Introduction
Madagascar is one of the most threatened biodiversity hotspots (Brooks et al., Reference Brooks, Mittermeier, da Fonseca, Gerlach, Hoffmann and Lamoreux2006). Most of the island's plant and animal species are endemic as a result of its long isolation from other continents (c. 80 million years; Dewar & Richard, Reference Dewar and Richard2007; Ali & Krause, Reference Ali and Krause2011), sympatric speciation (Buerki et al., Reference Buerki, Devey, Callmander, Phillipson and Forest2013) and relatively recent climate changes (Yoder & Nowak, Reference Yoder and Nowak2006; Buerki et al., Reference Buerki, Devey, Callmander, Phillipson and Forest2013; Mercier & Wilmé, Reference Mercier and Wilmé2013). Levels of endemism are as high as 82% in vascular plants (Callmander et al., Reference Callmander, Phillipson, Schatz, Andriambololonera, Rabarimanarivo and Rakotonirina2011) and 84% in vertebrates (Goodman & Benstead, Reference Goodman and Benstead2005). With ongoing deforestation, Madagascar's unique biodiversity is highly threatened (Brooks et al., Reference Brooks, Mittermeier, da Fonseca, Gerlach, Hoffmann and Lamoreux2006).
Deforestation inevitably causes defaunation. Both processes result in imbalanced, disturbed or even dysfunctional ecosystems. A critical consequence, which has so far received little attention from conservationists, is the breakdown of key plant–animal interactions, such as seed dispersal. The extinction of animal dispersers has catastrophic consequences for the long-term survival of animal-dispersed plant species (Howe & Smallwood, Reference Howe and Smallwood1982; Herrera, Reference Herrera, Herrera and Pellmyr2002; Caughlin et al., Reference Caughlin, Ferguson, Lichstein, Zuidema, Bunyavejchewin and Levey2014). Plant regeneration is limited in the absence of seed dispersers, resulting in an increasing aggregation of individuals and a strongly increasing probability of extinction (Terborgh et al., Reference Terborgh, Nuñez-Iturri, Pitman, Cornejo Valverde, Alvarez and Swamy2008; Caughlin et al., Reference Caughlin, Ferguson, Lichstein, Zuidema, Bunyavejchewin and Levey2014). Long-term conservation can therefore be successful only if gaps in dispersal mutualisms are identified and repaired.
For Madagascar, knowledge about plant species and plant–animal interactions is still scarce, and deforestation and defaunation are progressing rapidly. Traditional methods of studying ecological processes such as seed dispersal (e.g. long-term field observations) are too time-consuming to provide comprehensive information about the breakdown of vital plant–animal interactions. Dispersal syndromes (i.e. sets of fruit or seed traits) provide a practical concept for predicting plant–animal interactions and interdependencies in a community quickly and accurately (van der Pijl, Reference van der Pijl1982; Howe, Reference Howe2016; Kuhlmann & Ribeiro, Reference Kuhlmann and Ribeiro2016). In the absence of comprehensive data to infer dispersal syndromes in Madagascan ecosystems, we used data science tools to impute missing data on relevant plant traits. Using this approach we were able to assign dispersal syndromes to Madagascar's endemic plant species and to infer vital plant–animal relationships and interdependencies.
