Introduction
Radiation therapy (RT) is a safe and effective treatment for central nervous system tumours in both paediatric and adult patients.Reference Merchant, Gunderson and Tepper1–Reference Weller, van den Bent and Tonn3 Radiation necrosis of treated tissues can develop as an unintended complication of RT, which can affect patients’ performance status or quality of life. The incidence of brain radionecrosis (RN) ranges from 2.5% to 24% of treated patients, depending on diagnostic criteria used.Reference Chao, Ahluwalia and Barnett4 Radiation necrosis is more common after re-irradiation of brain tissues.Reference Chin, Ma and DiBiase5 Clinical manifestations of brain RN vary according to location but can include focal neurologic deficits or seizures, and the severity can range from an asymptomatic imaging finding to severe brain oedema causing death.
There are many treatments available for brain RN, but there is no clear standard of care. Patients who are asymptomatic can be observed, while those who are symptomatic are managed with corticosteroids, hyperbaric oxygen therapy (HBOT), bevacizumab, pentoxifylline, vitamin E, laser-induced thermal therapy and/or surgery.Reference Lubelski, Abdullah, Weil and Marko6–Reference Rao, Hargreaves, Khan, Haffty and Danish10 A recent Cochrane systematic review showed paucity of data on the topic, and was only able to include three comparative studies that used bevacizumab, edaravone and vitamin E.Reference Chung, Bryant and Brown11 HBOT is a non-invasive treatment that may stabilise necrosis, promote tissue repair and expedite neurological recovery.Reference Chuba, Aronin and Bhambhani12,Reference Feldmeier and Hampson13 Small retrospective studies have demonstrated high rates of benefit with either rates of stability or improvement estimated at 70%–80% of treated patients.Reference Chuba, Aronin and Bhambhani12,Reference Singh, Tsang, Khan and Merchant14–Reference Aghajan, Grover, Gorsi, Tumblin and Crawford18
Due to the sparsity of data on the use of HBOT for brain RN, this study aimed to review our institutional experience with HBOT and evaluate the efficacy of this treatment in stabilising or improving clinical symptoms and radiologic appearance of brain RN.
Methods
This was a retrospective cohort study. We included 13 adult patients diagnosed with symptomatic brain RN and who underwent HBOT at Toronto General Hospital between 2008 and 2018. Patients were permitted to have received single or multiple courses of RT, including stereotactic radiosurgery (SRS). Patients who had previous HBOT for an indication other than brain RN were excluded. The diagnosis of symptomatic RN based on the patient’s history, physical examination and concordant magnetic resonance imaging (MRI) findings was independently confirmed by the treating radiation oncologist, neuroradiologist and hyperbaric medicine physician. Establishing this diagnosis is important because only patients who have a “delayed radiation injury (soft tissue [or] bony necrosis)” are eligible for funding by provincial health insurance; all patients met this criterion for public insurance funding.Reference Ledez, Evans and Wherrett19,20 Demographic data, primary diagnosis and previous oncologic treatment, symptoms and diagnosis of brain RN, HBOT dose and administration, toxicities, radiologic and clinical outcomes were extracted. HBOT was administered at the Hyperbaric Medicine Unit at Toronto General Hospital using 2.0–2.4 atmospheric absolute (ATA) at 14.7–20 psi for 90 minutes daily. The number of daily dives given was at the discretion of treating physician; the median dive number was 30 (range 20–60). Post-treatment MRI studies were available in eight patients, which included three-dimensional gadolinium-enhanced T1 as well as T2 fluid attenuated inversion recovery (FLAIR) sequences. Magnetic resonance spectroscopy was not routinely performed.
