1 Introduction
From the very beginnings of modern psychiatry the disturbances of self that lead to loss of ego boundaries (i.e. a sense of awareness that there is a distinction between self and world, and self and others) were considered a core characteristic of psychosis and of schizophrenia spectrum disorders in particular (SSD) Reference Bleuler[1]. Recently, there has been a renaissance of the idea that self-disorders (i.e., alterations in the “minimal” experiential self associated with a variety of anomalous subjective experiences), including weak ego boundaries, are the core of psychotic disorders Reference Noel, Cascio, Wallace and Park[2], and are observed from the very early phase of psychosis Reference Nelson, Thompson and Yung[3–Reference Parnas, Jansson, Sass and Handest6]. Loss of, or weakened, ego boundaries is hypothesized to underlie severe reality- and self-disturbances. Based on the source monitoring framework Reference Johnson, Hashtroudi and Lindsay[7] that provided a theoretical background on the process of discrimination between different source of information, it was hypothesized that severe reality distortions observed in psychosis, such as hallucinations, may be explained by failures in reality monitoring (i.e., discrimination between internal and external sources; deficits result in inner/outer confusions) Reference Bentall[8, Reference Frith and Done9]. This hypothesis has become an influential cognitive model of psychotic symptoms Reference Brookwell, Bentall and Varese[10, Reference Waters, Woodward, Allen, Aleman and Sommer11]. Recently, Nelson et al. linked the cognitive model with the phenomenological analysis by hypothesizing that source monitoring Reference Nelson, Whitford, Lavoie and Sass[12], along with aberrant salience Reference Nelson, Whitford, Lavoie and Sass[13], may underlie self-disorders observed from very early phases of psychotic symptoms. The authors suggest that source monitoring, along with self-disorders, may precede the development of psychotic symptoms.
Numerous source-monitoring studies have reported a higher tendency to confuse sources of information in patients with psychosis. For instance, a consistent body of research has shown a tendency to misattribute internally-generated stimuli to external sources, which has been linked to auditory hallucinations Reference Waters, Woodward, Allen, Aleman and Sommer[11, Reference Brunelin, Combris, Poulet, Kallel, D’Amato and Dalery14, Reference Waters, Badcock and Maybery15] and delusions Reference Brébion, Amador, David, Malaspina, Sharif and Gorman[16, Reference Anselmetti, Cavallaro, Bechi, Angelone, Ermoli and Cocchi17]. Independent meta-analyses confirmed the role of source monitoring deficits, especially a tendency to misattribute internally generated events as being generated by external agents, in auditory hallucinations Reference Brookwell, Bentall and Varese[10, Reference Waters, Woodward, Allen, Aleman and Sommer11].
However, less is known about the role of discrimination between imagination and reality, i.e., self-monitoring, in psychosis. It has been shown that patients with schizophrenia make significantly more errors in discriminating between performed and imagined events Reference Franck, Rouby, Daprati, Daléry, Marie-Cardine and Georgieff[18, Reference Mammarella, Altamura, Padalino, Petito, Fairfield and Bellomo19]. Patients with schizophrenia confuse imagination with reality and vice versa, and they exhibit some difficulty in discriminating between two types of action presentation (e.g., verbal vs non-verbal instructions) Reference Gawęda, Moritz and Kokoszka[20]. Further studies have revealed that a specific misattribution pattern, i.e., imagined actions recognized as performed, is related to auditory hallucinations in SSD Reference Gawęda, Woodward, Moritz and Kokoszka[21] and in a group of alcohol dependent patients with a history of hallucinations Reference Gawęda, Mikuła, Szelenbaum and Kokoszka[22].