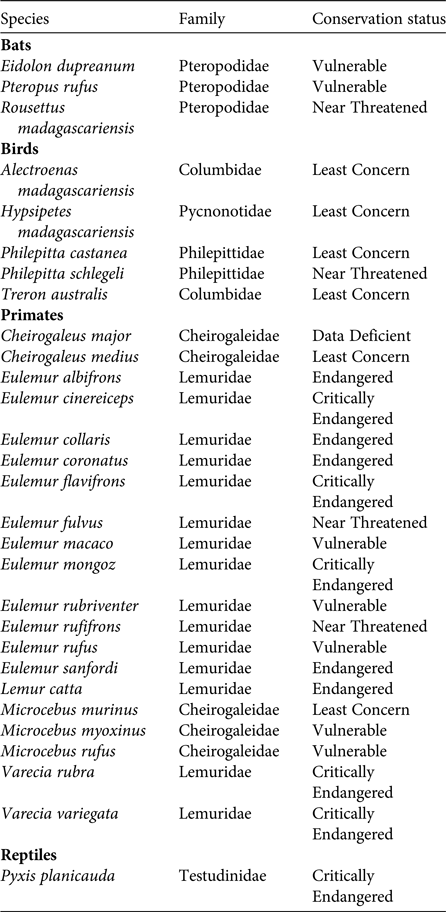
Of the various dispersal strategies plants have evolved, endozoochory is usually the most common in tropical and subtropical rainforests, where at least 50% but more often 80–90% of tree species bear fleshy fruits (Howe & Smallwood, Reference Howe and Smallwood1982; Jordano, Reference Jordano and Fenner2000; Willson & Traveset, Reference Willson, Traveset and Fenner2000; Osuri et al., Reference Osuri, Ratnam, Varma, Alvarez-Loayza, Astaiza and Bradford2016). Diaspores of endozoochorous species generally offer an edible reward to attract animal dispersers (e.g. fruit pulp, elaiosomes or arils). The predominance of a syndrome is not always proportional to the abundance of the corresponding dispersal agent in a community (Hughes et al., Reference Hughes, Dunlop, French, Leishman, Rice, Rodgerson and Westoby1994; Herrera, Reference Herrera, Herrera and Pellmyr2002); however, Madagascar is unique in that its extant frugivorous guild is strikingly small compared to other tropical areas (Goodman & Ganzhorn, Reference Goodman and Ganzhorn1997; Ganzhorn et al., Reference Ganzhorn, Arrigo-Nelson, Boinski, Bollen, Carrai and Derby2009). Only 20 lemur, three bat, five bird and one tortoise species on the island are mainly frugivorous (excluding granivores) (Table 1). Therefore, an analysis of the frequency of the various seed dispersal strategies present in Madagascar's endemic plant species (i.e. seed dispersal spectra; Hughes et al., Reference Hughes, Dunlop, French, Leishman, Rice, Rodgerson and Westoby1994) would be informative. The proportion of frugivorous species in Madagascar has always been small, even before the Holocene extinctions (Godfrey et al., Reference Godfrey, Jungers, Schwartz, Irwin, Fleagle and Gilbert2008). Hypotheses to explain the unusually low number of frugivores include the unpredictable pattern of fruit production on the island (Goodman & Ganzhorn, Reference Goodman and Ganzhorn1997; Wright, Reference Wright1999) and the lower absolute abundance of fruiting trees at any given time compared to other tropical areas (Federman et al., Reference Federman, Sinnott-Armstrong, Baden, Chapman, Daly and Richard2017). The most unusual aspect of Madagascar's disperser guild is that there are only five species of frugivorous birds on the island, whereas in other tropical areas birds are the most important and diverse group of frugivores (Fleming et al., Reference Fleming, Breitwisch and Whitesides1987).
Table 1 Extant frugivores in Madagascar. Only species for which fruits constitute the largest component of their diet during most of the year are included; granivores are excluded. Data from Goodman & Benstead (Reference Goodman and Benstead2003), IUCN (2015) and Mittermeier et al. (Reference Mittermeier, Louis, Richardson, Schwitzer, Langrand and Rylands2010).
Our aim was to answer the following questions: (1) Is the small size of Madagascar's disperser guild reflected in a lower than average proportion of endozoochorous plant species compared to other tropical areas? (2) Is the low proportion of birds among Madagascar's seed dispersers reflected in a lower than average proportion of species with a bird dispersal syndrome compared to other tropical areas? (3) Is there a difference in the frequencies of dispersal syndromes between Madagascar's various bioclimates and vegetation formations? Generally, forests and humid areas harbour a higher proportion of endozoochorous species than open vegetation (e.g. grasslands) and dry areas (Howe & Smallwood, Reference Howe and Smallwood1982; Willson et al., Reference Willson, Irvine and Walsh1989).
