The metabolic syndrome classically refers to a multi-component disorder that is characterized by abdominal obesity, hypertension, dyslipidaemia and impaired glucose tolerance(1). It is associated with a high risk of subsequent development of type 2 diabetes mellitus and CVD(Reference Grundy, Cleeman and Daniels2). The prevalence of the metabolic syndrome defined by the Adult Treatment Panel III criteria was 23·7 % in the Third National Examination Survey in the United States, but its prevalence was found to differ among ethnic groups(Reference Ford, Giles and Dietz3). In Korea, the prevalence of the metabolic syndrome was 29·0 % in men and 16·8 % in women aged 30–80 years when using Asian–Pacific waist criteria(Reference Oh, Hong and Sung4).
Serum fatty acid composition has been shown to predict the risk of diabetes(Reference Wang, Folsom, Zheng, Pankow and Eckfeldt5) and CVD(Reference Wang, Folsom and Eckfeldt6) and is related to components of the metabolic syndrome(Reference Tremblay, Despres and Piche7). n-3 PUFA such as EPA (20 : 5n-3) and DHA (22 : 6n-3) have beneficial effects on improving lipid profiles(Reference Mori, Burke and Puddey8, Reference Holness, Greenwood, Smith and Sugden9), reducing blood pressure(Reference Prisco, Paniccia and Bandinelli10), improving insulin resistance(Reference Mori, Burke and Puddey8, Reference Holness, Greenwood, Smith and Sugden9) and reducing markers of systemic inflammation(Reference Harris, Park and Isley11). On the other hand, intake of trans fatty acids was inversely related with HDL-cholesterol(Reference Troisi, Willett and Weiss12, Reference Mensink, Zock, Kester and Katan13) and positively related to LDL-cholesterol(Reference Troisi, Willett and Weiss12). Trans fatty acids also increased lipoprotein(a)(Reference Nestel, Noakes and Belling14), TAG(Reference Katan, Zock and Mensink15) and insulin resistance(Reference Hu, Manson and Stampfer16) and were associated with systemic inflammation and endothelial dysfunction(Reference Lopez-Garcia, Schulze and Meigs17, Reference Mozaffarian, Katan, Ascherio, Stampfer and Willett18). Thus, n-3 and trans fatty acid tissue levels may be a modifiable factor for the metabolic syndrome. The estimated dietary intake of fish is high in Korea(Reference Nogi, Yang, Li, Yamasaki, Watanabe, Hashimoto and Shiwaku19), so Korea is a particularly appropriate population to investigate the role of n-3 PUFA on the metabolic syndrome.
The purpose of the present study was to compare fatty acid composition of erythrocytes (RBC), plasma lipid profiles, high sensitivity C-reactive protein (hs-CRP), fasting glucose and insulin levels between Koreans with and without the metabolic syndrome. The Omega-3 index, a new blood test to measure EPA and DHA in RBC, as a good reflection of systemic n-3 PUFA status(Reference Harris and Von Schacky20) was also compared.
Subjects and methods
Subjects
Subjects were recruited from patients visiting for regular heath examinations at Pusan National University Hospital between August 2006 and January 2007. Cases consisted of patients diagnosed with the metabolic syndrome, defined by modified Adult Treatment Panel III criteria(1): presence of three or more of the following components: (1) a waist circumference ≥ 90 cm in men and ≥ 80 cm in women; (2) HDL-cholesterol levels < 400 mg/l in men and < 500 mg/l in women; (3) TAG level ≥ 1500 mg/l; (4) systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85mmHg or taking an anti-hypertensive medication; (5) fasting glucose levels ≥ 1100 mg/l or taking an anti-diabetic medication. We selected age- and sex-matched controls with less than three risk factors of the modified Adult Treatment Panel III criteria. The current study was approved by the Institutional Review Board of Pusan National University Hospital and informed, written consent was obtained from all subjects before participating.
Procedures
Medical history and lists of medications such as oral hypoglycaemic agents, insulin, lipid-lowering, anti-hypertensive or oestrogen agents were obtained. Weekly total fish intakes were additionally obtained from all subjects. Subjects taking a supplement containing n-3 fatty acids were excluded. Height and body weight was measured using a digital scale with the subjects wearing a light gown. BMI was calculated as weight (kg)/height (m2). Using a tape measure, waist circumference was measured up to 0·1 cm unit at the midpoint between the lower costal margin and the iliac crest by well-trained examiners. Total body fat (%) was determined using a bioelectric impedance analyser (Inbody 3·0; Biospace Co., Ltd., Seoul, Korea). Resting blood pressure was measured using an automatic sphygmomanometer (BP203RV-II; Nippon Colin, Japan) after>10 min at rest in a sitting position at 08.00 hours to 10.00 hours.
