The dietary glycaemic index (GI) is a way of evaluating food consumption that considers the glycaemic response to the group of different carbohydrates present in the diet(Reference Jenkins, Woleverm and Taylor1). The glycaemic load (GL) represents the overall glycaemic effect of the diet and is calculated by multiplying the GI by the number of g of carbohydrates(Reference Salmerón, Manson and Stampfer2,Reference Salmerón, Ascherio and Rimm3) . Low-GI and low-GL diets have been identified as potential measures for preventing chronic diseases in adults(Reference Jenkins, Kendall and Augustin4,Reference Ludwig, Astrup and Willett5) .
Based on these findings, a positive association between GI and GL diets and gestational diabetes and gestational weight gain (GWG) has been hypothesised, which should protect against excessive GWG and macrosomia, both prevalent and relevant issues around the world(Reference Beta, Khan and Khalil6,Reference Goldstein, Abell and Ranasinha7) . Some studies have tried to test this hypothesis, though the literature shows inconclusive results, as discussed below.
In a meta-analysis of randomised controlled trials, compared with control diets, low-GI diets reduced fasting glycaemia and the proportion of newborns large for gestational age. These benefits are especially valid for pregnant women at risk of developing glucose-related disorders and have no negative associations with the health outcomes of the newborn. A lower GWG and birth weight were also observed, but with no significant differences. However, the authors drew attention to the limited number of studies and the high heterogeneity among the same(Reference Zhang, Han and Chen8). In a cohort study, pregnant women in the first quintile of dietary GL were compared with those in the fifth quintile; the latter showed a greater risk of large-for-gestational-age babies, and higher GWG rates were detected among normal-weight and overweight women(Reference Knudsen, Heitmann and Halldorsson9).
As presented in the review(Reference Zhang, Han and Chen8), most of the studies on this topic investigated specific groups of pregnant women: diabetics, pre-gestational overweight or those who have had macrosomic babies. Thus, the purpose of the present study was to investigate the relationship between the dietary GI and GL of pregnant women and GWG and newborn birth weight among usual-risk pregnant women by determining the contribution of GI and GL on outcomes, not only in comparisons with broader categories of GWG classification. Our hypothesis is that the GI and GL have a positive association with GWG and newborn birth weight.
Participants and methods
Study design and population
Between November 2012 and June 2013, all the pregnant women in the municipality of Botucatu, SP, Brazil, who enrolled in prenatal care of the primary health care system (SUS), were invited to participate in the study. The data gathered from the cohort followed between November 2012 and February 2014 form the basis of the results discussed below.
Originally, this cohort was used to investigate the effectiveness of an intervention in the training of primary health care professionals to promote healthy eating practices and physical activity during their prenatal visits(Reference Malta, Carvalhaes and Takito10). Briefly, during their consultations with pregnant women, the physicians and nurses of the nine primary health care units involved in Family Health Strategy (Estratégia de Saúde da Família) were trained to promote walks during leisure time at least 5 d/week and five feeding practices: three daily portions of fruit; two portions of vegetables and two of beans, at least 5 d/week; and only sporadic consumption of soft drinks and industrially processed cookies. The pregnant women attended by these health professionals consisted of the intervention cohort (cohort A). Health professionals from the other eight health units received no training regarding these activities during the same period. Therefore, the pregnant women whom they attended (cohort B) received routine prenatal care, according to the Brazilian Ministry of Health directives, which includes general guidelines on healthy eating and physical activity during pregnancy. In the present study, investigating the effects of the intervention was not our intention, since the health professionals were not instructed on the possible relevance of dietary GI and GL, nor they were oriented regarding the consumption of low-GI foods or lower carbohydrate consumption. However, in all the analyses performed, the cohort (A or B) was considered as an adjustment variable on the outcomes under study.
The pregnant women considered eligible for the study were those aged 18 years or older who were in the first trimester of gestation (<14 weeks). Cases of twins, the presence of diseases or complications identified during the study, such as diabetes, hypertension, cardiopathy or any adverse condition involving rest or a reduction in physical activity were excluded.