Methods
Data collection
Trait data of endemic Madagascan plant species were compiled from the literature, public databases (Madagascar Catalogue, 2013) and examination of herbarium specimens at the Royal Botanic Gardens, Kew (2,519 specimens) and at the Muséum National d'Histoire Naturelle, Paris (364 specimens). Traits recorded included life form (tree, shrub, herb, liana), fruit type (indehiscent, including achene, camara, caryopsis, lomentum, utricle, samara; dehiscent, including capsule, follicle, coccum, legume, follicarium; and externally fleshy, including berry, drupe, epispermatium, syncarpium, syconium; Spjut, Reference Spjut1994), diaspore colour, thickness of fruit epicarp (thin: < 1 mm vs thick: ≥ 1 mm), pulp type (i.e. fleshy, non-fleshy (e.g. dry, spongy, sticky, fibrous, farinose) and aril), fruit length and width, seed length and width, bioclimate (humid, subhumid, subarid, dry, montane; Cornet, Reference Cornet1973) and vegetation formation (forest/woodland, bushland/thicket, shrubland, grassland/wooded grassland, mangrove, anthropic). We coded diaspore colours as follows: black (including dark-bluish and reddish black), blue, purple, brown (including dull dark red), red (including scarlet and pink), green, yellow, orange, grey and white.
Imputation of missing data
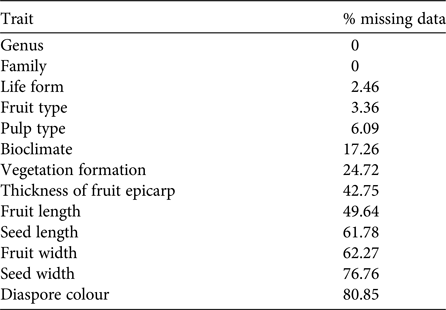
Knowledge about Madagascar's dispersal ecology is still incomplete. Our dataset of plant traits relevant for the identification of dispersal syndromes contained many gaps (29% missing data). Given the random occurrence of missing values across the dataset, a complete case analysis in which all observations with one or more missing values were omitted would include only 281 species (i.e. only 3% of the original 8,788 species). This would lead to non-negligible loss of power, with misleading results and biased estimates of parameters (Ambler et al., Reference Ambler, Omar and Royston2007; Giorgi et al., Reference Giorgi, Belot, Gaudart and Launoy2008). We therefore decided to perform multiple imputations of missing values (Rubin, Reference Rubin1987). The percentage of missing data per trait was 0–80.85% (Table 2), and the percentage of missing data per species was 0–100% (mean = 28.71 ± SD 22.07%). Firstly, we removed the four species (Euphorbia ambatomenahensis, E. beuginii, E. nicaisei and Venana cauliflora) for which we had no data at all (i.e. 100% missing values). We then used multivariate imputations by chained equations (van Buuren & Groothuis-Oudshoorn, Reference van Buuren and Groothuis-Oudshoorn2011), assuming a missing at random mechanism (Penone et al., Reference Penone, Davidson, Shoemaker, DiMarco, Rondinini and Brooks2014). Multivariate imputations by chained equations have proved to perform well for continuous (Penone et al., Reference Penone, Davidson, Shoemaker, DiMarco, Rondinini and Brooks2014) and categorical data (Giorgi et al., Reference Giorgi, Belot, Gaudart and Launoy2008; Akande, Reference Akande2015), even for high-dimensional data (Deng et al., Reference Deng, Chang, Ido and Long2016). We used the predictive mean matching for continuous data to preserve non-linear relationships (van Buuren & Groothuis-Oudshoorn, Reference van Buuren and Groothuis-Oudshoorn2011), the logistic regression for binary variables, the multinomial logistic regression for categorical variables with more than two factors, and the proportional odds model for ordered categorical data. Fruit length and width, and seed length and width were log-transformed to produce a normal distribution. To generate each imputed dataset, we repeated the algorithm with 10 iterations to obtain stable predictions for the missing data (van Buuren & Groothuis-Oudshoorn, Reference van Buuren and Groothuis-Oudshoorn2011). We generated 20 imputed datasets to capture most of the uncertainty underlying the imputation process (Graham et al., Reference Graham, Olchowski and Gilreath2007; Bodner, Reference Bodner2008; White et al., Reference White, Royston and Wood2011). Given the moderate rate of missing data (i.e. 29%) in the original dataset and the large number of observations (8,784 species), we kept all species with at least one data entry for the multivariate imputations by chained equations (Hardt et al., Reference Hardt, Herke, Brian and Laubach2013). The algorithm converged for all traits (Supplementary Fig. S1), even when the rate of missing values was high (e.g. diaspore colour). We analysed each imputed dataset individually and identically, and pooled the estimates from each dataset to obtain the final result (Rubin, Reference Rubin1987). We used the packages mice and miceadds in R v. 3.3.1 (R Development Core Team, 2013). The corresponding scripts are available from AAD upon request.
