Introduction
The prevalence of noncommunicable diseases are increasing globally. 1 Risk factors for these conditions are passed on from one generation to another. According to the concept Developmental Origin of Health and Disease, the prenatal environment does not only affect fetal growth, but may also influence adult health. Reference Grissom and Reyes2
Common definitions of high birthweight include a birthweight above two standard deviation scores (SDS) or a birthweight above the 90th or 95th percentile. Previous studies have reported associations between a high birthweight and adult obesity as well as metabolic disease, Reference Walsh and McAuliffe3-Reference Schellong, Schulz, Harder and Plagemann6 while others instead indicate beneficial effects of a high birthweight. Reference Baker, Olsen and Sorensen5,Reference Wennerstrom, Simonsen and Melbye7,Reference Andersen, Angquist and Eriksson8 However, few studies on adult health have isolated subjects with a very high birthweight (>3 SDS) from those with a moderately high birthweight (2–3 SDS). Reference Schellong, Schulz, Harder and Plagemann6 This distinction might be of interest since data from our group indicate that subjects with a very high birthweight differ from those with a moderately high birthweight with respect to risk of adult disease. Reference Johnsson, Haglund, Ahlsson and Gustafsson4
Excessive body weight during pregnancy is associated with several complications for both the mother and the offspring. A pregnant woman with overweight (body mass index (BMI) ≥25 kg/m2) or obesity (BMI ≥30 kg/m2) runs an increased risk for preeclampsia, gestational diabetes mellitus (GDM), instrumental delivery, and cesarian section. Reference Cedergren9,Reference Dodd, Grivell, Nguyen, Chan and Robinson10 The offspring of a woman with overweight or obesity has an increased risk for a high birthweight, shoulder dystocia, breathing problems, hypoglycemia, and to be admitted to the neonatal intensive care unit. Reference Vasudevan, Renfrew and McGuire11
GDM strongly affects the fetal environment and increases the risk of having a macrosomic offspring. Reference Sridhar, Ferrara, Ehrlich, Brown and Hedderson12 The prevalence of GDM is increasing, but differs depending on ethnic and socioeconomic background. Reference Ferrara13 In Sweden, GDM is reported in 1%–2% of the pregnancies. Reference Ignell, Claesson, Anderberg and Berntorp14 Gestational diabetes and type 2 diabetes (T2DM) share several risk factors such as increased BMI, increased age, as well as a family history of diabetes. Reference Ben-Haroush, Yogev and Hod15,Reference Bellamy, Casas, Hingorani and Williams16 Furthermore, a positive association between birthweight and later GDM risk has been reported. Reference Lagerros, Cnattingius, Granath, Hanson and Wikstrom17 In addition, a meta-analysis covering many different ethnic populations reveals a sevenfold increased risk of manifest T2DM after GDM, Reference Bellamy, Casas, Hingorani and Williams16 and a Swedish study reports that 25% of females with GDM develop diabetes, mainly T2DM, within 8–14 years after delivery. Reference Wahlberg, Ekman and Nystrom18
As maternal BMI, GDM, T2DM, and offspring birthweight seem closely related, factors influencing the incidence of these conditions may have long lasting effects on public health. This study aimed to investigate how maternal birthweight is related to early pregnancy obesity, GDM, and offspring birthweight. Particularly, we intended to focus on differences between females with a moderately high (2–3 SDS) and those with a very high (>3 SDS) birthweight.
Materials and methods
Data sources
The Swedish Birth Register, founded in 1973, contains data on more than 99% of all births in Sweden. 19 Information is collected prospectively during the pregnancy, beginning with the first antenatal visit. Data are recorded on maternal demographic factors and reproductive history, as well as on complications during pregnancy, delivery, and the neonatal period. All births and deaths are validated yearly against the Register of the Total Population (kept by Statistics Sweden), using the mother’s and the infant’s unique individual identification number.