The primary endpoint of the study was clinical improvement of a presenting symptom after initiation of HBOT which included a decrease in corticosteroid dose. RN was retrospectively graded using the Common Toxicity Criteria for Adverse Events version 5.0, using the “Central nervous system necrosis” subscale, as follows: Grade 1 – Asymptomatic, clinical or diagnostic observations only; Grade 2 – Moderate symptoms, corticosteroids indicated; Grade 3 – Severe symptoms, medical intervention indicated; Grade 4 – Life-threatening consequences, urgent intervention indicated; Grade 5 – Death. Specifically, those requiring inpatient hospitalisation were assigned grade 3 or greater.21
Radiologic improvement was determined using brain MRI and defined as a decrease in lesion enhancement intensity (by neuroradiologist determination) or lesion size (on enhanced T1 sequences) and/or brain oedema (on FLAIR sequences). Clinical characteristics were reported descriptively. Vital status and date of death of patients were collected through the medical chart; those who were lost to follow-up were found through publicly accessible records (i.e. obituaries). Overall survival was calculated from the first day of HBOT to date of death using the Kaplan–Meier method; those still alive or lost to follow-up were censored. Time to first clinically apparent necrosis improvement was counted from the first day of HBOT. Analyses were performed using SPSS v23.0 (IBM, IL, USA). The study was approved by the research ethics board of University Health Network.
Results
Thirteen patients had a diagnosis of brain RN and were treated with HBOT (Table 1; the complete version is available as Supplementary Table 1). The median age was 46 years (range 21–63 years); 39% were male. The initial oncologic diagnoses that required radiotherapy were brain metastasis (6; 47%), ependymoma (2; 15%), nasopharyngeal carcinoma (NPC, 2; 15%), medulloblastoma (1; 8%), meningioma (1; 8%) and cavernoma (1; 8%). Four patients received focal brain RT, two had received intensity modulated radiotherapy (IMRT) for nasopharyngeal cancer with exposure of brain tissue to high RT doses, two had SRS, three underwent whole brain radiotherapy (WBRT), one had craniospinal irradiation (CSI) upfront and one had missing radiotherapy details. Among these, eight had repeat brain RT with fractionated focal brain RT (3), accelerated fractionation focal IMRT for nasopharyngeal cancer (1) and SRS (2), WBRT (1) and CSI (1). All patients had previously underwent craniotomy as part of the course of their primary treatment except for four patients with cavernoma (n = 1), brain metastasis (n = 1) and NPC (n = 2), the latter received definitive chemoradiotherapy. Seven and four patients received chemotherapy and targeted therapy as part of their primary oncologic treatment, respectively.
The median time from last brain RT to presenting symptoms of brain RN was 6 months (range 1–351 months). Common presenting symptoms were hemiparesis, vision change and balance/gait issues followed by hearing change, alteration in sensorium, swallowing problems, dysarthria, diplopia, cognitive issues, seizures and headache. All of the patients were diagnosed radiologically and none underwent biopsy or resection of presumed brain RN. Available imaging was retrospectively re-reviewed; of 12 patients with available MRI source data, 11 (92%) patients had enhancement of the necrotic lesion and 11 (92%) had oedema seen on T2 FLAIR sequences. One patient did not have MRI available because the patient was referred for HBOT from another province, while one patient had necrosis of the abducens nerve which was not well seen on diagnostic MRI. Dosimetric data were available for 10 patients; among patients who received SRS, the radiation plan’s point maximum dose was 16.65 Gy, while in fractionated RT the cumulative, composite prescription was 120.6 Gy.
Table 1: Listing of cases. Some patients’ grade of toxicity were not assessable, but the medical chart documented clear clinical improvement.