It is estimated that about 80–90% Reference Yung and McGorry[23] SSD and other psychotic disorders cases are preceded by warning signs of imminent onset of disorder (i.e., the prodromal phase). The “ultra-high risk” (UHR) for psychosis criteria, introduced about two decades ago Reference Yung, McGorry, McFarlane, Jackson, Patton and Rakkar[24], attempt to prospectively identify this pre-onset stage of the illness. The criteria include Reference Yung, Yuen, McGorry, Phillips, Kelly and Dell’Olio[25] attenuated positive symptoms, states of brief frank psychosis that resolve within a week without treatment, or trait vulnerability (i.e., schizotypal personality disorder or first-degree relatives with psychosis), in combination with a significant decrease or chronic low social functioning as assessed with Social and Occupational Functioning Assessment Scale (SOFAS) Reference Goldman, Skodol and Lave[26]. The UHR concept has attracted clinicians’ and researchers’ attention mostly due to the fact that investigation of the markers that underlie this phase might provide knowledge about mechanisms that drive the onset of full-blown psychotic episodes.
Source monitoring deficits have been observed among UHR individuals Reference Johns, Allen, Valli, Winton-Brown, Broome and Woolley[27] and first episode psychosis (FEP) patients Reference Kambeitz-Ilankovic, Hennig-Fast, Benetti, Kambeitz, Pettersson-Yeo and O’Daly[28], as well as among first-degree relatives Reference Brunelin, d’Amato, Brun, Bediou, Kallel and Senn[29] and individuals with schizotypal traits Reference Peters, Smeets, Giesbrecht, Jelicic and Merckelbach[30]. This has led to the hypothesis that source-monitoring deficits may be an early risk factor for psychosis Reference Versmissen, Myin-Germeys, Janssen, Franck, Georgieff and Campo[31, Reference Hommes, Krabbendam, Versmissen, Kircher, van Os and van Winkel32]. Most of the studies that have investigated source monitoring in UHR or FEP have focused on discrimination between internally and externally generated events, i.e., reality monitoring. The question about the role of self-monitoring, i.e., discrimination between two internal sources of information (imagination and reality) in early risk for psychosis remains unanswered. Some preliminary results showing confusion between imagination and reality were observed in non-clinical individuals with high schizotypal traits Reference Peters, Smeets, Giesbrecht, Jelicic and Merckelbach[30]. However, to the best of our knowledge, deficits in discrimination between imagination and reality have not been investigated in UHR or FEP groups.
Furthermore, in psychotic disorders, incorrect decisions made during various cognitive tasks are accompanied by higher confidence when compared to healthy controls Reference Moritz, Woodward and Ruff[33]. Similarly, it has been shown that patients with schizophrenia usually make incorrect self-monitoring responses with significantly higher confidence than healthy controls Reference Gawęda, Moritz and Kokoszka[20, Reference Gawęda, Woodward, Moritz and Kokoszka21]. Recently, it was suggested that confidence disruptions may be related to the risk of psychosis based on results showing a high knowledge corruption index (KCI), i.e. a high proportion of failure decisions made with high confidence in relation to the total number of failure responses, in UHR and FEP patients Reference Eisenacher, Rausch, Ainser, Mier, Veckenstedt and Schirmbeck[34].
In the present study, we investigated both the role of self-monitoring and response confidence in the UHR and FEP groups. We utilized the Action Memory Task Reference Moritz, Ruhe, Jelinek and Naber[35] that allows for investigating two types of source monitoring (self-monitoring – discrimination between imagined and performed actions; external source monitoring – discrimination between verbally and non-verbally presented actions). We hypothesized that patients with UHR and FEP will make more self-monitoring biases compared to healthy controls, and that both clinical groups will express a higher degree of confidence in their inaccurate judgments compared to healthy controls. Given that both patients with UHR and FEP experience reality distortions that are related to self-monitoring in particular Reference Waters, Woodward, Allen, Aleman and Sommer[11], based on a previous study Reference Gawęda, Woodward, Moritz and Kokoszka[21], we expected no group differences on verbal vs. non-verbal actions discrimination. Finally, we hypothesized that self-monitoring biases will be related to hallucinations in particular.
2 Methods
2.1 Participants
Participants included 36 individuals at ultra-high risk (UHR) for psychosis, 25 patients with first episode psychosis (FEP) and 33 healthy controls (CON). Participants were aged between 15 and 24 years. Participants with neurological diseases or IQ<70 as determined using the WASI-II Reference Wechsler[36] were excluded, as well as participants with lack of proficiency in English. The study was approved by the local ethic committee.