Table 2 Percentage of missing data per trait, before imputation, for the Madagascan flora.
Definition of dispersal syndromes
We defined endozoochorous and non-endozoochorous species based on the presence of edible rewards, such as fruit pulp or aril (Table 3) (van der Pijl, Reference van der Pijl1982). Subsequently, where possible, we assigned either the primate dispersal syndrome or the bird dispersal syndrome to each endozoochorous species (Table 3; Plate 1). Using a dual approach we distinguished between these dispersal syndromes by applying two alternative definitions based on (1) diaspore colour only, and (2) diaspore colour and fruit epicarp thickness. We used these two definitions in parallel because they are both commonly used in the literature (Voigt et al., Reference Voigt, Bleher, Fietz, Ganzhorn, Schwab and Böhning-Gaese2004). Diaspore colour has proved to be an important and reliable selection factor for primates and birds (Lomáscolo & Schaefer, Reference Lomáscolo and Schaefer2010). However, as there is no widely accepted definition of colours characterizing the bird and primate dispersal syndromes, we inferred our own definitions from published seed dispersal studies based in Madagascar (Supplementary Table S1). We checked that birds and primates consumed diaspores with different colours by performing a χ2 test of independence, followed by post-hoc pairwise tests applying a Bonferroni correction (χ2 = 81.704, d.f. = 18, P < 0.0001; post-hoc test: bird vs primate: P < 0.0001). We used the function pairwiseNominalIndependence in the package rcompanion (Mangiafico, Reference Mangiafico2016). We then performed a multiple correspondence analysis to find the colours most frequently associated with each disperser type. We used the function MCA in the package FactoMineR. We found that birds consumed predominantly diaspores with contrasting blue or purple colours, whereas primates consumed mainly brown, green or white diaspores (Supplementary Fig. S2). Therefore we used these colours to define the respective dispersal syndromes (Table 3). Fruit epicarp thickness has also often been used in the definition of syndromes (Janson, Reference Janson1983; Gautier-Hion et al., Reference Gautier-Hion, Duplantier, Quris, Feer, Sourd and Decoux1985): primates can eat fruits with thin as well as thick epicarps, whereas birds cannot eat fruits with thick epicarps. Seed dispersal syndromes reflect the adaptation of a plant species to be primarily but not exclusively dispersed by a certain agent, their primary disperser. Some plant species may have lost their primary dispersers (e.g. giant lemurs) but they continue to display their dispersal syndromes (e.g. primate syndrome), a circumstance referred to as dispersal anachronism (Janzen & Martin, Reference Janzen and Martin1982; Guimarães et al., Reference Guimarães, Galetti and Jordano2008; Andriantsaralaza et al., Reference Andriantsaralaza, Pedrono, Tassin, Edmond and Danthu2010).

Plate 1 Plant species displaying a bird syndrome: (a) Erythrina madagascariensis and (b) Commiphora sp.; and a primate syndrome: (c) Adansonia rubrostipa and (d) Adansonia suarenzensis (showing the inside of the fruit). Photographs by David Du Puy (a) and Wolfgang Stuppy (b, c, & d).
Table 3 Definition of seed dispersal syndromes.

* Seeds that deceptively suggest the presence of a fleshy edible reward (i.e. fruit pulp or aril) (van der Pijl, Reference van der Pijl1982).
Data analysis
We analysed the variation of traits between endozoochorous and non-endozoochorous species, and between species displaying a primate and a bird syndrome (following the definitions above). We fitted models using generalized least squares and maximizing the log-likelihood, with traits as dependent variables and syndrome as an independent variable, and compared them to null models using the likelihood-ratio test. We used the function anova combined with the function gls in the nlme package (Pinheiro et al., Reference Pinheiro, Bates, DebRoy and Sarkar2016). We performed all analyses in R v. 3.3.1. Data on traits of endemic Madagascan plant species before and after imputation of missing values are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.dt940.