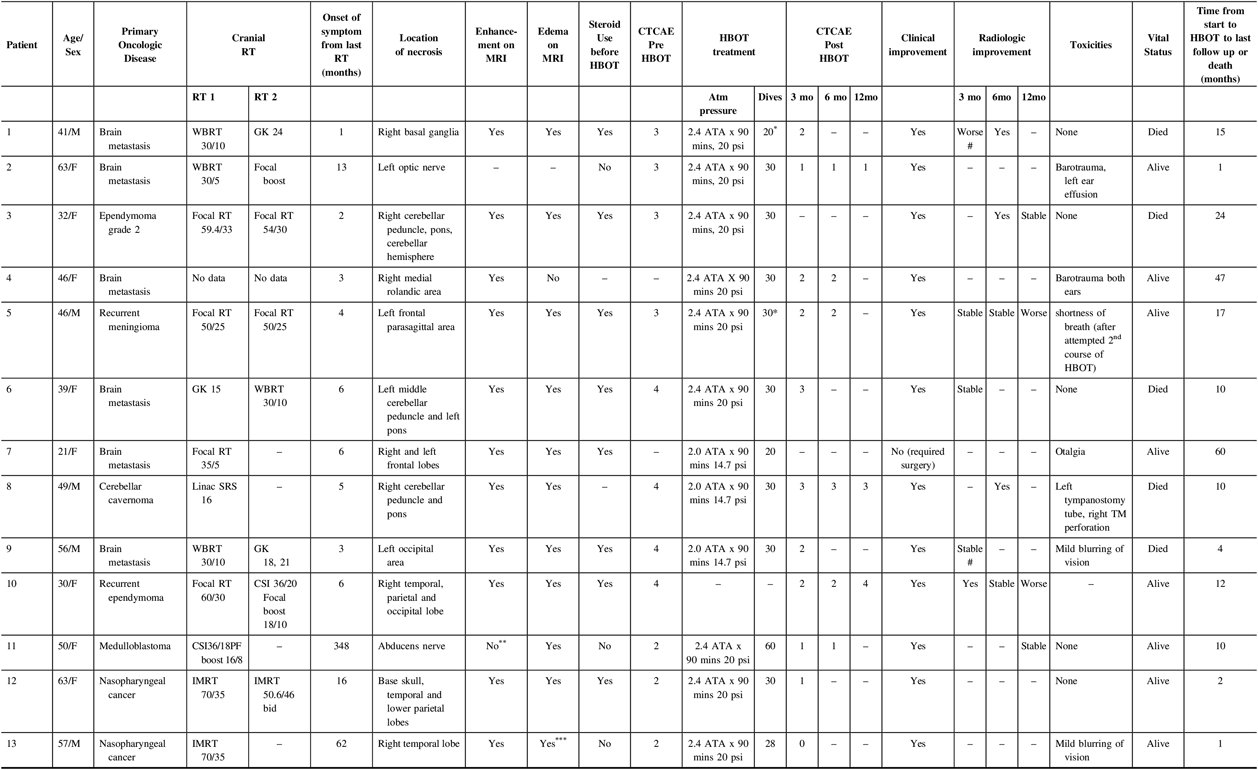
HBOT - hyperbaric oxygen therapy, WBRT - whole brain radiotherapy, GK - gamma knife stereotactic radiosurgery, RT - radiotherapy, SRS - stereotactic radiosurgery, PF boost - posterior fossa boost, TM - tympanic membrane
* prescribed course of HBOT not completed
** abducens nerve not well seen on MRI (T1 contrast-enhanced sequence was acquired with 4 mm slice thickness)
*** observed on T2 sequence (FLAIR sequence not done because study was protocolled as a head-and-neck study)
# MRI done at the end of HBOT
All patients underwent a single course of HBOT except for one who had a second course for recurrent RN. Six patients were treated between 2008 and 2011, while seven patients were treated between 2012 and 2018. The median time from presenting symptoms to start of HBOT was 4 months (range 0–11 months). Seven patients reported some treatment toxicity: mild barotrauma (2), ear effusion (1), otalgia (1), tympanic membrane perforation requiring tympanostomy tube insertion (1), transient, mild blurring of vision (2) and shortness of breath (1). All patients finished the prescribed course of HBOT except for two, which were discontinued due to uncertain benefit in one (patient 1) and dyspnea in the case of second course of HBOT (patient 5).
Twelve patients (92%) developed clinical improvement: eight during the course of HBOT and four within 1 month after HBOT completion (Figure 1), including the two patients who stopped HBOT. A single patient did not experience clinical improvement and required surgical intervention for RN (patient 7). The median time from initiation of HBOT to symptom improvement was 33 days (range 1–109 days). Among these 12 patients, 10 had evaluable necrosis toxicity grades pre-HBOT and post-HBOT; all had improvement by 3 months after HBOT. One patient had clinical initial improvement at 3 and 6 months, but worsened to grade 4 at 12 months; therefore, one patient did not have durable symptom improvement after HBOT (patient 5). Two more patients had documented clinical improvement but insufficient information to grade toxicity before and after HBOT. The medical records of 11 patients (85%) described steroid use prior to HBOT. Three were never started on steroids, five were using steroids throughout the HBOT and three were able to taper their steroid dose during treatment. The steroid use after HBOT was not available for review.