Subjects refrained from smoking or ingesting caffeine for 30 min prior to having their blood samples drawn. Fasting blood samples (>12 h) were collected from the antecubital vein to determine serum concentrations of total cholesterol, TAG, HDL-cholesterol, fasting glucose, insulin, hs-CRP, alanine aminotransferase (ALT) and alkaline phosphatase. All biochemical analyses were carried out within 2 h of blood sampling, using an autoanalyser (model 7600-110; Hitachi Corp., Tokyo, Japan) and commercially available kits. LDL-cholesterol was calculated using the Friedewald formula(Reference Friedwald, Levy and Fredrickson21). Homeostasis model assessment insulin resistance (HOMA-IR) was calculated from the fasting concentration of insulin and glucose using the following formula(Reference Matthews, Hosker and Rudenski22):
RBC were used for fatty acid analysis(Reference Harris and Von Schacky20). Boron trifluoride methanol-benzene (B1252; Sigma-Aldrich, MO, USA) was added to RBC and samples were methylated for 10 min at 100°C. Fatty acid methyl esters were analysed by GC (Shimadzu 2010AF; Shimadzu Scientific Instrument, Japan) with a 100 mm SP2560 capillary column (Supelco; Bellefonte, PA, USA). Standard (GLC-727; Nu-Check Prep, Elysian, MN, USA) was used for identifying fatty acids and correcting inter-assay variation. In the standard, 18 : 1t peak was the mixture of 18 : 1n-12t, C18 : 1n-9t and 18 : 1n-7t, while 18 : 2n-6t peak contained 18 : 2n-6tt. The Omega-3 index was calculated as the sum of EPA and DHA in RBC. The control sample composed of pooled RBC and CV was 6·2 %.
Statistical analysis
All data were expressed as mean with their standard errors of the mean. Subjects with and without the metabolic syndrome were compared using the independent t test, and correlation between variables was tested by partial correlation analysis after adjusting age and sex. OR were computed for specific fatty acids of interest using multivariable logistic regression analysis after adjusting for age, sex, height, weight, blood pressure, aspirate aminotransferase, ALT, hs-CRP, glucose, insulin, TAG, total cholesterol, HDL-cholesterol, LDL-cholesterol and waist circumference. Fatty acid values were categorized into quartiles based on the control data only and then second and third quartiles were combined. Statistical analysis was performed using SPSS 12.0 (SPSS Inc., Chicago, IL, USA). A P value of < 0·05 was considered statistically significant.
Results
General characteristics of subjects
The characteristics of subjects are shown in Table 1. Weight, BMI and waist circumference were significantly (P < 0·001) higher in the patients with the metabolic syndrome than those without it. Waist circumference (95·5 %) was the most common determinant risk factor in cases, and there were 63·6 % of cases with three risk factors and 34·1 % and 2·3 % of cases with four and five risk factors, respectively. On the other hand, 52·4 %, 23·8 %, 23·8 % of controls had one, none, two risk factor(s), respectively.
Table 1 General characteristics of subjects†
(Mean values with their standard errors)
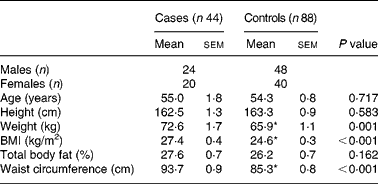
Mean values were significantly different: *P < 0·001 (independent t test).
† For details of subjects and procedures, see Subjects and methods.
Metabolic parameters and fish intake
Diastolic and systolic blood pressure, ALT, hs-CRP, fasting blood glucose, serum insulin, HOMA-IR and TAG were significantly (P < 0·05) higher in the patients with the metabolic syndrome than those without it (Table 2). On the other hand, HDL-cholesterol was significantly (P < 0·001) lower in the patients than in controls. There was no significant difference in the weekly fish consumption between the patients and the control subjects (2·80 servings/week v. 3·11 servings/week). However, subjects with higher fish consumption had a greater Omega-3 index (Fig. 1).