Results
Given estimations from the imputed datasets, all life forms are well represented within the Madagascan endemic flora, with shrubs dominating (35%), followed by trees (30%), herbs (21%) and lianas (14%). Fruits are mainly dehiscent (49%) or externally fleshy (34%), with indehiscent fruits accounting for only 17% of the flora. The distribution of Madagascar's endemic plant species across the various bioclimates is as follows: 49% of species occur in sub-humid, 45% in humid, 25% in dry and 18% in sub-arid areas. Seventy-five percent of the island's endemic species occur in closed vegetation (i.e. forest, woodland, bushland or thicket). Almost half of the species (46%) occur only in forests, and 8% occur in two or more vegetation formations. Of the endemic Madagascan flora (N = 8,784 species), 38% of species are endozoochorous. When bird and primate dispersal syndromes are defined based on diaspore colour only, 26% of the endozoochorous species qualify for primate dispersal, 20% qualify for bird dispersal and 55% are ambiguous (i.e. although they are endozoochorous, they possess fruits that are not specifically adapted to be dispersed by either primates or birds). Focusing on trees of humid forests, the distribution of endozoochorous species shows the same pattern (31% primate syndrome vs 26% bird syndrome). When we add fruit epicarp thickness to the syndrome definitions, the distribution pattern is even more biased towards species with a primate syndrome: 41% of the endozoochorous plant species display a primate syndrome, 17% display a bird syndrome and 42% are ambiguous. Endozoochorous and non-endozoochorous species differ significantly in terms of life form, fruit type (Fig. 1a), bioclimate and vegetation formations (Table 4). Species with a bird or primate syndrome differ significantly in terms of fruit type (Fig. 1b), regardless of the definition of syndromes used for the analyses. They also differ with a very low level of significance in terms of bioclimate when diaspore colour only is taken into account (Table 5). When fruit epicarp thickness is also included in the definition of syndromes the difference in bioclimate is no longer significant.