In the first gestational trimester, 353 pregnant women were interviewed and only 3·0 % refused to continue participating in the study. In addition, some follow-up losses occurred, due to changes in the location of prenatal consultations (private health care provider, or prenatal consultations at a reference hospital for high-risk pregnancies), several women moved to another city and miscarriages. Thus, in the second trimester, 281 pregnant women participated, and in the third, 267 participated. Weight gain data from 259 women and the birth weight of 260 infants were collected; however, no information was registered concerning the sex of one newborn, making it impossible to classify the child’s birth weight z-score (Fig. 1).
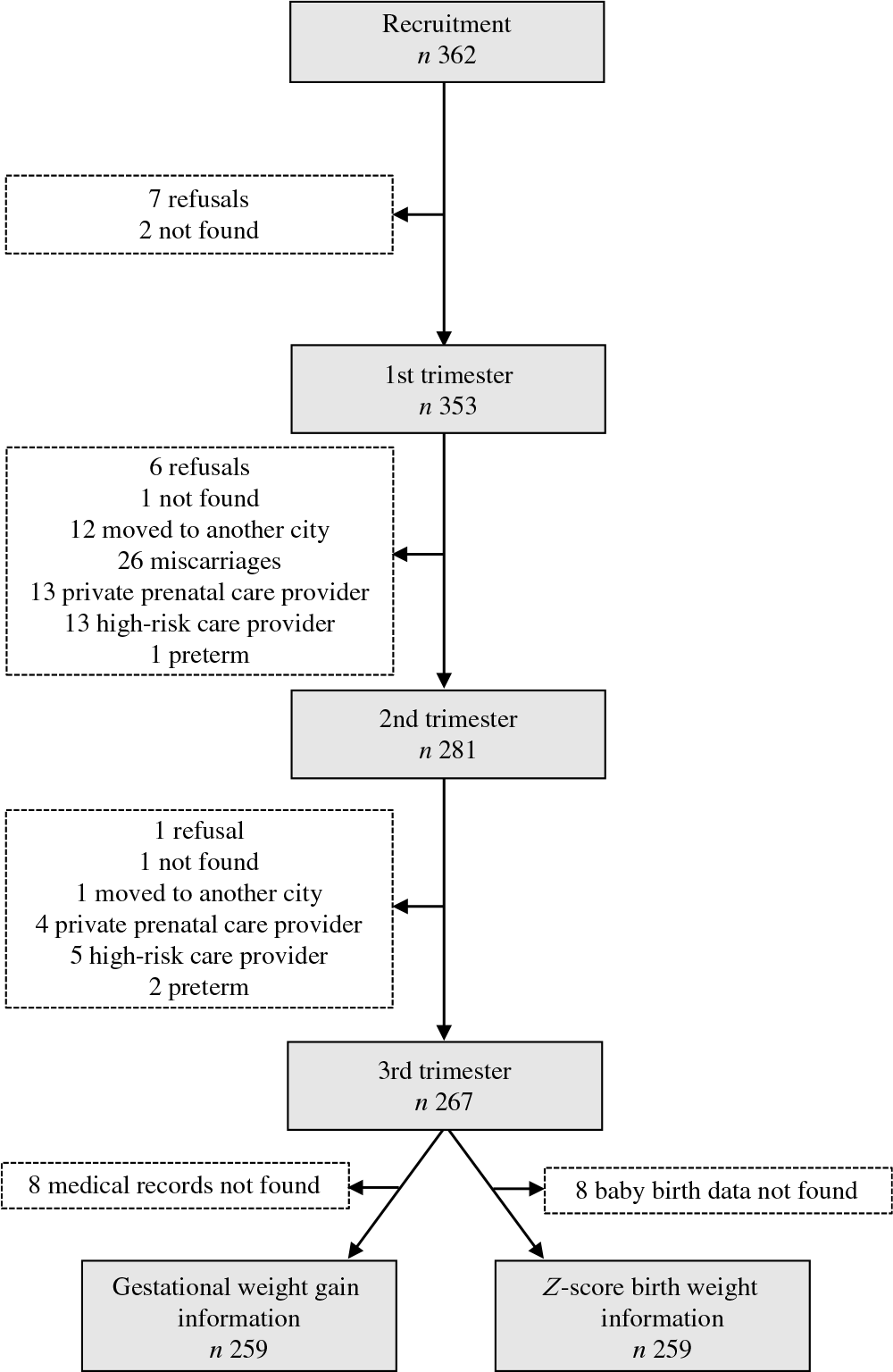
Fig. 1. Flow chart of the follow-up of the pregnant women, Botucatu, SP, Brazil, 2012–2014.