The UHR patients were recruited from a specialist early intervention service for UHR patients, the PACE clinic in Melbourne Reference Yung, McGorry, Francey, Nelson, Baker and Phillips[37]. The UHR criteria were assessed using the Comprehensive assessment of at risk mental states (CAARMS) Reference Yung, Yung, Pan Yuen, Mcgorry, Phillips and Kelly[38] and consisted of three help-seeking groups:
• Attenuated Psychotic Symptoms (APS): young people who have experienced attenuated positive psychotic symptoms during the past year;
• Brief Limited Intermittent Psychotic Symptoms Group (BLIPS): young people who have experienced episodes of frank psychotic symptoms that have not lasted longer than a week and have spontaneously abated;
• Trait risk factor group: young people who have a schizotypal personality disorder or who have a first-degree relative with a psychotic disorder.
All groups must have experienced a decline of 30% in social functioning, as assessed with Social and Occupational Functioning Assessment Scale (SOFAS) Reference Goldman, Skodol and Lave[26] or have had chronic low social functioning (scores of 50 or below in SOFAS) over the year prior to referral.
The FEP group was defined as having daily positive psychotic symptoms for longer than one week and was recruited from the first episode psychosis clinical service (EPPIC) at Orygen, Melbourne. Only FEP patients that were stabilized from acute psychotic symptoms (based on clinical impression) and with schizophrenia spectrum diagnoses (schizophrenia, schizophreniform disorder, schizoaffective disorder, delusional disorders) were recruited. This was determined based on the diagnosis provided by the clinical team and confirmed via a SCID research interview. Diagnoses of patients according to the DSM-IV were as follow:
• three patients were diagnosed with schizoaffective disorder;
• two patients had a diagnosis of paranoid schizophrenia;
• one patient had delusional disorder;
• one patient had a diagnosis of major depressive disorder with psychotic features;
• eighteen patients had a diagnosis of psychotic disorder not-otherwise specified.
The CON group consisted of participants who had not been treated or diagnosed for psychiatric disorders in the past. The CON group was recruited through advertising in the local geographical area of Orygen, Melbourne (eg. community centers, universities, colleges, online, local newspaper). All participants were screened with a short screening questionnaire based on the Structured clinical interview for DSM-IV (SCID-I) – Screening Questions over the phone. Participants having relatives with psychosis were excluded from the study.
Psychopathology was assessed in all groups with the structured clinical interview – Brief Psychiatric Rating Scale (BPRS) Reference Overall[39]. Symptoms were calculated according to the five-factor solution Reference Shafer[40] with following factors: positive symptoms, negative symptoms, affect, resistance and activation.
2.2 Source monitoring assessment
Source monitoring was assessed with the Action Memory Task Reference Moritz, Ruhe, Jelinek and Naber[35]. Participants are presented with either verbal instructions or nonverbal pictograms cuing actions. Instructions set in a green frame have to be performed (see stimuli examples in supplementary materials), whereas action instructions set in a red frame have to be imagined but not performed.
Eighteen verbal and 18 nonverbal action instructions were presented, with each part (9 items each) requiring the participant to either perform or imagine each item. Before the recognition phase, a filler task was administered that took 10 minutes. Then, participants were required to indicate whether the corresponding instruction had appeared either as text (verbal), pictogram (nonverbal), or was new (presentation type differentiation) and whether or not the action was performed or imagined (self-monitoring) and graded for confidence (binary scale – unsure vs. sure). All items were randomized both in the learning and recognition phase. The experiment was programmed in MATLAB (MathWorks, Natick, MA).
2.3 Data analysis
Data analysis was performed using SPSS 24. All groups were contrasted with one-way ANOVA. Post-hoc comparisons were calculated using Bonferroni correction. At the first step, correct and incorrect recognitions of new and old actions were calculated. Percentage of hits (old actions identified as old) and false alarms (new actions identified as performed or imagined) were considered throughout the analyses. Further, sensitivity d’ index and response bias c index were calculated according to signal detection theory Reference Stanislaw and Todorov[41]. D’ index was calculated as follow: z(H)- z(F); higher d’ values denote higher sensitivity to discriminate old actions from new actions. C index was calculated with the following formula: c=–[z(H)+z(F)]/2 (z(F) refers to standardized z-score for false alarms and z(H) refers to standardized z-score for hits); higher (positive) values of the c index refer to a tendency to respond “new action”.