At the first antenatal visit around 10–12 weeks of gestation (95% occurring prior to 15 weeks of gestation), pregnant women are interviewed about current heath, lifestyle, and family history. Weight is measured and height is self-reported or measured. Blood glucose is measured routinely four to six times during the pregnancy since the early 1980s. Women with a blood glucose >8 mmol/l or with risk factors for GDM are offered a 75-g oral glucose tolerance test (OGTT) in order to diagnose possible GDM. Reference Ignell, Claesson, Anderberg and Berntorp14,Reference Persson, Winkvist and Mogren20 The main diagnostic criteria for GDM are fasting capillary whole blood glucose >6.1 mmol/l, and/or OGTT 2 h blood glucose >9.0 mmol/l. Reference Ignell, Claesson, Anderberg and Berntorp14,Reference Lagerros, Cnattingius, Granath, Hanson and Wikstrom17
Study population
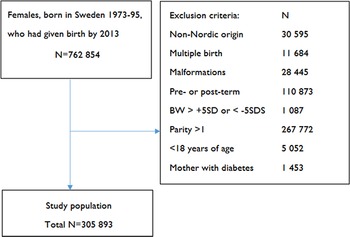
The study cohort (Fig. 1) comprised all females born term and singleton in Sweden 1973–1995 by a mother of Nordic origin. Subjects with heredity for diabetes, that is, those whose mothers had a diabetes diagnosis (identified by the International Classification of Disease (ICD) codes 250 (ICD-8) and 648A+W (ICD-9)), were excluded. Subsequently, all subjects who, at the age of 18 years or older, delivered a first born offspring between 1991 and 2013 were extracted (N = 305,893). Data on the cohort, their mothers (born 1924–1976), and their offspring were collected. The study was approved by the Regional Ethical Review Board in Uppsala (Dnr: 2014/104).

Fig. 1. Description of study cohort and exclusion criteria.
Data analysis
Information on birthweight, gestational age, early pregnancy weight and height, smoking habits, as well as maternal and offspring diagnoses was obtained from the Birth Register. Smoker was defined as smoking at the time of registration in maternity care. Maternal age was defined as the age in years at the time of delivery. Maternal data were linked to offspring data through the individual identification number of each Swedish citizen.
Maternal birthweight represented the exposure. It was transformed into SDS according to Niklasson et al., Reference Niklasson, Ericson and Fryer21 and classified into four groups: small-for-gestational age (SGA), that is, birthweight <−2 SDS, appropriate for gestational age (AGA), that is, birthweight −2 to +2 SDS, moderately large-for-gestational age (LGA 2–3) with a birthweight 2–3 SDS and very large-for-gestational (LGA >3) with a birthweight >3 SDS.
Main outcomes were early pregnancy BMI (25.0–29.9 and ≥30.0), GDM (identified by ICD codes 648W (ICD-9) and O24.4 (ICD-10)), and offspring birthweight (2–3 SDS and ≥3 SDS). In addition, we performed analyses on the relation between maternal birthweight and offspring birthweight classified by sex. BMI was calculated as kg/m2 and categorized as underweight (BMI <18.5), overweight (BMI ≥25.0), and obesity (BMI ≥30.0) according to WHO criteria. 22
During the study period, three versions of ICD were used in Sweden. From study start until 1986, ICD-8 was in use, followed by ICD-9 from 1987 to 1996, and ICD-10 from 1997 and forward. The majority of the subjects (96%) had their first offspring in 1997 or later, that is, after introduction of ICD-10. The ICD-8 and ICD-9 do not define the type of diabetes in pregnancy (coded as 250 (ICD-8) and 648A+W (ICD-9)). The ICD-10 contains the following diabetes diagnoses: GDM (O24.4), type 1 diabetes mellitus (E10 or O24.0), T2DM (E11 or O24.1), and unspecified diabetes (E12–14 or O24.9). Pregnant women with diabetes before or during pregnancy (250 (ICD-8), 648A+W (ICD-9), and E10, E11, and O24.0–9 (ICD-10)) were excluded from the analyses evaluating gestational diabetes and offspring macrosomia. In the analysis evaluating offspring macrosomia, pregnancies with multiple fetuses (1.2%) as well as pre- and post-term births were also excluded.