The present study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Research Ethics Committee of Botucatu Medical School, under protocol no. CAAE: 32407314.0.0000.5411. All the pregnant women were informed regarding the purpose of the study and participants signed a term of free, informed consent.
Data collection
The data were collected in the pregnant women’s homes by trained interviewers at three time points: during the first gestational trimester (<14 weeks), in the second trimester (24–27 weeks) and in the third trimester (31–34 weeks). All the questionnaires were reviewed weekly by the field study coordinator, and when inconsistencies were observed, the pregnant women were contacted quickly. The first interview took place before the first prenatal visit, when a socio-demographic, obstetric and lifestyle questionnaire was applied.
In the present study, we used the following variables: age range, 18–19, 20–29, 30 years old or over; education, <8, 8–11, ≥11 years; socio-economic status, class B, class C, class D/E, according to the classification criteria provided by the Brazilian Association of Research Institutes, which is divided into A, B, C, D and E, with A being the highest economic class, considering both household assets (i.e. car, television, washing machine, etc.) and the education level of the head of the household(11); paid work, yes or no; living with a partner, yes or no; parity, primiparous, one birth, two or more births; pre-gestational tobacco use, yes or no; pre-gestational nutritional status according to BMI(12), normal weight, underweight, overweight or obese; and number of prenatal consultations, 4–6, ≥7. These variables were used to describe the cohort and to identify possible adjustment factors that should be included in the investigation of the associations of interest.
To obtain the food consumption data, in each of the gestational trimesters, two 24-h dietary recalls (24-h DR) were applied, the first in person and the second by telephone. In each trimester, we were very careful to record 24-h DR for 1 d on the weekend or a holiday and one for a weekday, on non-consecutive days(Reference Monteiro, Hassan and Estima13).
The 24-h DR were applied using the multiple-pass method, which helps the interviewee remember the previous day’s diet and reduces errors in the dietary measures(Reference Moshfegh, Rhodes and Baer14). Information on the consumption of beverages was also obtained in the dietary recall.
Food consumption data were input into the software Nutrition Data System for Research, version 2010. Prior to their input, the foods and their preparation were subject to standardisation and quantification in g or ml, according to tables of Brazilian household measures(Reference Pinheiro, Lacerda and Benzecry15,Reference Fisberg and Villar16) . After each recall was recorded, the foods and type of preparations were verified, paying particular attention to the units of measurement, as well as periodic consistency analyses to determine the presence of outliers in portions, weights, energy and nutrients. 24-h DR showing an energy intake of over 25 104 kJ or <2510 kJ were excluded, since these levels of consumption are considered implausible(Reference Oken, Kleinman and Olsen17). Only 2·5 % of 24-h DR recorded presented these levels, and only these were excluded; the remainder was kept for analysis.
The dietary GI and GL values of each 24-h DR were obtained directly from the Nutrition Data System for Research software. The GI is a ranking of foods based on their effect on glycaemia compared by a reference food(Reference Jenkins, Woleverm and Taylor1), in our case, glucose reference. The GL quantifies the glycaemic effect of a food portion, calculated as: GL = (GI × CHO content)/100(Reference Salmerón, Manson and Stampfer2,Reference Salmerón, Ascherio and Rimm3) . The exposure variables were dietary GI and GL in the second and third gestational trimesters, considering the mean values of these trimesters.
The data referring to the outcomes of interest were obtained after the birth of the newborns. The height and weight measured in the prenatal consultations were obtained directly from medical records in the respective health units. It is worth emphasising that prenatal consultations are frequent in Brazil. Consultations occur monthly up to the final month of gestation, when the frequency is weekly(18).
Pre-gestational weight used was that registered on the medical records. This was compared with the initial weight measured during the first trimester of pregnancy, and when the difference between these was >2 kg, the latter was used. This standardisation was adopted to prevent under or overestimation of pre-gestational weight, which has been frequently reported(Reference Kleinman, Oken and Radesky19,Reference Kac and Velásquez-Meléndez20) . The BMI was calculated using these data and for classification of pre-gestational nutritional status(12).