Correct self-monitoring attributions for performed and imagined actions were then divided and responses were analyzed separately. Two separate self-monitoring biases were computed and analyzed, namely performed actions misattributed as being imagined and imagined actions recognized as being performed. The second type of source monitoring was analyzed with the same rationale, i.e., general correct and incorrect responses for discrimination between verbal and non-verbally presented actions were calculated. Each type of response was then analyzed separately. Two types of correct (i.e., verbal recognized as verbal and non-verbal recognized as non-verbal) and two types of incorrect source monitoring biases (i.e., verbal recognized as non-verbal and non-verbal recognized as verbal) were considered when analyzing the response pattern for discrimination between the types of actions’ presentation (verbal and non-verbal).
Confidence ratings provided for self-monitoring responses were analyzed. “Sure” and “unsure” confidence ratings that proceeded correct and incorrect self-monitoring responses were calculated and analyzed. Mixed ANOVA was performed with two types of responses (correct and incorrect), two types of confidence (sure and unsure) and three groups. Further, according to previous studies Reference Gawęda, Moritz and Kokoszka[20, Reference Eisenacher, Rausch, Ainser, Mier, Veckenstedt and Schirmbeck34], we calculated the knowledge corruption index (KCI Reference Moritz, Woodward, Cuttler, Whitman and Watson[42]), which is the proportion of failure self-monitoring responses that were followed by a high degree of confidence (“sure”) to all other responses given with high confidence. Confidence gap Reference Moritz, Woodward and Rodriguez-Raecke[43] was referred to as the difference score between correct high confidence responses and incorrect high confidence responses. Group differences in the KCI and knowledge corruption were calculated with one-way ANOVA.
Finally, the relationship between the calculated variables and symptom severity was calculated for the combined sample of UHR and FEP patients with Pearson's correlational coefficient.
3 Results
3.1 Sociodemographic and clinical characteristics
Characteristics of groups are presented in Table 1. There were no differences with regards to age, sex and years of education.
3.2 Old/new actions recognition
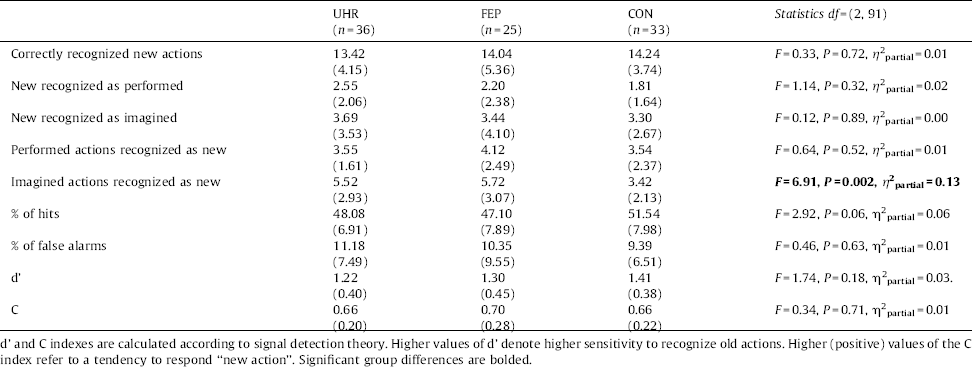
No group differences were found regarding to the percentage of false alarms (new actions recognized as old) and signal detection parameters d’ and c. Results are presented in Table 2. There was a statistical tendency (P =0.06) in group differences for percentage of hits, Bonferroni post-hoc revealed a tendency (P =0.09) for less percentage of hits in the FEP group when comparing to CON group.
All three groups performed similarly regarding correct recognition of new actions and incorrect recognition of new actions as being performed. More detailed analysis showed that there were no group differences in recognising old actions that were performed, but groups differed in recognising old actions that were imagined.