Statistical analyses were performed in IBM SPSS Statistics 25 (IBM Corp., Armonk, NY, USA) and SAS 9.3 (SAS Institute Inc., Cary, NC, USA). A two-sided P value of <0.05 was considered indicating statistical significance. Multiple logistic regression models were used to evaluate the associations of maternal birthweight with early pregnancy BMI, GDM, and offspring birthweight. Adjustments were made for early pregnancy BMI, maternal age, and smoking status at first antenatal visit. The results were expressed as odds ratios (OR) with 95% confidence intervals (CI).
Results
Mean maternal birthweight was 3 459 ± 465 g. The birthweights were distributed as follows: 2.3% SGA, 95.1% AGA, 2.3% LGA 2–3, and 0.3% LGA >3. Mean age of the subjects at the time of delivery was 26.5 ± 4.3 years. At the first antenatal care visit, 2.8% of the females were underweight, 65.2% normal weight, 22.1% overweight, and 10.0% obese. The mean BMI of the cohort was 24.2 ± 4.4 kg/m2 and the prevalence of GDM was 0.5%.
Maternal birthweight in relation to early pregnancy BMI
As seen in Table 1, a maternal birthweight between 2–3 SDS was associated with 30% greater odds of overweight (OR 1.30, CI 1.23–1.38) and 52% greater odds of obesity (OR 1.52, CI 1.42–1.63) in early pregnancy compared with a normal birthweight. For subjects with a birthweight >3 SDS, the odds were even higher (OR 1.46, CI 1.25–1.72 for overweight, and OR 2.06, CI 1.71–2.49 for obesity).
Table 1. Prevalence and likelihood of overweight and obesity in early pregnancy in relation to subject birthweight

Data are presented as odds ratios (OR) with 95% confidence intervals (CI).
BMI, body mass index; SDS, standard deviation score.
* ref.
a Adjustments were made for maternal age and smoking.
Maternal birthweight in relation to GDM
A low maternal birthweight (<−2 SDS) was associated with an increased likelihood of GDM (OR 2.49, CI 2.00–3.12), compared with a normal birthweight. There was no association between a high birthweight and GDM (Table 2). In addition, there was a positive association between early pregnancy BMI and the likelihood of GDM, which was doubled for overweight subjects (OR 2.13, CI 1.87–2.42), and almost sevenfold increased for obese subjects (OR 6.71, CI 5.97–7.54) (Table 2). Increased maternal age and smoking were also associated with an increased likelihood of GDM (Table 2).
Table 2. Prevalence and likelihood of gestational diabetes (GDM) in relation to maternal birthweight, early pregnancy BMI, maternal age, and smoking

Data are presented as odds ratios (OR) with 95% confidence intervals (CI).
BMI, body mass index; GDM, gestational diabetes mellitus; SDS, standard deviation score.
* ref.
aAdjusted for the other variables listed in the table.
Maternal birthweight in relation to offspring birthweight
Offspring birthweights, in subjects with singleton, term pregnancies and no diagnosis of diabetes in pregnancy, were distributed as follows: 1.6% SGA, 96.3% AGA, 1.9% LGA 2–3, and 0.2% LGA >3 SDS.
A high maternal birthweight was associated with greater odds of a high offspring birthweight (Table 3). Subjects with a birthweight 2–3 SDS had almost a fourfold increased likelihood of an offspring birthweight 2–3 SDS (OR 3.83, CI 3.44–4.26), compared with mothers with a normal birthweight. The odds of having an offspring with a birthweight >3 SDS in this group were 3.55 (CI 2.54–4.97) (Table 3). Corresponding figures for subjects with a birthweight >3 SDS were 5.38 (CI 4.12–7.01) and 6.98 (CI 3.57–13.65), respectively (Table 3).