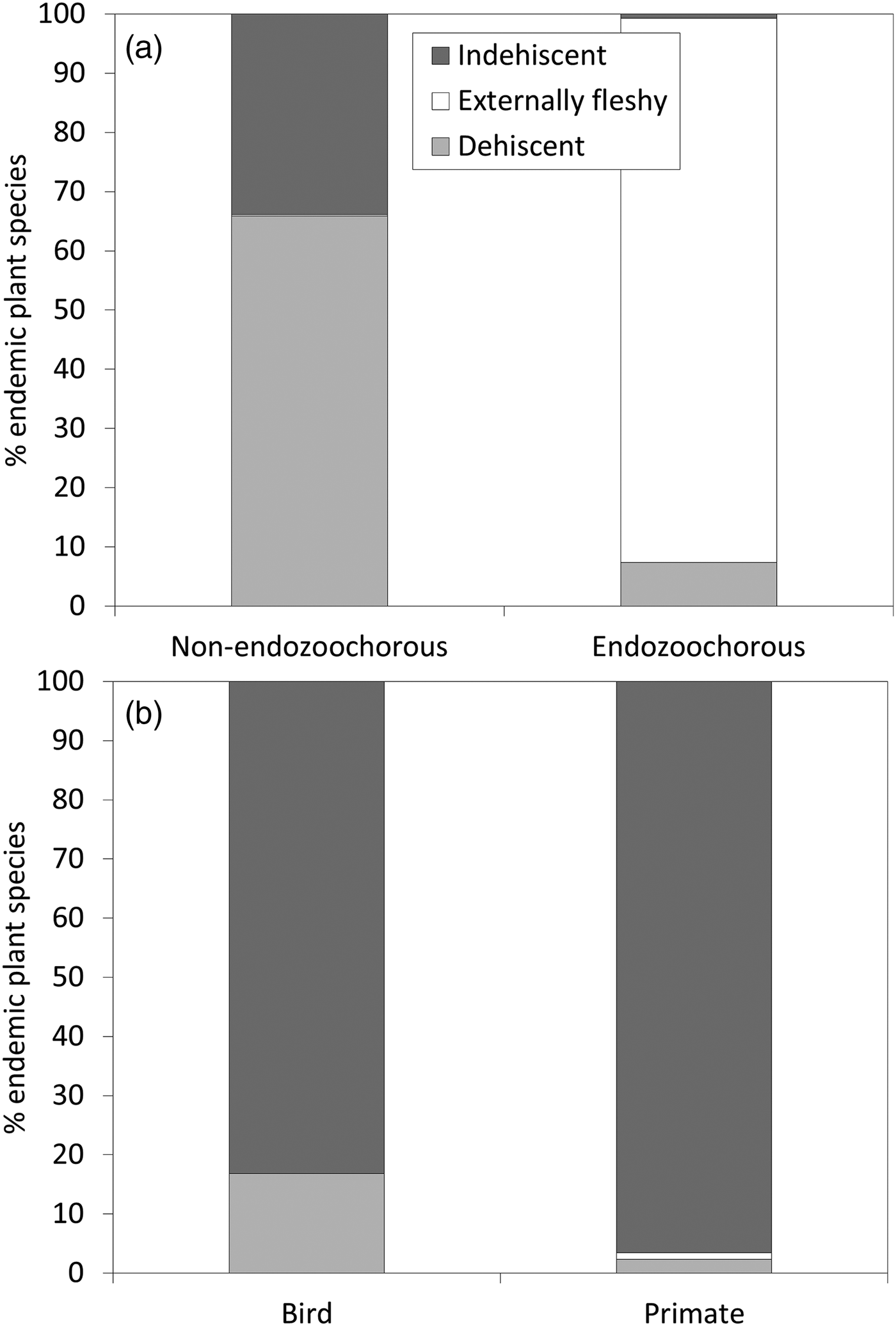
Fig. 1 Percentage of endemic Madagascan plant species (N = 8,784) producing each fruit type, comparing (a) non-endozoochorous and endozoochorous syndromes, and (b) bird and primate syndromes (with syndromes defined according to diaspore colour only).
Table 4 Characteristics linked to endozoochorous and non-endozoochorous endemic Madagascan plant species.
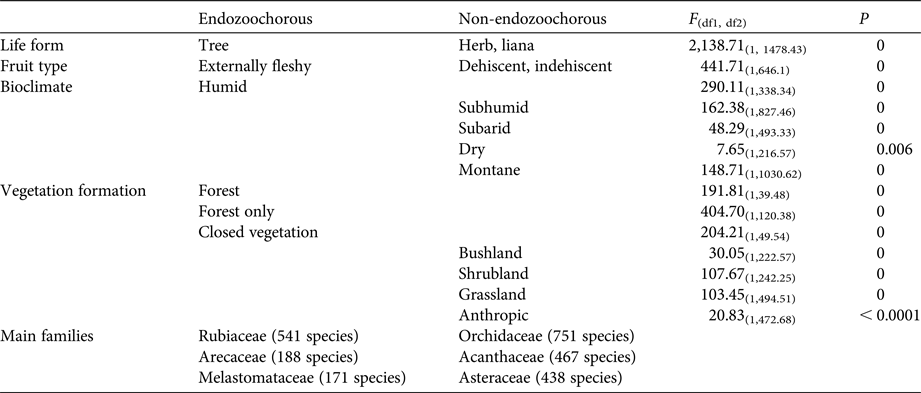
Table 5 Characteristics linked to endemic Madagascan plant species with a bird or primate syndrome, when syndromes are defined according to diaspore colour only.

Discussion
Our analyses indicated that, overall, slightly more than a third of Madagascar's endemic plant species display adaptation to endozoochory. Of these, 53% are trees. Focusing on humid forest tree species, the percentage of endozoochorous species is 70%, which is low compared to other tropical areas. In most tropical and subtropical moist broadleaf forests the percentage of animal-dispersed tree species is 80–90% (Osuri et al., Reference Osuri, Ratnam, Varma, Alvarez-Loayza, Astaiza and Bradford2016). The only exception is in South-east Asia, where some forest tree communities comprise > 50% of abiotically dispersed species. This result could be explained by the low number of frugivores in Madagascar (only 29 vertebrate species) compared to other tropical areas, where > 100 vertebrate frugivorous species may occur (Fleming et al., Reference Fleming, Breitwisch and Whitesides1987). Another explanation for the high proportion of non-endozoochorous species in Madagascar could be the presence of vast areas of grasslands where they are predominant. However, the question as to whether Madagascar's extensive grasslands are natural or the result of human interference has yet to be resolved (Bond et al., Reference Bond, Silander, Ranaivonasy and Ratsirarson2008; Crowley & Samonds, Reference Crowley and Samonds2013; Vorontsova et al., Reference Vorontsova, Besnard, Forest, Malakasi, Moat and Clayton2016); an anthropogenic and therefore recent origin of grasslands would make this last explanation irrelevant.