3.3 Self-monitoring: performed vs imagined action discrimination
Group differences regarding correct self-monitoring responses bordered significance, F(2, 91)=3.02, P =0.05, η 2partial=0.06. Fig. 1 shows that controls gave correct attribution of only imagined actions more often than UHR and FEP participants, F(2, 91)=5.03, P=0.008, η 2partial =0.10.
We found a specific misattribution self-monitoring response pattern: there were only significant group differences for imagined actions attributed as being performed, F(2, 91)=6.99, P =0.001, η 2partial =0.13 (Fig. 1). Importantly, after controlling for false positive responses (new actions recognized as performed) in the ANCOVA analysis, as recommended by some authors Reference Woodward and Menon[44], the results did not change, F(1, 91)=5.85, P =0.004, η 2partial =0.12.
3.4 Source monitoring: verbal vs non-verbal action discrimination
With regard to correct action's presentation discrimination there were group differences at the level of statistical tendency, F(2, 91)=3.09, P =0.05, η 2partial =0.06. Detailed analysis showed that the group differences were significant only for correct recognition of actions presented verbally F(2, 91)=5.20, P =0.007, η 2partial =0.10 (Fig. 2.
Table 1 Demographic and clinical characteristics of groups.

BPRS35: the Brief Psychiatric Rating Scale; the CAARMS: Comprehensive Assessment of at Risk Mental States; APS: Attenuated Psychotic Symptoms; TR: Trait risk factor group; BLIPS: Brief Limited Intermittent Psychotic Symptoms group; SOFAS: Social and Occupational Functioning Assessment Scale.
a Please note that data for BPRS were available for all UHR individuals, 18 FEP patients and 26 CON. For seven patients from FEP group were assessed with PANSS (subscales scores are not presented, but numbers of patients with hallucinations and delusions are derived from P1 and P3 items).
Table 2 Group differences in old/new recognition for self-monitoring.
There were no group differences with regard to incorrect discrimination between two types of action presentation (verbal v non-verbal), F(2, 91)=2.26, P =0.11, η 2partial =0.05.
3.5 Confidence
Mixed ANOVAs were performed with group as between subject factors, as well as Accuracy (correct and incorrect) and confidence (sure and unsure) as within subject factors. Results are presented in Fig. 3. The main effect of the group was insignificant, F(2, 91)=0.20, P =0.82, η2partial =0.01. However, a two-way interaction group×response was significant, F(2, 91)=5.09, P =0.008, η 2partial =0.12. Further, the three way interaction was significant, F(2, 91)=7.01, P =0.001, η2partial =0.13, because controls displayed high confidence in correct and lower confidence in incorrect responses relative to the two clinical groups. Subsequent one-way ANOVA revealed significant group differences in correct, F(2, 91)=3.97, P =0.02, η 2partial =0.08 and incorrect, F(2, 91)=5.96, P =0.004, η 2partial =0.12, self-monitoring responses given with high confidence (Fig. 3). Bonferroni post-hoc comparisons revealed that UHR group made significantly less correct responses with high confidence than CON (P =0.003). UHR group made significantly more incorrect responses with high confidence than CON (P =0.03); FEP patients made more errors with high confidence than CON at the level of statistical tendency (P =0.09). Both UHR and FEP groups had significantly lower confidence gap than CON, F =6.69, P =0.002, η 2partial =0.13, suggesting that patients had more difficulties in discriminating correct from incorrect decisions with an accurate confidence. Bonferroni post-hoc comparison revealed that both UHR and FEP groups had lower confidence gap as compared to CON (Fig. 3).
Our analysis revealed significant group differences with regard to the KCI, F =7.50, P =0.001, η 2partial =0.14. Bonferroni post-hoc comparisons suggested that both UHR and FEP groups had significantly higher KCI indexes than the CON group, suggesting that both clinical groups made their incorrect self-monitoring responses with less doubt.
3.6 Relation with symptom severity
No significant relations were found between symptom severity as measured with the BPRS, old/new recognition, self-monitoring or confidence. All correlation coefficients with symptom severity are presented in the Supplementary materials.