The weekly GWG was calculated based on the difference in weight between the final measurement in the third gestational trimester and the first measurement in the second trimester, divided by the number of gestational weeks in that interval, according to the recommendations of the Institute of Medicine(Reference Rasmussen and Yaktine21).
We choose to evaluate weekly GWG in the second and third trimesters, together with GI and GL consumption, because this period is recognised as the most important phase for maternal and newborn weight gain(Reference Rasmussen and Yaktine21). We excluded the first trimester because eating disorders and restrictions are more common in this period. Brazilian maternity hospitals do not routinely weigh pregnant women upon admission for birth; thus, the data on weekly GWG provide better-quality information for an assessment of total GWG, which would normally depend on knowing the pre-gestational weight and the weight on the final day of gestation for precise estimation. The average weekly GWG can be easily determined using data obtained from the medical records of pregnant women, since this is recorded during prenatal consultations.
Data on newborn birth weight, in g, and gestational age at birth, in weeks, were obtained from the national Live Birth Information System (Sistema de Informações de Nascidos Vivos, SINASC-Brazil). This system uses the Declaration of Live Birth as its data source, a national, individualised, standardised document that is filled in at the maternity ward. The coverage, completeness and accuracy of SINASC data have been evaluated by Brazilian researchers, and good performance was observed(Reference Pedraza22). The birth weight z-scores in relation to gestational age and according to sex were calculated using the international standard developed by the Intergrowth-21st Project(Reference Villar, Cheikh Ismail and Victora23). We used the free tool available on the Intergrowth-21st website(24).
Statistical analyses
The analyses included only the pregnant women followed through the third trimester of gestation (n 267). Descriptive analyses (frequency for categorical variables, mean and standard deviation for continuous variables with normal distributions) of the socio-demographic, obstetric and life habits of pregnant women were initially performed. The Kolmogorov–Smirnov test was used to verify the normality of the outcome variables, and GWG and birth weight z-score were in normal distributions (P = 0·376 and P = 0·433, respectively).
Linear regression analysis was used to determine the association of GI and GL with the weekly GWG between the second and third trimesters, and the birth weight z-score, according to gestational age and sex. Initially, crude analyses were performed and covariates associated with the outcomes under study, considering P < 0·25 for multiple models(Reference Hosmer and Lemeshow25).
Since the variables mentioned above all had a possible relationship with the outcomes supported by the literature, those that met the criteria for statistical significance in the univariate analyses (P < 0·25) were included and maintained as possible confounding factors in the multiple model, regardless of the P value they presented later. In addition to these variables, to control for the possible effect of belonging to cohort (A or B), this variable was included in the multiple model, as was the average total energy consumed in the second and third gestational trimesters.
For GWG (g/week), the multiple model was adjusted for: the living with a partner, socio-economic status, years of education, age, self-reported skin colour, pre-gestational BMI and number of births(Reference Rasmussen and Yaktine21). Similarly, for the birth weight z-score, the multiple model was adjusted for: living with a partner, socio-economic status, years of education, age, self-reported skin colour, pre-gestational BMI and number of births(Reference Frederick, Williams and Sales26–Reference Kramer28).
All analyses were performed using the software STATA, version 14.2, considering P < 0·05 as the level of significance.
Results
The mean age of the pregnant women was 25·9 (sd 5·8) years old and varied from 18 to 46 years old. The most prevalent pre-gestational nutritional status was normal weight (50·6 %), followed by overweight (26·8 %) and obesity (17·7 %). Approximately half of the women had studied for 11 years or over, 67·8 % were from class C, 51·7 % did not work outside the home, 74·2 % lived with a partner, 40·5 % were primiparous, 62·8 % white and a quarter of the women were smokers before becoming pregnant. The vast majority attended more than seven prenatal consultations (91·1 %), and 5·9 % delivered between 34 and 36 weeks, with 6·5 % of newborns presenting low birth weight and 3·5 % presenting macrosomia. According to the classification of weekly GWG, 74·5 % did not gain the appropriate weight (Table 1). The mean weekly GWG, birth weight and birth weight z-score according to gestational age are also shown in Table 1, together with the mean energy intake, GI and GL of the maternal diet in the second and third gestational trimesters.
