performance of rainbow trout fed SBM-based diets. In addition, the growth-promoting effect of dietary cholesterol is associated with the cholesterol-stimulating action when the supplemental cholesterol is increased by up to 0·9 %, which suggests the possibility that inadequate endogenously produced cholesterol might be limiting normal growth of rainbow trout fed SBM-based diets(Reference Schoknecht, Ebner and Pond16). Thus, the growth-promoting action of dietary cholesterol seems to compensate partially for the inadequate endogenously produced cholesterol activity(Reference Schoknecht, Ebner and Pond16). However, in agreement with previous studies with fish(Reference Yun, Mai and Zhang19, 36), the present study also shows that DGC is significantly lower in fish fed the C15 diet compared with fish fed the C12 diet, which indicates that excessive cholesterol inclusion may retard the growth of rainbow trout. Previous studies have demonstrated that cholesterol-rich diets induce the endothelial dysfunction and coronary atherosclerosis in salmon(Reference Farrell, Saunders and Freeman69). Thus, the impaired growth of rainbow trout fed the C15 diet is probably due to the dysfunction of cholesterol metabolism, but the exact mechanisms need further investigation.
In summary, data from the present study suggest the possibility of inadequate endogenous production of cholesterol in rainbow trout fed SBM-based diets. The growth-promoting effects of dietary cholesterol supplementation (0·6–1·2 %) may be related to alleviation of the negative effects of SBM and/or make up for the deficiency of cholesterol in rainbow trout. However, the underlying mechanism remains unclear and needs to be determined in future studies.
Acknowledgements
The present research was financially supported by the National Natural Science Foundation of China (grant no. 30960298, 31160533 and 31260639). The authors thank Xiaowen Long for help in feeding and sampling. The contribution of the authors to the present paper is as follows: X. Z. developed the principal design of the study. J. D. contributed to the more detailed planning and was the main author during the preparation of the manuscript. B. B., Q. W. and L. K. performed the practical part of the study and participated in the data analyses. B. K. provided inputs on the trial design and methodology. All the authors had inputs during the writing of the manuscript. The authors declare no conflicts of interest.