By applying the concept of dispersal syndromes to identify plant–animal interactions we were able to confirm what has previously been suggested by several studies of Madagascan ecosystems, namely the unusually strong reliance on primates as seed dispersers compared to other tropical areas (Bleher & Böhning-Gaese, Reference Bleher and Böhning-Gaese2001; Bollen et al., Reference Bollen, Elsacker and Ganzhorn2004; Voigt et al., Reference Voigt, Bleher, Fietz, Ganzhorn, Schwab and Böhning-Gaese2004). Our results are in line with those of Voigt et al. (Reference Voigt, Bleher, Fietz, Ganzhorn, Schwab and Böhning-Gaese2004), who found that the proportion of plant species with a primate syndrome was higher in Madagascar than in South Africa. In other words, in Madagascar primate dispersal plays a greater role than in other tropical areas, where the majority of plants have a bird syndrome. This reflects the much higher proportion of primates compared to birds in the Madagascan frugivore assemblage (Bleher & Böhning-Gaese, Reference Bleher and Böhning-Gaese2001; Bollen et al., Reference Bollen, Elsacker and Ganzhorn2004). Although Hughes et al. (Reference Hughes, Dunlop, French, Leishman, Rice, Rodgerson and Westoby1994) stated that ‘the availability of specific dispersal vectors seems rarely to be an important determinant of dispersal mode’, Madagascar could be the exception, given its strikingly small frugivore assemblage and the unusually strong importance of primates as dispersers. More studies are necessary to confirm that the dispersal spectra we observed in Madagascar are linked to the proportion of dispersers and not only to the presence of particular abiotic factors (Herrera, Reference Herrera, Herrera and Pellmyr2002). In particular, it would be interesting to compare the biomass of frugivorous birds and lemurs. Moreover, it is important to note that we did not take fruit bats into account. Although fruit bats are usually important dispersers in tropical areas (Muscarella & Fleming, Reference Muscarella and Fleming2007), including Madagascar (Bollen & Elsacker, Reference Bollen and Elsacker2002; Picot et al., Reference Picot, Jenkins, Ramilijaona, Racey and Carrière2007), we found a strong correlation between the proportion of each colour in the endozoochorous flora and the proportion of colours in species eaten by bats (r = 0.91), which suggests that bats do not seem to select fruits according to their colour. It is likely that fruit bats arrived by dispersal on the island much later than lemurs, encountering plants that were probably already largely adapted to dispersal by lemurs (Hutcheon, Reference Hutcheon, Goodman and Benstead2003).
Typically we found the following associations between fruit type, life form and vegetation formation: fleshy fruits (endozoochorous) were associated with trees and forests, and dry fruits (non-endozoochorous) were associated with herbs and grasslands (Armesto & Rozzi, Reference Armesto and Rozzi1989; Willson et al., Reference Willson, Rice and Westoby1990; Herrera, Reference Herrera, Herrera and Pellmyr2002; Bolmgren & Eriksson, Reference Bolmgren and Eriksson2005; Lorts et al., Reference Lorts, Briggeman and Sang2008). Moreover, endozoochorous (i.e. fleshy) fruits were more strongly associated with humid areas than dry fruits. As other studies have shown (Willson et al., Reference Willson, Rice and Westoby1990; Bunney, Reference Bunney2014), these results follow the metabolic costs hypothesis proposed by Willson et al. (Reference Willson, Irvine and Walsh1989), which assumes that plant species require some critical amount of water to produce fleshy fruits. Another explanation could be the lower proportion of arboreal frugivores in open areas.
Plant species with a primate syndrome more often produce externally fleshy fruits, whereas species with a bird syndrome more often produce dehiscent fruits (Table 5). Arillate seeds are, almost as a logical consequence, associated with dehiscent fruits, as an aril has to be exposed (visually or olfactorily) to attract animals for dispersal. Moreover, arillate seeds are often associated with ornithochory (van der Pijl, Reference van der Pijl1982). Therefore, it is unsurprising to observe that a high proportion of Madagascar's species with a bird syndrome produce dehiscent fruits.