Table 1. Frequency of socio-economic, obstetric and anthropometric characteristics of the pregnant women and glycaemic index (GI) and glycaemic load (GL) during second and third trimesters according to these characteristics, Botucatu, SP, Brazil, 2012–2014 (n 267)*
(Numbers and percentages; mean values and standard deviations)
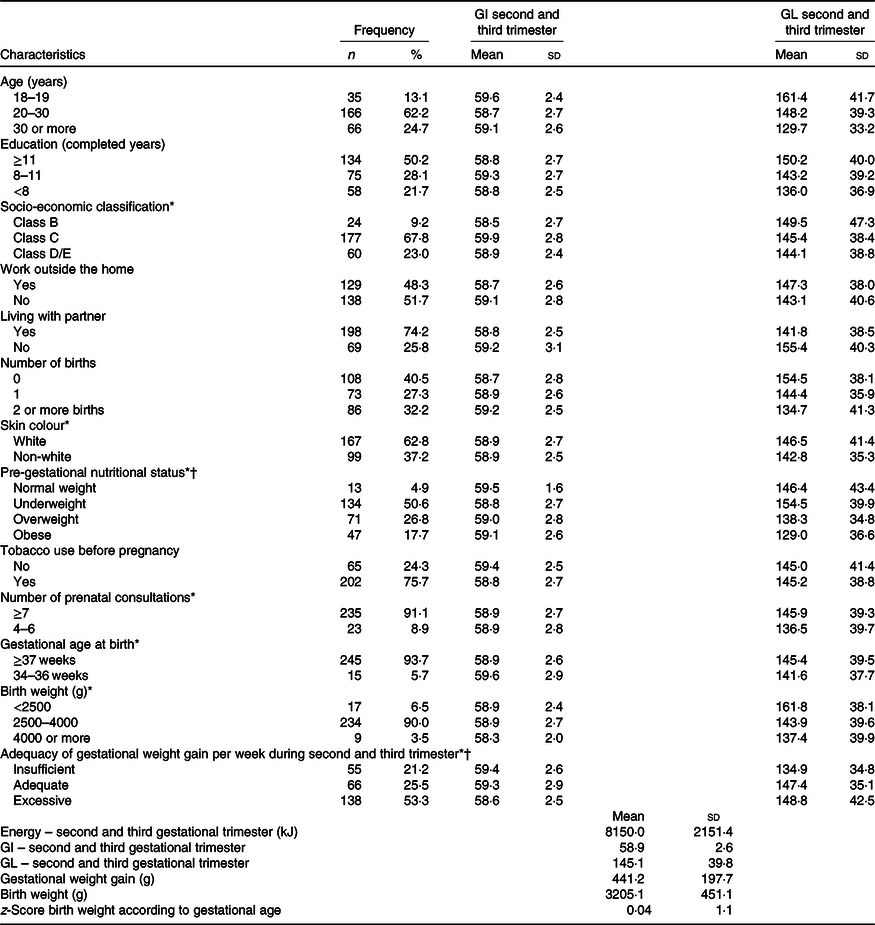
* Differences refer to missing data.
† Institute of Medicine (2009)(Reference Rasmussen and Yaktine21).
Table 2 shows the results of the crude and adjusted linear regression analyses for the association of dietary GI and GL with GWG in the second and third gestational trimesters. Crude analyses indicated that both dietary GI and GL were associated with GWG (GI: β = –12·02; 95 % CI –21·13, –2·91; and GL: β = 1·20; 95 % CI 0·60, 1·80). However, in the adjusted analyses, a one-point increase in GI meant a mean reduction of 12·9 g in weekly GWG (β = –12·86; 95 % CI –21·48, –4·24), while GL lost its association.