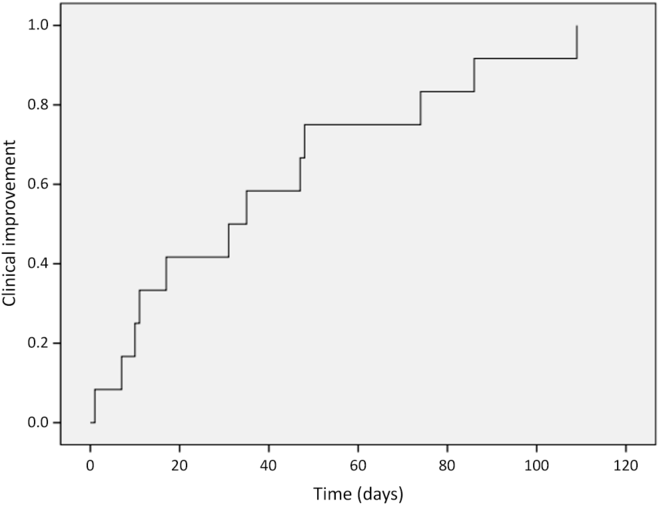
Figure 1: Time to clinical improvement after initiation of HBOT.
Eight patients had evaluable MRI at the end of or after HBOT; four patients had improved MR appearances, while the remaining four had stable necrosis appearance on MRI on follow-up. Two patients (patients 5 and 10) had deterioration in MRI appearances at 17 months after HBOT and 9 months from the start of HBOT, respectively, in the background of initial radiologic stability or improvement. Patient 1 had MRI after 20 dives which showed worse appearance of MRI; this led to discontinuation of HBOT and reinstitution of targeted therapy (erlotinib). Interestingly, the same patient had clinical improvement during HBOT with reduction in steroid dosage and had radiologic improvement after 6 months.
The overall median follow-up time from HBOT was 10 months (1–60 months). Eight patients were alive at last contact and five died (Figure 2); median survival was 15 months. The causes of death were tumour progression 17 months after HBOT (1; patient 3), complications of brain RN after transient improvement (2) and unclear aetiology (2; lost to follow-up). Patient 3’s tumour recurrence was within the original tumour bed in the fourth ventricle, rather than within the right cerebellar peduncle (where the treated necrosis was located).
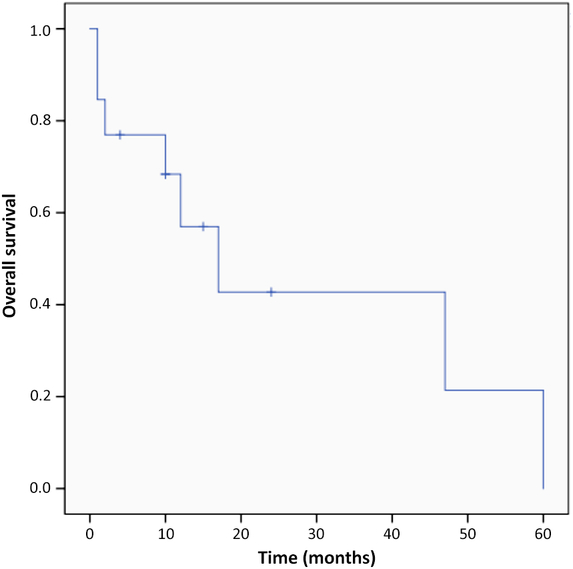
Figure 2: Overall survival of all patients from the first day of HBOT.
Discussion
This retrospective review presents the largest single institution experience on the use of HBOT for adult brain RN. Our series demonstrates that HBOT was helpful in stabilising or improving the symptoms of brain RN in most patients. Moreover, symptom improvement persisted in a majority of cases, except for one patient. Some patients did develop side effects from HBOT, though most were limited to otologic complications.
Tissue necrosis is a known infrequent side effect of radiotherapy that can occur in bones, soft tissues and brain.Reference Strojan, Hutcheson and Eisbruch22,Reference Greene-Schloesser, Robbins, Peiffer, Shaw, Wheeler and Chan23 HBOT has been used to treat air embolism, arterial insufficiencies, carbon monoxide poisoning, myonecrosis, compromised soft tissue grafts and flaps, crush injuries, decompression sickness, acute sensorineural hearing loss, intracranial abscess, necrotizing soft tissue infections, refractory osteomyelitis, severe anemia, thermal burns and delayed radiation injuries.Reference Weaver24 Small retrospective studies had previously reported the benefit of HBOT in treating brain RN. However, this is the largest known published series of adults treated with HBOT, which supports a clinical benefit of HBOT for this indication.