We found that in Madagascar, similarly to other tropical areas (Turner, Reference Turner2001), plants with a bird syndrome belong mainly to the families Rubiaceae and Melastomataceae. However, plants with a primate syndrome belong mainly to the Rubiaceae, Pandanaceae and Malvaceae, whereas in other tropical areas, primate dispersal occurs mostly in members of the Sapindaceae, Meliaceae and Euphorbiaceae (Turner, Reference Turner2001). It would have been interesting to test whether the distribution of dispersal syndromes across plant families in Madagascar is phylogenetically biased; for example, a phylogenetic signal in dispersal syndromes has already been found in the plant species of Ranomafana National Park (Razafindratsima, Reference Razafindratsima2015). However, a comprehensive molecular phylogeny for the entire Madagascan flora is not yet available and the most comprehensive phylogenetic tree published (reconstructed by Hinchliff et al., Reference Hinchliff, Smith, Allman, Burleigh, Chaudhary and Coghill2015) includes only 2,745 of the 8,784 species analysed in this study.
Conclusion
Our results confirm that Madagascar's flora is unique with respect to the unusual dispersal spectra expressed among endemic plants. Seed dispersal syndromes are a useful tool for predicting plant–animal interactions and interdependencies. However, they must be applied cautiously in any attempts to conserve and restore seed-dispersal processes (Howe, Reference Howe2016). Although syndromes probably reflect specific interactions with dispersers, the frugivore assemblage and the effectiveness of each disperser species may have changed over time and across regions (Howe, Reference Howe2016). Although we do not conclude or suggest that all lemurs provide equally effective seed dispersal to plant species with a primate syndrome, it is clear that the protection of lemurs in Madagascar is of primary importance for the maintenance of plant communities. During the last 2,000 years 12 frugivorous lemur species have become extinct (Burney et al., Reference Burney, Burney, Godfrey, Jungers, Goodman, Wright and Jull2004; Crowley, Reference Crowley2010). Sixteen of the 20 extant frugivorous lemurs are threatened with extinction (i.e. Vulnerable, Endangered or Critically Endangered sensu IUCN, 2015) and many of these species have already disappeared from some regions (Godfrey et al., Reference Godfrey, Jungers, Simons, Chatrath, Rakotosamimanana, Rakotosamimanana, Rasamimanana, Ganzhorn and Goodman1999). The complete disappearance of lemurs would change the natural regeneration cycle and recruitment of primate-dispersed plant species (Jordano et al., Reference Jordano, Forget, Lambert, Böhning-Gaese, Traveset and Wright2011) and increase the risk of extinction of these plants (Caughlin et al., Reference Caughlin, Ferguson, Lichstein, Zuidema, Bunyavejchewin and Levey2014). Moreover, the fact that 54.57% of the endozoochorous plant species were found to occur only in forests highlights the urgent need not only to prevent deforestation in Madagascar but also to promote forest restoration.
Acknowledgements
We thank Kathy Willis for her support and encouragement; Gwilym Lewis for his support and advice on all matters related to legumes; Stuart Cable, William Baker, Wolf Eiserhardt, Hélène Ralimanana, Franck Rakotonasolo, Guy Onjalalaina and Romer Rabarijaona for sharing their knowledge about Madagascar and its flora; Ian Willey, Janet Terry, Patricia Wood, Aurelie Grall, Charlotte Couch, Nina Davies and Joanna Osborne for their assistance with the Millennium Seed Bank and herbarium collections of the Royal Botanic Gardens, Kew; the Muséum National d'Histoire Naturelle for permission to work on their collections, and particularly Peter Lowry and Thomas Haevermans; and Martin Fisher and two anonymous reviewers for their helpful comments. This study was part-funded by The Joseph Jones and Daisy and Graham Rattenbury Charitable Trust.
Author contributions
AAD and WS designed the research; AAD and SP collected data; AAD conducted the research and analysed the data; and all authors wrote the article.
Biographical sketches
Aurélie Albert-Daviaud is a specialist in seed dispersal by animals. She studies the impact of dispersers on forest maintenance and regeneration, and the impact of human disturbance on seed dispersal interactions and networks. Sarah Perillo’s research focuses on distribution and dispersal of Arecaceae in Madagascar, with interests in policy and governance. Wolfgang Stuppy is a specialist in seed and fruit morphology and development. His main research interests are the structure and biology of fruits and seeds, especially adaptations to dispersal by animals.