4 Discussion
To the best of our knowledge, this is the first study showing that UHR and FEP patients tend to misattribute imagined actions as being performed, but not vice versa, when compared to healthy controls. The findings are consistent with previous studies of action self-monitoring in patients with schizophrenia with auditory hallucinations Reference Gawęda, Woodward, Moritz and Kokoszka[21]. Furthermore, in accordance with previous studies in schizophrenia Reference Gawęda, Moritz and Kokoszka[20, Reference Gawęda, Woodward, Moritz and Kokoszka21, Reference Moritz, Woodward and Ruff33, Reference Köther, Veckenstedt, Vitzthum, Roesch-Ely, Pfueller and Scheu45] and UHR Reference Eisenacher, Rausch, Ainser, Mier, Veckenstedt and Schirmbeck[34] populations, our UHR and FEP groups tended to be over-confident in their self-monitoring judgments compared with healthy controls. Hence, the specific self-monitoring deficits and disruptions in confidence appear to be an early cognitive marker for the risk of psychosis. No differences between UHR and FEP patients in terms of self-monitoring and cognitive confidence suggest that these cognitive processes may be involved in psychotic symptoms development at the pre-onset phase and may not decline during the first phase of psychosis.

Fig. 1 Self-monitoring. Number of correctly recognized imagined or performed actions, as well as the number of imagined actions recognized as performed and performed actions recognized as imagined for the control, UHR and FEP groups.

Fig. 2 External source monitoring. Correctly recognized verbal and non-verbal actions, as well as verbally presented actions recognized as non-verbal and vice-versa for the control, UHR and FEP groups.

Fig. 3 Confidence ratings for self-monitoring responses. Correctly and incorrectly recognized actions with high and low confidence for the control, UHR and FEP groups (A). Knowledge Corruption Index (KCI) and confidence gap in the control, UHR and FEP groups (B).
Our results on self-monitoring are consistent with phenomenological and empirical studies showing the confusion between imagination and reality Reference Franck, Rouby, Daprati, Daléry, Marie-Cardine and Georgieff[18–Reference Gawęda, Moritz and Kokoszka20]. Contrary to a previous study among schizophrenia patients Reference Gawęda, Moritz and Kokoszka[20], we did not observe a significant decline in the sensitivity of discriminating between old and new actions in the UHR and FEP groups. Patients with UHR have more difficulties than the CON group in correctly remembering verbal actions. However, we did not observe exaggerated patterns of misattribution when discriminating between action presentation type (verbal vs non-verbal). This suggests that the results we obtained with regard to self-monitoring are unlikely to be related to general difficulties in the performance in the Action memory task. Both clinical groups not only misattributed imagined actions as being performed more often than controls, but at the same time they exhibited a higher tendency to forget imagined actions. This may suggest general difficulties in consolidating memory for imagined actions, but not for performed actions. This explanation is in line with a previous study Reference Brodeur, Pelletier and Lepage[46] showing preserved “enactment effect” among schizophrenia patients; that is, actions that were performed were remembered better compared to those that were observed or imagined. There is still a lack of research on the relationship between self-monitoring and cognitive impairments in schizophrenia, with some studies suggesting that decline in organizational sequencing may be related to more self-monitoring errors Reference Shakeel and Docherty[47]. However, the meta-analysis revealed that cognitive deficits have no significant impact on source monitoring biases observed in patients with hallucinations Reference Waters, Woodward, Allen, Aleman and Sommer[11]. Furthermore, we did not observe group differences in false positive responses (i.e., new actions recognized as performed and new actions identified as imagined), and controlling for false positive responses in the analysis of covariance, as suggested by some authors Reference Woodward and Menon[44], did not change the results. Moreover, although our UHR and FEP sample were considerably younger than schizophrenia patients included in the prior studies on action self-monitoring Reference Mammarella, Altamura, Padalino, Petito, Fairfield and Bellomo[19, Reference Gawęda, Moritz and Kokoszka20], the differences in the results are unlikely to be attributable to age, as we have recently demonstrated that age does not impact significantly action self-monitoring Reference Gaweda[48].