Radiation Necrosis
Several mechanisms have been proposed for the development of brain RN. Release of vascular endothelial growth factor stimulated by endothelial cell damage triggers this pathologic process; these cascading events lead to microvascular permeability, oedema and necrosis.Reference Li, Chen, Jain, Reilly and Wong25–Reference Coderre, Morris and Micca27 Other possible mechanisms include astrocyte hyperplasia and hypertrophy, oligodendrocyte damage and demyelination or perturbation of the fibrinolytic pathway.Reference Burger, Boyko, Gutin, Leibel and Sheline28–Reference Sawaya30 However, the mechanism of action of HBOT is not fully understood. HBOT has been used as a treatment for other radiation-induced injuries in various tissues.Reference Hart and Mainous31 It has been proposed that HBOT produces a positive oxygen gradient and subsequently promotes cellular and vascular repair.Reference Marx, Johnson and Kline32,Reference Marx, Ehler, Tayapongsak and Pierce33 In addition, previous animal studies have shown that HBOT decreases anaerobic conditions in the brain.Reference Kapp, Phillips, Markov and Smith34 Adverse radiation effect can occur early on which is mainly due to oedema and is frequently relieved by a course of corticosteroids, but those patients with necrosis occurring after 6 months are typically brain RN and more difficult to treat.Reference Buboltz and Tadi35
The incidence of brain RN ranges from 2.5% to 24% of treated patients, depending on diagnostic criteria used.Reference Chao, Ahluwalia and Barnett4 The variation may be due to improvement in RN diagnosis, awareness and length of oncologic follow-up.Reference Vellayappan, Tan and Yong36 Brain RN classically has a median time of onset of 6–12 months from last brain RTReference Chuba, Aronin and Bhambhani12,Reference Singh, Tsang, Khan and Merchant14–Reference Aghajan, Grover, Gorsi, Tumblin and Crawford18,Reference Ruben, Dally, Bailey, Smith, McLean and Fedele37 , though prolonged expression of injury can occur as long as 4 years after SRS.Reference Leber, Eder, Kovac, Anegg and Pendl38 RN is associated with dose–volume parameters, prior radiation treatment, concurrent chemotherapy, location, primary cancer histology, planning treatment volume and intrinsic idiopathic radiosensitivity.Reference Vellayappan, Tan and Yong36 Higher rates of RN can be seen in for patients receiving concurrent chemoradiation compared to radiation aloneReference Ruben, Dally, Bailey, Smith, McLean and Fedele37; in our study, two patients received concurrent chemotherapy for NPC. In our cohort of patients, the median time to develop symptoms after brain RT was 6 months. Interestingly, one patient in the present study developed symptoms of brain RN 29 years after radiotherapy, likely due to an exacerbation of chronic tissue damage. Previous studies have shown that those who manifest symptoms early after brain RT have a better prognosis compared to those who become symptomatic late and are considered as a late radiation injury.Reference Sheline, Wara and Smith39 Thus, there may be a difference in the pathogenesis of early- and late-onset brain RN.