Table 2. Association of the glycaemic index and glycaemic load with weekly gestational weight gain (GWG), Botucatu, SP, Brazil, 2012–2014 (n 259)
(β-Coefficients and 95 % confidence intervals)
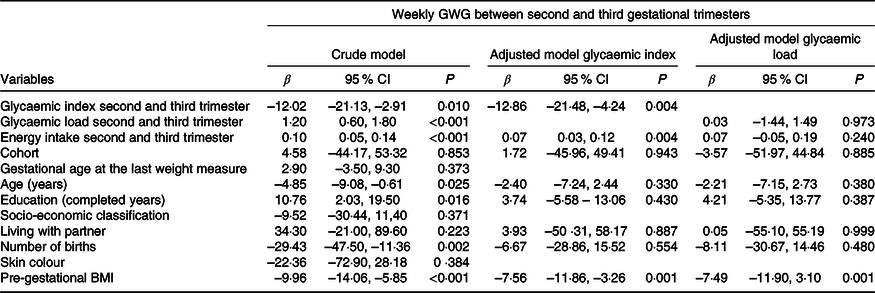
Table 3 presents the results of the crude and adjusted linear regression analyses showing the independent associations of dietary GI and GL with newborn birth weight. In the crude analyses, only dietary GL was associated with the birth weight z-score (β = –0·004, 95 % CI –0·01, –0·0004); however, it lost its significance when adjusted for confounding variables (β = –0·004; 95 % CI –0·01, 0·004).
Table 3. Association of the glycaemic index and glycaemic load with the z-score of newborn birth weight, Botucatu, SP, Brazil, 2012–2014 (n 259)
(β-Coefficients and 95 % confidence intervals)
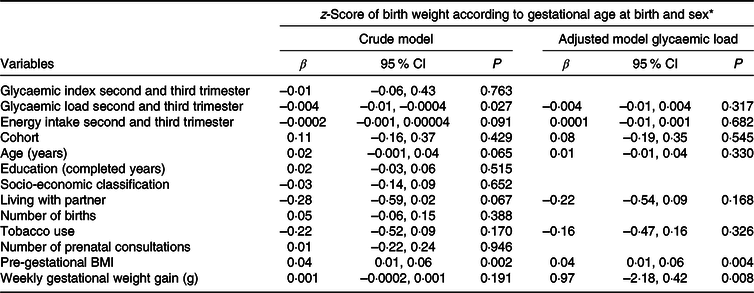
* z-Score of birth weight according to gestational age calculated according to the Intergrowth-21st Project standard(Reference Villar, Cheikh Ismail and Victora23).
Discussion
We hypothesised that the GI and GL have a direct association with GWG and newborn birth weight z-score, and the results contradicted our hypothesis. Analysis of the results obtained herein indicate that a one-point increase in maternal dietary GI, consumed in the second and third gestational trimesters, resulted in a mean decrease of 12·9 g in GWG. Dietary GL, however, showed no association with GWG, and no association was observed between GI, GL and birth weight z-scores.
A reduction in weekly GWG while dietary GI increased seems contrary to our initial hypothesis and differs from the results obtained by a clinical trial involving Irish pregnant women, in which the women in the intervention group (counselling on low-GI diet) gained less weight than those in the control group(Reference Walsh, McGowan and Mahony29). It is worth highlighting that the GI values reported in the Irish study are close to those obtained in the present study (59·0) and to those of a recent Brazilian study on maternal dietary GI (2nd tertile, 57·5–58·2)(Reference Ellery, Sampaio and Carioca30).
In the Danish cohort of births, GL showed a significant association with GWG. Weight gain was 30 g/week higher among overweight pregnant women, when comparing women from the first and fifth quintiles(Reference Knudsen, Heitmann and Halldorsson9). In the present study, an apparently positive association of GL with GWG, identified in the crude analysis, was not observed in the adjusted model. In complementary analyses (not shown), we identified that after inserting the variable energy consumption in the multiple model, the association of GL was not significant. The variable that seems to influence GWG is total energy intake rather than GL, a fact reinforced by the strong correlation between GL and dietary energy during the period evaluated (r 0·9063). This result contradicts the idea that the effect of GL is independent from energy consumption. This assumption is reinforced by the results of another cohort study on pregnant women: only energy density was significantly associated with total GWG, the GL was not associated with either outcome of GWG(Reference Deierlein, Siega-Riz and Herring31).
Therefore, the influence of GL on GWG is in fact due to the association between GL and energy. According to Ludwig et al.(Reference Ludwig, Willett and Volek32), a low-GL, low-fat, high-fibre diet promotes satiety and helps avoid overconsumption of food, probably due to increased GLP-1, a mechanism that maybe responsible for the prevention of diabetes, obesity and hyperglycaemia and that requires further requires investigation(Reference Runchey, Valsta and Schwarz33).