In line with our hypotheses, UHR and FEP patients made more over-confident responses in their incorrect self-monitoring compared to healthy controls (more than double). According to previous work Reference Moritz, Woodward and Ruff[33, Reference Moritz, Woodward, Jelinek and Klinge49], patients with schizophrenia tend to liberally accept decisions with a high degree of confidence, precluding corrective experiences; discomfirmatory evidence is overlooked so that reality distortion is maintained (e.g., delusions). Recently, it was shown Reference Eisenacher, Rausch, Ainser, Mier, Veckenstedt and Schirmbeck[34] that people at risk for psychosis and FEP patients tended to make their false decision with higher confidence in their judgments, even when the accuracy of memory recognition in the false memory task was preserved in both groups. A combination of misattributing imagined actions as performed and an overconfidence in these misattributions might be early risk factor for psychosis, especially delusions and hallucinations.
Although our results on the relationship between the risk of psychosis and early psychosis and self-monitoring are in line with previous results on reality monitoring Reference Johns, Allen, Valli, Winton-Brown, Broome and Woolley[27], these two source monitoring types may represent phenomenologically distinct processes. On the one hand, inner/inner confusion resulting from self-monitoring deficits may lead to self-disturbances described as “perceptualization” Reference Rasmussen and Parnas[50]. Two processes may be involved here. First, abnormal brain activity Reference Kompus, Westerhausen and Hugdahl[51, Reference Allen, Larøi, McGuire and Aleman52] may lead to “over-perceptualization”, which is then experienced as an enhanced vividness of imagination as shown by studies on schizophrenia Reference Oertel, Rotarska-Jagiela, van de Ven, Haenschel, Grube and Stangier[53, Reference Sack, van de Ven, Etschenberg, Schatz and Linden54]. Second, the process of discrimination between imagined and performed events may engage more cognitive resources, as suggested by a study showing hyper-activity in frontoparietal brain regions among healthy people with high imagery abilities during self-monitoring task Reference Stephan-Otto, Siddi, Senior, Munoz-Samons, Ochoa and Sanchez-Laforga[55]. Interestingly, greater activity of frontoparietal regions is also related to over-confident false responses as suggested by fMRI study on healthy subjects Reference Kim and Cabeza[56]. It is also related to increased risk of psychosis Reference Schmidt, Smieskova, Simon, Allen, Fusar-Poli and McGuire[57–Reference Orr, Turner and Mittal60]. Hence, dysfunctions of frontoparietal networks may be involved in the cognitive mechanisms underlying risk of psychosis. However, given the fact that we used only behavioral measures, future neuroimaging studies are required to verify the neuronal correlates of self-monitoring and disrupted confidence in UHR and FEP patients. Furthermore, neuroimaging studies may address the question about the specific neural correlates for different types of source monitoring, as our results suggest different results for self-monitoring (impaired) and discrimination between verbal and non-verbal events (preserved).
On the other hand, inner/outer confusions that are caused by deficits in the reality monitoring may explain why these perceptual abnormalities are experienced by some patients as external (e.g., alien voices). It has been shown that inner/outer confusions in schizophrenia is related to aberrant connectivity between the medial prefrontal cortex and left superior temporal gyrus Reference Wang, Metzak and Woodward[61], which is different to the regions typically associated with self-monitoring deficits. However, some similarities in brain functioning have been found and the findings suggest that the prefrontal lobe is engaged in different types of source monitoring. Hence, research on different types of source monitoring and interrelations between them is required for a better understanding of cognitive markers of the risk for psychosis. The hypothesis that deficits in self-monitoring (inner/inner confusion) may be involved in the development of perceptual anomalies, whereas reality monitoring may underlie an external attribution of these to external sources Reference Waters, Woodward, Allen, Aleman and Sommer[11] is worth considering for future studies.