Table 2 Metabolic parameters of subjects†
(Mean values with their standard errors)
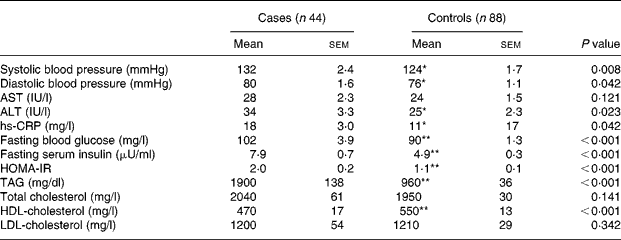
AST, aspirate aminotransferase; ALT, alanine aminotransferase; hs-CRP, high sensitivity C-reactive protein; HOMA-IR, homeostasis model assessment of insulin resistance, calculated from (fasting insulin (μU/ml) × fasting glucose (mg/dl))/405.
Mean values were significantly different: *P < 0·05; **P < 0·001 (independent t test).
† For details of subjects and procedures, see Subjects and methods.
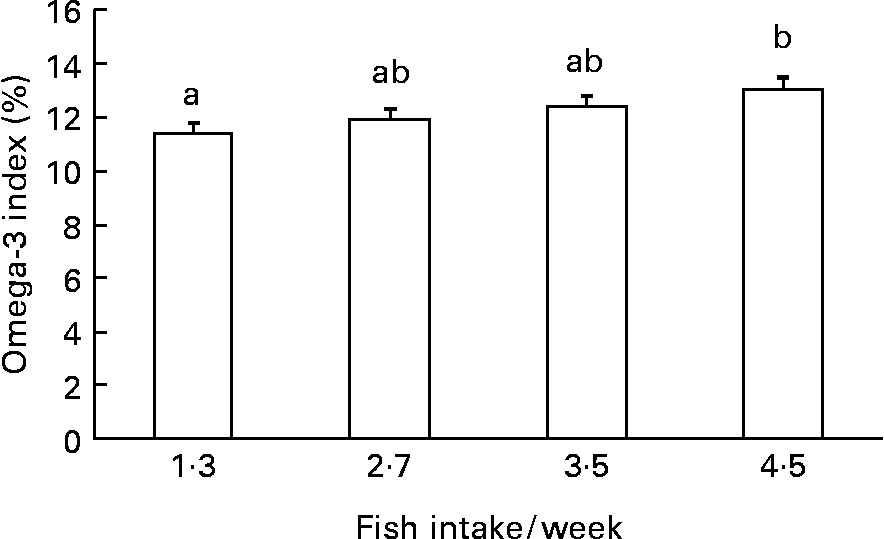
Fig. 1 Quartile of weekly fish intake and Omega-3 index (thirty-three in each). a,b Mean values with different letters were significantly different: P < 0·05 (ANOVA with post-hoc by Tukey's test).
Erythrocyte fatty acid composition
Fatty acid composition of RBC is presented in Table 3. Trans fatty acids of RBC were significantly (P = 0·043) higher in patients than controls, while the Omega-3 index did not differ between groups. Total PUFA was significantly lower, but 14 : 0, 16 : 1n-t, 18 : 1n-9c, 18 : 2n-6t, 18 : 3n-3 and MUFA were significantly (P < 0·05) higher in the patients with than those without the metabolic syndrome. However, multivariable-adjusted regression analysis showed that only total trans fatty acids and 18 : 2n-6tt were positively (P < 0·05) associated with risk of the metabolic syndrome after adjusting for age, sex, height, weight, blood pressure, aspirate aminotransferase, ALT, hs-CRP, glucose, insulin, TAG, total cholesterol, HDL-cholesterol, LDL-cholesterol and waist circumference (Table 4). Subjects in the highest quartile of total trans fatty acids and 18 : 2n-6tt had seven and fourteen times higher risk of the metabolic syndrome, even after adjusting for all confounding variables. Total trans fatty acids (P = 0·01), 16 : 1n-7t (P = 0·045) and 18 : 2n-6tt (P = 0·007), 14 : 0 (SFA; P < 0·001) and MUFA were positively (P = 0·014) related with waist circumference (Table 5). BMI was positively associated with 14 : 0 (P < 0·001) and MUFA (P = 0·031), but negatively associated with PUFA (P = 0·023) and n-6 PUFA (P = 0·006). TAG levels were positively related with 14 : 0 (P < 0·001), 18 : 1n-9c (P < 0·001), 16 : 1 n-7t (P = 0·009) and MUFA (P < 0·001). C-reactive protein was positively related with SFA (P = 0·023), but negatively with PUFA (P = 0·004) and n-6 PUFA (P = 0·036). In addition, 14 : 0 was positively associated with glucose (P = 0·009), insulin (P < 0·001) and HOMA-IR (P < 0·001).