Diagnosis of brain RN is challenging because the location of the high-dose radiation area is also a frequent site of local disease progression, which is always on the differential diagnosis of symptoms or imaging findings. The time to develop RN with initial RT is usually similar to the time to develop tumour recurrence. Histopathology is the gold standard for diagnosis; however, in our cohort of patients, biopsy was not performed to confirm the diagnosis of brain RN due to the need to start empiric therapy or an inaccessible lesion location. Brain MRI can sometimes differentiate brain RN through the use of diffusion-weighted imaging (DWI) and spectroscopy, but was not consistently available for our study cohort.Reference Zhang, Ma, Wang, Zheng, Wu and Xu40–Reference Rock, Scarpace and Hearshen42
Treating Necrosis
The initial management of brain RN is observation for an asymptomatic patient, while steroids are given in symptomatic patients to allow for rapid symptom relief.Reference Giglio and Gilbert9 A previous case report described brain necrosis that symptomatically improved after steroid initiation but took 6 months to taper the dose.Reference Tada, Matsumoto, Nakagawa, Tamiya, Furuta and Ohmoto43 Some studies use steroids as a comparator arm which gives insight on the effectiveness of steroids alone in brain RN. In a study investigating the role of edaravone, the control arm (steroids alone) was only able to provide improvement in 38.5% of patients after 3 months on the Late Effects Normal Tissue Task Force-Subjective, Objective, Management, Analytic criteria (LENT-SOMA) scale and on brain MRI.Reference Tang, Rong and Hu44 Long-term use of steroids is associated with many complications and doses should be decreased as soon as possible. Bevacizumab can also be given in RN due to its anti-angiogenesis properties. A small randomised control trial showed bevacizumab was able to reduce oedema, post-gadolinium contrast enhancing lesion size, and neurologic and clinical symptoms compared to a control group.Reference Levin, Bidaut and Hou45
In our cohort, a median of 30 HBOT dives were administered (range 20–60); this is consistent with prior reports, where the number of dives varied between 20 and 60 dives.Reference Chuba, Aronin and Bhambhani12,Reference Singh, Tsang, Khan and Merchant14–Reference Kohshi, Imada, Nomoto and Yamaguchi17,Reference Leber, Eder, Kovac, Anegg and Pendl38,Reference Alyahya, Mittal and Rowe46 Our cohort showed that 92% of the patients improved: 61.5% responded clinically during the course of HBOT, while 30.5% responded within 1 month of HBOT. This is consistent with previous reports that some patients attain clinical response even during a course of HBOT.Reference Kohshi, Imada, Nomoto and Yamaguchi17,Reference Leber, Eder, Kovac, Anegg and Pendl38 Data from the present study support HBOT as a treatment option for radiation necrosis.
Selected studies from the literature are presented in Table 2; similar to our data, the proportion of patients who develop clinical and radiologic improvement or stability is high. Among these, three studies included both paediatric and young adults in their population. Two case reports even reported patients who had symptomatic recurrence after the course of HBOT; in these studies, the patients were given another course of HBOT which afforded relief.Reference Cihan, Uzun, Yildiz and Dönmez16,Reference Kohshi, Imada, Nomoto and Yamaguchi17 In our cohort, one patient had received two courses of HBOT, but that patient was not able to complete the second course due to the development of dyspnea.
Table 2: Previous studies, published and unpublished, on use of hyperbaric oxygen for brain necrosis
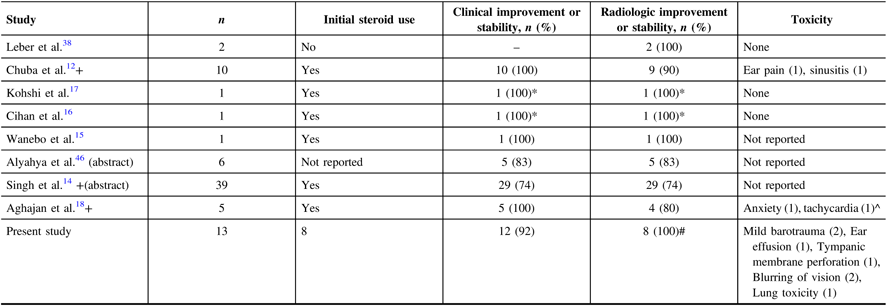
* Had two courses of HBOT.
# Only 8 out of the 12 patients had evaluable MRI post-HBOT.
^ Total population is seven patients which included brain tumours, the patients who had developed these toxicities were not specified.
+ Included paediatric population.
Our cohort included some patients who were treated initially with steroids and further improvement was seen with the addition of HBOT. We recognise that the effect of steroids may have contributed to the clinical and radiological improvement of this cohort and cannot be definitively isolated from the effect of HBOT. This practice is in concordance with typical clinical practice, wherein multiple modalities of treatments are instituted for brain RN. However, baseline status was determined at the initiation of HBOT; thus, improvements are more likely attributable to hyperbaric oxygen as opposed to steroids. In fact, all of the previous studies except one that evaluated HBOT for brain RN found that their patients were on steroids either before or during HBOT (Table 2).Reference Chuba, Aronin and Bhambhani12,Reference Singh, Tsang, Khan and Merchant14–Reference Aghajan, Grover, Gorsi, Tumblin and Crawford18,Reference Leber, Eder, Kovac, Anegg and Pendl38,Reference Alyahya, Mittal and Rowe46 Furthermore, the effect of HBOT may be effective against both the reversible and irreversible components of brain RN, but this retrospective study is not able to determine this difference. One of the limitations of the present study was that the steroid use after HBOT was unavailable for review.