Regarding pregnant women, the present study evaluated these dietary factors pregnant women at usual risk for obstetric outcomes, that is, those without diabetes mellitus or hypertension or without high risk of complications during pregnancy. In addition, we identified an association of GI that was in disagreement with our hypothesis: pregnant women who consume diets with a higher GI had lower weight gains. This result is probably because most of the studies in the literature that show an effect of GI or GL on the weight gain in pregnant women are clinical trials in which the population has or is at risk of diabetes mellitus or excess weight. It is possible that dietary GI and GL can help minimise the effects of a negative condition, such as pre-gestational obesity and gestational diabetes in a previous pregnancy, but do not play a relevant role in the outcomes investigated for usual-risk pregnant women. In our population, the results indicate that energy consumption, regardless of the GI or GL, has a direct association with GWG.
Our findings regarding birth weight are in agreement with those reported in previous studies. The birth weight z-score was not associated with maternal dietary GL and that GI showed no association even prior to the adjusted analysis. Two large clinical trials with low-GI diets during gestation also reported no effect on newborn birth weight(Reference Walsh, McGowan and Mahony29,Reference Moses, Casey and Quinn34) . However, in the Danish cohort, a positive association was observed between GL and newborn birth weight(Reference Knudsen, Heitmann and Halldorsson9).
Although the influence of dietary GI and GL is considered plausible, our results identified no evidence that supports the use of either GI or GL as parameters for defining dietary guidelines for pregnant women. Thus, further research is required, especially among populations of pregnant women without co-morbidities. In a systematic review and meta-analysis on the quality of carbohydrates and health outcomes of healthy adult population, involving approximately 135 million person-years, Reynolds et al. only reported minimal or no risk reduction for health outcomes in relation to dietary GI and GL(Reference Reynolds, Mann and Cummings35). Moreover, they classified numerous studies as being of poor and very poor quality, which reinforces the merits for further research, together with issues that have produced conflicting results. One important consideration for future studies should be the methodological differences between studies, including the use of different tables for evaluation and 24-h DR and FFQ, since these may be the cause of such disagreements.
One of the strong points of the present study is how food consumption data were collected using six 24-h DR throughout the pregnancy, in contrast to the majority of international studies, which used FFQ, instruments that are more easily subject to memory error and quantification difficulties(Reference Biró, Hulshof and Ovesen36). Brazilian researchers consider FFQ to be inadequate for evaluating food intake in Brazilian pregnant women, hence our decision to use 24-h DR(Reference Barbieri, Crivellenti and Nishimura37). In addition, information on dietary GI and GL was obtained directly from the Nutrition Data System for Research software, one of the most widely used and recognised for food consumption analyses. This avoids the use of different tables and estimates, which is a common problem in studies on issue.
Another important aspect that supports the validity of our results is the few losses that occurred over this longitudinal study. Multiple adjustments were also made in relation to possible confounders of the relationships under investigation, since the outcomes we studied are known to be multi-determined(Reference Rasmussen and Yaktine21,Reference Frederick, Williams and Sales26–Reference Kramer28) . Since the present study was a secondary analysis of an intervention study, all the analyses involved cohort adjustments, though the results obtained indicated no influence, independent of the intervention. We sought to isolate the specific associations of GI and GL and verified that GL positively influences GWG and birth weight only when this variable is considered without energy adjustment. This fact stems from the strong correlation between dietary GL and total energy.
Conclusion
In a cohort of pregnant women considered to be at usual risk for obstetric complications, maternal dietary GI was negatively associated with weekly GWG in the second and third gestational trimesters, with a one-point increase representing a mean decrease of 12·9 g in GWG; dietary GL was not associated with this outcome. The birth weight z-score, according to gestational age and sex, was not associated with dietary GI or GL.
Acknowledgements
The authors are grateful to all the pregnant women who participated in the study, to Research Unit on Collective Health (UPeSC) of Botucatu Medical School for technical and logistical assistance and to Botucatu city Health Department for support.
M. H. D. B was funded by the São Paulo Research Foundation with research support (FAPESP 2011/18579-0).
M. H. D. B and M. B. M. formulated the research question and designed the study. M. A. B. L. C. formulated the research question, designed the study and performed statistical analysis. C. O. G. and J. L. F. A. performed the statistical and methodological assessment. C. B. G. carried out the study, performed statistical analysis and wrote the manuscript. All authors reviewed and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.