Table 3 Fatty acid composition of erythrocytes in subjects†
(Mean values with their standard errors of the mean)

Omega-3 index, DHA+EPA.
Mean values were significantly different: *P < 0·05; **P < 0·001 (independent t test).
† For details of subjects and procedures, see Subjects and methods.
Table 4 OR and 95 % CI associated with fatty acid composition and the risk of the metabolic syndrome by multivariable regression analysis*

RBC, erythrocytes.
* For details of subjects and procedures, see Subjects and methods.
† Second quartile+third quartile.
‡ OR was adjusted for age, sex, height, weight, blood pressure, aspirate aminotransferase, alanine aminotransferase, high sensitivity C-reactive protein, glucose, insulin, TAG, total cholesterol, HDL-cholesterol, LDL-cholesterol and waist circumference.
Table 5 Correlation between fatty acid composition and metabolic parameters by partial correlation analysis†
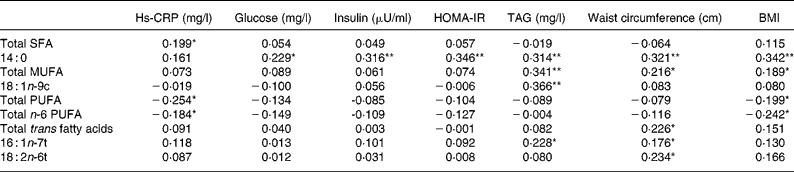
hs-CRP, high sensitivity C-reactive protein; HOMA-IR, homeostasis model assessment of insulin resistance, calculated from (fasting insulin (μU/ml) × fasting glucose (mg/dl))/405.
All values were significantly different: *P < 0·05; **P < 0·001 (partial correlation coefficient adjusted by age and sex).
† For details of subjects and procedures, see Subjects and methods.
Discussion
In the present study, we observed that RBC trans fatty acids were higher in patients with than without the metabolic syndrome and associated with risk of the metabolic syndrome after adjusting for age, sex, height, weight, blood pressure, aspirate aminotransferase, ALT, hs-CRP, glucose, insulin, TAG, total cholesterol, HDL-cholesterol, LDL-cholesterol and waist circumference. However, the Omega-3 index might not be associated with the metabolic syndrome in this population. Total MUFA, PUFA, trans fatty acid, 14 : 0, 16 : 1n-7t, 18 : 1n-9c, 18 : 2n-6tt and 18 : 3n-3 differed between patients and controls, but after adjusting for all metabolic parameters only trans fatty acids and 18 : 2n-6tt were significantly different. Correlation analysis showed that RBC fatty acids were significantly related with mostly TAG, waist circumference and BMI, which might be the major factors accounted for the OR analysis.
Trans fatty acids are positively related with lipoprotein(a)(Reference Nestel, Noakes and Belling14), systemic inflammation, endothelial dysfunction and plasma TAG levels(Reference Katan, Zock and Mensink15) and strongly associated with CVD(Reference Mozaffarian, Katan, Ascherio, Stampfer and Willett18, Reference Sun, Ma, Campos and Hankinson23). Thus, it is possible that trans fatty acids play a role in the development of the metabolic syndrome and type 2 diabetes mellitus(Reference Lopez-Garcia, Schulze and Meigs17, Reference Mozaffarian, Katan, Ascherio, Stampfer and Willett18). In a large prospective study of women, intake of trans fatty acids was positively associated with type 2 diabetes(Reference Salmeron, Hu and Manson24). Sun et al. (Reference Sun, Ma, Campos and Hankinson23) reported that the risk for CHD among subjects in the highest quartile of erythrocyte trans fatty acid content was three times higher than that of subjects in the lowest quartile. Troisi et al. (Reference Troisi, Willett and Weiss12) reported that intake of trans fatty acids was positively related to LDL-cholesterol and inversely related to HDL-cholesterol. Meta-analysis of Mensink et al. (Reference Mensink, Zock, Kester and Katan13) showed that trans fatty acids raised the serum total cholesterol:HDL ratio. Koh-Banerjee et al. (Reference Koh-Banerijee, Chu, Spiegelman, Rosner, Colditz, Willett and Rim25) reported a positive association between intake of trans fatty acids and abdominal adiposity in the prospective cohort. Kavanagh et al. (Reference Kavanagh, Jones, Sawyer, Kelley, Carr, Wagner and Rudel26) also showed that trans fatty acids were an independent factor for abdominal fat deposition and impaired insulin sensitivity, both of which are linked to the metabolic syndrome.