Table 3. Likelihood of offspring birthweight between 2–3 SDS or >3 SDS, in relation to maternal birthweight SDS, BMI in early pregnancy (kg/m2), age (years), and smoking

Data are presented as odds ratios (OR) with 95% confidence intervals (CI).
BMI, body mass index; BW, birthweight; SDS, standard deviation score.
* ref.
a Adjustments were made for early pregnancy BMI, age, and smoking.
The analyses evaluating maternal birthweight in relation to offspring birthweight classified by sex showed higher odds of LGA among males compared with females (Supplementary Tables 1 and 2).
There was also an association of early pregnancy BMI with offspring birthweight. The risk of having an infant with a birthweight >3 SDS was almost seven times higher for obese subjects (OR 6.78, CI 5.41–8.51), compared with normal weight mothers (Table 3). The effect of age was modest with lowest risk for high offspring birthweight in subjects who were 30–34 years old (Table 3).
Discussion
This large population based register study demonstrated a positive and independent association of maternal birthweight with early pregnancy BMI and offspring birthweight. In addition, subjects with a low birthweight had greater odds of developing GDM.
Our results showed that the mother’s own birthweight was positively associated with early pregnancy BMI, which is in line with previously reported data. Reference Derraik, Ahlsson, Diderholm and Lundgren23 In our cohort, 22.1% of the mothers were overweight in early pregnancy and 10.0% were obese. These figures are lower compared with those of other populations. Reference Huda, Brodie and Sattar24,Reference Mitchell and Shaw25 However, the proportion of overweight and obese women entering pregnancy is escalating in Sweden. Reference Derraik, Ahlsson, Diderholm and Lundgren23 Consequences of rising maternal weights include increasing prevalence of GDM and offspring macrosomia. Reference Catalano and Ehrenberg26
We found that a low maternal birthweight was associated with greater odds of GDM. This is consistent with data of a previous study reporting a twofold increased likelihood of GDM in mothers who themselves had a birthweight <10th percentile compared with those who had a normal or high birthweight. Reference Seghieri, Anichini and De Bellis27 In contrast, others report no correlation between maternal birthweight and later development of GDM. Reference Plante28 However, the latter study evaluated a supposedly healthy cohort since the women were between 24 and 26 years of age.
In our study, the likelihood of GDM was not increased in mothers with high birthweights compared with those with a normal birthweight. This finding is in contrast with previous studies reporting a U-shaped relation between female birthweight and later development of GDM. Reference Lagerros, Cnattingius, Granath, Hanson and Wikstrom17,Reference Pettitt and Jovanovic29 Hence, according to these studies, women born SGA or LGA (>2 SDS) are more prone to develop GDM compared with those with a normal birthweight. Reference Lagerros, Cnattingius, Granath, Hanson and Wikstrom17 These inconsistent results could possibly be explained by differences in cohort characteristics. For example, we only included nulliparous subjects, whilst Lagerros et al. Reference Lagerros, Cnattingius, Granath, Hanson and Wikstrom17 also included parous women. Nulliparous women are younger and have a lower risk of developing GDM compared with women who are older. Reference Solomon, Willett and Carey30 Moreover, parous women are heavier and gain more weight during pregnancy compared with nulliparous women. Reference Sebire, Jolly and Harris31 A high body weight and excessive weight gain in pregnancy are well-known risk factors for the development of GDM. Reference Solomon, Willett and Carey30
In this study, the low GDM prevalence of 0.5% may be partly explained by the exclusion of groups with a higher risk of GDM, for example, parous women, older women, and women with a non-Nordic origin. Reference Solomon, Willett and Carey30 Additionally, some cases of GDM might not be captured by the screening. Reference Ferrara13,Reference Ben-Haroush, Yogev and Hod15,Reference Fadl, Ostlund, Magnuson and Hanson32 Since 1998, GDM prevalence has been steady around 0.9%–1.3%. This should be compared to a prevalence of 2.2% in a Swedish region where OGTT was routinely performed in all pregnant women. Reference Ignell, Claesson, Anderberg and Berntorp14