Seven of the patients reported toxicities related to HBOT, although most of them were treatable. The most common was ear barotrauma, which can be prevented or treated with tympanostomy tubes. One patient (patient 5) had treatment-limiting toxicity (dyspnea), which necessitated discontinuation of a second course of HBOT. A study by Chuba et al. reported that HBOT was tolerable and resulted only in mild toxicity including ear pain requiring myringotomy and sinusitis.Reference Chuba, Aronin and Bhambhani12 Two studies reported two patients each and neither reports documented any toxicity from HBOT, even after repeated courses of treatment.Reference Cihan, Uzun, Yildiz and Dönmez16,Reference Kohshi, Imada, Nomoto and Yamaguchi17
HBOT costs 350 CAD (260 USD) per dive. A typical course of treatment of 20–30 dives would cost 7000–10,500 CAD. This might be viewed as costly burden to the healthcare system, but if effective, it may translate to healthcare savings because more costly surgical intervention and complications of prolonged steroids will be averted. Moreover, the cost of bevacizumab, another common therapy for RN, is 12,600 CAD per course (7.5 mg/kg intravenous every 4 weeks for four cycles)Reference Levin, Bidaut and Hou45,47 ; this amount does not include nursing or chemotherapy administration costs.
As of our knowledge, this retrospective review is the largest published series of patients investigates the use of HBOT in brain RN. This study does have limitations, however. Use of advanced MRI techniques was limited to standard enhanced T1 and FLAIR sequences; DWI was not always available and spectroscopy was not performed. Because histopathologic diagnosis was not obtained, tumour progression cannot be completely ruled out. This study is unable to compare the efficacy of HBOT as compared with bevacizumab. Varying tumour diagnoses and radiation treatments were included, which is a limitation inherent to retrospective outcomes research. The HBOT treatment protocol varied slightly between patients, typically ranging from 20 to 30 dives and 2.0 to 2.4 ATA; however, all treatments were delivered at a single hyperbaric facility, ensuring consistent treatment protocols and reporting. It would be ideal to measure the patient-reported quality of life outcomes after radiation necrosis and treatment with HBOT, ideally in a prospective manner. In such a study, there should be standardised reporting of clinical outcomes and toxicities of HBOT. We recognise the limited evidence on this topic; there is presently a phase 2, single-arm trial named Adverse Radiation Effects After Gamma Knife Radio Surgery and Hyperbaric Oxygen Therapy from Italian investigators which will expect to finish accrual in May 2019 (ClinicalTrials.gov identifier, NCT02714465). Bevacizumab is also emerging as an efficacious treatment for radiation necrosis; however, efforts to study this have been challenging. There is a multi-institutional, cooperative group study that is evaluating bevacizumab for radiation necrosis after SRS (ClinicalTrials.gov identifier, NCT02490878), though this study closed in late 2018 due to poor accrual.
Conclusions
HBOT renders favourable outcomes in the treatment of brain RN; HBOT led to clinical and radiologic improvement or stability in most patients. Many individuals were able to avoid or reduce their dose of corticosteroids during or after HBOT. HBOT can safely be administered with a tolerable toxicity profile, though otologic complications were common. Further study is required to a) prospectively evaluate HBOT as a treatment for radiation necrosis and b) compare its efficacy with other interventions such as bevacizumab.
Funding
No funding was used in the completion of this study.
Conflict of Interest
NL has received an honorarium from Abbvie, outside the submitted work. Dr AWE is the medical director at Medical Oxygen Repair, a HBOT facility in Toronto, Canada. DST’s institution received funds from Varian Medical Systems, Mevion Medical Systems, RayStation Laboratories, Hitachi, IBA and ProTom, outside the submitted work. The other authors have no conflict of interest to disclose.
Statement of Authorship
Conception and design – JC, RK and DT.
Data extraction – JC, MD and DT.
Synthesis and analysis – all authors.
Manuscript preparation and review and final approval – all authors.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2019.290.