Some of the limitations of our study should be noted. Most of our patients in both UHR and FEP groups had hallucinations or delusions with little variance in terms of their severity which might have led to non-significant association between self-monitoring, confidence and psychotic symptoms. Furthermore, this might be also a cause of no differences between UHR and FEP group in self-monitoring deficits as these deficits have been linked to the presence of clinical symptoms rather than to the diagnosis per se (FEP vs UHR) Reference Waters, Woodward, Allen, Aleman and Sommer[11]. Future studies may benefit from comparing patients with and without psychotic symptoms on self-monitoring and cognitive confidence. Although self-monitoring deficits as assessed with the Action memory task may be described as a behavioral expression of self-disturbances or anomalous self-experiences Reference Hur, Kwon, Lee and Park[62], future studies may also benefit from directly linking self-monitoring or reality monitoring deficits as well as cognitive confidence with self-disturbances, which are observed from the very early manifestation of SSD Reference Nelson, Thompson and Yung[3, Reference Parnas, Jansson, Sass and Handest6] and may be operationalized with structured interviews Reference Parnas, Moller, Kircher, Thalbitzer, Jansson and Handest[63] or self-report scales Reference Cicero, Neis, Klaunig and Trask[64]. Recently, a theoretical basis has been proposed as to the link between source monitoring Reference Nelson, Whitford, Lavoie and Sass[12, Reference Nelson, Whitford, Lavoie and Sass13] (and other cognitive processes Reference Nelson, Whitford, Lavoie and Sass[65, Reference Sass and Borda66]) and self-disturbances related to the risk of psychosis. Future studies may benefit from controlling for neurocognitive performance when investigating self-monitoring and confidence in UHR and FEP patients. We observed preserved old/new recognition as well as discrimination between verbal and non-verbal action's presentation, which suggests that our findings of specific self-monitoring misattributions patterns is unlikely to be due to a decline in general performance in the task. Furthermore, it should be noted that the analyses of “high-confidence” vs. “low-confidence” responses were based on a binary scale (sure vs. unsure) that was used in the Action memory task. Future studies may benefit from an extension of the confidence scale to the wider Likert-scale responses. The cross-sectional design of our study does not allow for causal inferences. Importantly, the rationale to investigate early markers of psychosis is to elucidate early mechanisms that may be used in early detection and intervention. Hence, reliable factors should not only be related to UHR state, but should also predict a transition to psychosis or persistent attenuated psychotic symptoms. Some longitudinal studies have reported that self-disturbances predict transition to psychosis in UHR Reference Nelson, Thompson and Yung[3, Reference Parnas, Carter and Nordgaard67]. Our result of no differences in self-monitoring deficits between UHR and FEP patients is in line with the studies showing that cognitive deficits are relatively stable from early development of psychosis Reference Barder, Sundet, Rund, Evensen, Haahr and Ten Velden Hegelstad[68, Reference Bozikas and Andreou69]. However, future longitudinal studies are required to investigate whether early self-monitoring and cognitive confidence deficits predict transition to psychosis or persistent attenuated psychotic symptoms among UHR patients, and whether improvements in psychotic symptoms among FEP are related to improvements in self-monitoring and cognitive confidence disturbances.
Contributions
B.N. and Ł.G. designed the study; T.W. programmed the experiment; E.L. collected the data and prepared the dataset; Ł.G. calculated the data and wrote the first draft of the manuscript; all authors interpreted the results; S.M., B.N., S.L. and T.W. edited the final version of the manuscript; all authors approved the finale version of the manuscript.
Role of funding source
Ł.G. was supported by ministry of Higher Education and Science of Republic of Poland (0295/E-393/STY/10/2015, 1258/MOB/IV/2015/0) and by National Science Centre, Poland (2016/21/B/HS6/03210). BN was supported by a NARSAD Independent investigator grant from the Brain & Behavior Research Foundation and a University of Melbourne, faculty of MDHS fellowship. T.W. is supported by an NHMRC career development fellowship (APP1090507) and discovery projects from the Australian Research Council (DP170103094; DP140104394).
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
The authors would like to express their gratitude to all participants for their valuable contribution.
Appendix A Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eurpsy.2017.09.003.








Comments
No Comments have been published for this article.