We found that a high maternal birthweight was positively associated with offspring birthweight after adjustments for early pregnancy BMI. The likelihood was greatest for women who themselves had a very high birthweight (>3 SDS). Moreover, the ORs were higher for males compared with females. Our results are in good agreement with those of a previous study reporting associations of maternal birthweight with offspring birthweight among mothers who themselves had a normal birthweight or macrosomia. Reference Ncube, Gavin and Williams33 Like our findings, the associations differed depending on offspring sex. Our results also demonstrated an association between early pregnancy BMI and offspring birthweight, which has been reported by other investigators. Reference Schellong, Schulz, Harder and Plagemann6,Reference Fadl, Ostlund, Magnuson and Hanson32,Reference Ehrenberg, Mercer and Catalano34-Reference Pirkola, Pouta and Bloigu36 The increasing prevalence of high birthweights is worrying as it is associated with increased risk of adverse perinatal complications, such as asphyxia, shoulder dystocia, and hypoglycemia, as well as later risk of obesity. Reference Walsh and McAuliffe3 A high birthweight also increases the risk of maternal injuries during delivery. Reference Walsh and McAuliffe3
Although being born moderately LGA is associated with adult overweight, some studies indicate advantages of an increased birthweight. Thus, two Danish studies Reference Baker, Olsen and Sorensen5,Reference Wennerstrom, Simonsen and Melbye7 demonstrated decreased overall mortality for individuals born with birthweights above the 90th percentile compared with AGA, Reference Baker, Olsen and Sorensen5 and for birthweights between 3.7 and 4.2 kg compared with both higher and lower birthweights. Reference Wennerstrom, Simonsen and Melbye7 Furthermore, the risk of coronary heart disease has been reported to be lower for individuals with a moderately high birthweight compared with those with birthweights between 3 and 4 kg. Reference Andersen, Angquist and Eriksson8
A major strength of this study was the use of a nationwide register with high coverage, making it possible to analyze birthweight subgroups separately. The register also provides a possibility to study intergenerational effects, related to pregnancy and the perinatal period. Since data are forwarded to the register at the time of delivery, recall bias is minimized. Moreover, the homogeneity of the cohort represents another strength. Hence, the results reflect the situation in a Nordic population.
A weakness of this study was the limited possibility to control for genetic factors, Reference Robitaille and Grant37 due to the incompleteness of grandmother BMI and GDM data. Other limitations include that we did not have access to comprehensive socioeconomic data, or information on maternal weight gain during pregnancy. Yet another possible limitation to this study is that we did not have information on father’s size. Paternal birthweight is associated with offspring birthweight, Reference Mattsson and Rylander38,Reference Derraik, Pasupathy and McCowan39 whereas there is no association between paternal BMI during pregnancy and birthweight of the offspring. Reference Derraik, Pasupathy and McCowan39
In conclusion, our study demonstrated that being born with a high birthweight was associated with an increased likelihood of adult overweight and obesity as well as offspring macrosomia. This was most pronounced in subjects with a very high birthweight (>3 SDS). A low, but not a high, birthweight was associated with an increased likelihood of gestational diabetes.
Supplementary materials
For supplementary material for this article, please visit https://doi.org/10.1017/S2040174421000751.
Acknowledgments
We gratefully acknowledge the support by the Gillbergska Foundation and the Birth Foundation. We are also grateful for the assistance of data management by Patrik Öhagen, Uppsala Clinical Research Center.
Financial support
This study was funded by the Gillbergska Foundation and the Birth Foundation.
Conflicts of interest
The authors declare no competing interests.
Ethical standards
The study was approved by the Regional Ethical Review Board in Uppsala (Dnr: 2014/104), and performed in accordance with relevant national and international guidelines for medical research.