Recently, the Korean Food and Drug Administration reported that the average intake of trans fat was estimated as 0·7 % total energy or 1·54 g trans fat per d, which was lower than the WHO recommendation of less than 1 % total energy(Reference Hunter27). The average trans fatty acid of the current subjects was 0·73 % in RBC, which was also lower than those of the USA (2·0 %) and Denmark (1·2 %)(Reference Hunter27, Reference Dyerberg, Eskesen and Andersen28). Although RBC trans fatty acids were relatively low in all subjects, they were different between patients and controls, possibly suggesting the association between trans fatty acid and the metabolic syndrome.
Interestingly, we found higher 18 : 2n-6tt and lower 18 : 1t in RBC of subjects as compared with those of Westerners. The main contributors for trans 18 : 2n-6tt are vegetable oils (maize oil, soyabean oil, sesame oil), mayonnaise and canned tuna, while dairy products such as cheese, milk, ice cream, sour cream and butter contain 18 : 1t(Reference Karabulut29, Reference Baylin, Siles, Donovan-Palmer, Fernandez and Campos30). It is known that consumption of dairy products is relatively low among Koreans. Thus, this discrepancy can be explained by the dietary pattern between Koreans and Westerners.
Studies have suggested that the genetic regulatory effects of PUFA may protect against the adverse signs of the metabolic syndrome by mediating insulin and carbohydrate control of lipogenic and glycolytic genes, inhibiting fat storage and promoting fat oxidation(Reference Clarke31, Reference Clarke32). Tai et al. (Reference Tai, Corella and Demissie33) found significant gene–nutrient interactions between the PPAR-α gene-leucine to valine (PPARα-L162V) polymorphism and PUFA intake on plasma TAG and apo C-III concentrations in a Framingham Heart Study. In addition, the fatty acid composition in patients with insulin resistance and the metabolic syndrome is typically characterized by high levels of SFA and low levels of PUFA(Reference Warensjö, Sundström, Lind and Vessby34). The present study did not find a difference in PUFA between patients and controls, but PUFA was negatively correlated with hs-CRP and BMI.
n-3 PUFA have beneficial effects in reducing plasma TAG(Reference Mori, Burke and Puddey8), blood pressure(Reference Troisi, Willett and Weiss12) and markers of systemic inflammation such as hs-CRP(Reference Mensink, Zock, Kester and Katan13) and also in improving the lipoprotein profile by decreasing the fraction of atherogenic small dense LDL-cholesterol and improving insulin resistance(Reference Mori, Burke and Puddey8, Reference Holness, Greenwood, Smith and Sugden9). However, studies showed that EPA and DHA were not significantly different in patients with the metabolic syndrome(Reference Warensjö, Sundström, Lind and Vessby34, Reference Klein-Platat, Drai, Oujaa, Schlienger and Simon35). We also found no significant association between fish consumption and the metabolic syndrome, and between the Omega-3 index and the metabolic syndrome. In the present study, average fish consumption was three servings per week and was greater than that of US residents, who consumed less than one serving/week(Reference Sands, Reid, Windsor and Harris36, Reference Mozaffarian, Bryson and Lemaitre37). Although the Omega-3 index was not significantly (11·8 (sem 2·5) % v. 12·4 (sem 1·9) %, P = 0·123) different between patients with and without the metabolic syndrome, it was higher than in US residents and Europeans (4 %). The current subjects consumed more than the American Heart Association recommendation of fish(Reference Kris-Etherton, Harris and Appel38) and had a higher Omega-3 index than the recommended value of 8–10 % to prevent CHD(Reference Harris and Von Schacky20). This may be explained by the fact that Pusan is a major port for the Korean fish market; thus, most people may have higher fish consumption than the general population. The average Omega-3 index was 4 % in the USA(Reference Albert, Campos and Stampfer39) and Europe(40); thus, Korea is a particularly appropriate population to investigate the role of n-3 PUFA.
In conclusion, this study showed that RBC trans fatty acid content was higher in patients with the metabolic syndrome compared with controls and was associated with risk of the metabolic syndrome after adjusting for age, sex and metabolic parameters. Although causal relationships cannot be ascertained in such a case–control study, these findings have suggested that trans fatty acids might contribute to the metabolic syndrome phenotype. Even though there was no relationship with the Omega-3 index, further studies in populations with lower fish intake regarding the relationship between fish intake and the metabolic syndrome should be undertaken.
Acknowledgements
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant founded by the Korean government (MOST) (R01-2007-000-10 613-0).








