Introduction
Integrated cropping systems once dominated agriculture across the globe, but over the last century agroecosystems have become specialized and have decoupled crop and animal production (Russelle, Entz, and Franzluebbers, Reference Russelle, Entz and Franzluebbers2007; Hilimire, Reference Hilimire2011a; Garrett et al., Reference Garrett, Ryschawy, Bell, Cortner, Ferreira, Garik, Gil, Klerkx, Moraine, Peterson, Dos Reis and Valentim2020). While specialization of United States (US) agroecosystems has increased crop and animal rate of production (USDA ERS, 2020) it has also created negative side effects in relation to agriculture and the environment. These side effects include greater reliance on synthetic fertilizer in cropping systems, problems with storage and handling of animal manure in large-scale animal production, and possible general declines in soil and environmental health (Pimentel et al., Reference Pimentel, Harvey, Resosudarmo, Sinclair, Kurz, McNair, Crist, Shpritz, Fitton, Saffouri and Blair1995; Kraft and Stites, Reference Kraft and Stites2003; Foley et al., Reference Foley, DeFries, Asner, Barford, Bonan, Carpenter, Chapin, Coe, Daily, Gibbs, Helkowski, Holloway, Howard, Kucharik, Monfreda, Patz, Prentice, Ramankutty and Snyder2005; Savian et al., Reference Savian, Neto, de David, Bremm, Schons, Genro, do Amaral, Gere, McManus, Bayer and de Faccio Carvalho2014; Congreves and Van Eerd, Reference Congreves and Van Eerd2015). Managing soil quality is a challenge in intensive vegetable production systems (Rudisill et al., Reference Rudisill, Bordelon, Turco and Hoagland2015), especially in organic systems when growers must balance organic principles with crop nutrient demands and economic sustainability. Organic vegetable production is challenging especially when growers have to rely primarily on cover crops, green manure, composts, and various off-farm sources (Øvsthus et al., Reference Øvsthus, Breland, Hagen, Brandt, Wold, Bengtsson and Seljåsen2015) to manage soil fertility and health. Organic principles highlight the importance of on-farm nutrient cycling (Watson et al., Reference Watson, Bengtsson, Ebbesvik, Løes, Myrbeck, Salomon, Schroder and Stockdale2002) therefore; trucking plant nutrients from off-farm is somewhat incompatible with the principles of organic production. Could re-integrating animal and crop production bring organic growers closer to closed-loop regenerative soil fertility and health goals (Lemaire et al., Reference Lemaire, Franzluebbers, de Faccio Carvalho and Dedieu2014; Russelle, Entz, and Franzluebbers, Reference Russelle, Entz and Franzluebbers2007)?
Crop-animal integrated agriculture harnesses the synergistic relationships between plants and animals, efficiently transferring resources resulting in a semi-closed or closed-loop system (Hendrickson et al., Reference Hendrickson, Hanson, Tanaka and Sassenrath2008; Garrett et al., Reference Garrett, Niles, Gil, Gaudin, Chaplin-Kramer, Assmann, Assmann, Brewer, de Faccio Carvalho, Cortner, Dynes, Garbach, Kebreab, Mueller, Peterson, Reis, Snow and Valentim2017). Integrated systems have many benefits, most of which are antidotes to the adverse side effects created by decoupling crops and animals, including improved soil health (Hendrickson et al., Reference Hendrickson, Hanson, Tanaka and Sassenrath2008; Salton et al., Reference Salton, Mercante, Tomazi, Zanatta, Concenço, Silva and Retore2014), farm income diversification (Poffenbarger et al., Reference Poffenbarger, Artz, Dahlke, Edwards, Hanna, Russell, Sellers and Liebman2017), pest management (Lantinga, Oomen, and Schiere, Reference Lantinga, Oomen and Schiere2004, Lubchansky and Tracy, Reference Lubchansky and Tracy2006), and increased biodiversity (Lemaire et al., Reference Lemaire, Franzluebbers, de Faccio Carvalho and Dedieu2014).
Research and implementation of integrated systems have been focused on row crop and forage production (Sulc and Franzluebbers, Reference Sulc and Franzluebbers2014), and there is limited work on integrating animals and vegetables (Clark and Gage, Reference Clark and Gage1996; Balkcom et al., Reference Balkcom, Reeves, Kemble, Dawkins and Raper2010). Despite the paucity of research, vegetable cropping is amenable to the integration of many livestock categories (Clark and Gage, Reference Clark and Gage1996; Balkcom et al., Reference Balkcom, Reeves, Kemble, Dawkins and Raper2010) specifically pastured poultry, as it requires minimal infrastructure and relatively low capital investment (Pesch, Reference Pesch2016). The ability to raise broilers (meat chickens) that are quick to market (Hilimire, Reference Hilimire2011b) and their size and ease of handling make broiler chickens an intriguing option for animal integration on vegetable farms. In addition to the revenue from meat, the addition of chicken manure can improve indicators of soil health such as increased total soil carbon, microbial biomass, and soil aggregate stability (Adeli et al., Reference Adeli, Tewolde, Sistani and Rowe2010). Additionally, chicken manure provides necessary crop nutrients (Chastain, Camberato, and Skewes, Reference Chastain, Camberato and Skewes1999; Demir et al., Reference Demir, Sahin, Kadioglu, Pilbeam and Gunes2010). The benefits work both ways. Significant amounts of non-saleable plant material are often left in the field after vegetable harvest. By introducing chickens after vegetable harvest, this residue becomes feed for the animals, and manure for the next season's crop (Hu et al., Reference Hu, Zuo, Wang, Pan, Zheng, Qian and Zou2011).
Organic certification of poultry requires outdoor access (Hermansen, Strudsholm, and Horsted, Reference Hermansen, Strudsholm and Horsted2004; Fanatico et al., Reference Fanatico, Pillai, Cavitt, Owens and Emmert2005; USDA, NOP 205.239(a)(1), 2000). However, the amount of space and time outdoors is not specified (Fanatico, Owens, and Emmert, Reference Fanatico, Owens and Emmert2009). In poultry production, chickens are mainly raised indoors with limited outdoor access (Fanatico, Owens, and Emmert, Reference Fanatico, Owens and Emmert2009). In contrast, pastured poultry is the seasonal production of poultry solely outdoors with constant access to pasture. Broilers intended for pasture rearing are placed outside starting at 21–28 days of age and moved to fresh pasture each day (Skřivan et al., Reference Skřivan, Pickinpaugh, Pavlu, Skřivanová and Englmaierovám2015) by way of a movable coop (Fanatico, Reference Fanatico2006). Consumption of pastured poultry products is becoming increasingly popular due to a perception that poultry raised outdoors results in a happier healthier bird (Fanatico et al., Reference Fanatico, Pillai, Cavitt, Owens and Emmert2005; Hilimire, Reference Hilimire2011c; Sossidou et al., Reference Sossidou, Dal Bosco, Elson and Fontes2011). However, minimal research has compared the welfare of pastured and indoor raised chickens, Moyle et al. (Reference Moyle, Arsi, Woo-Ming, Arambel, Fanatico, Blore, Clark, Donoghue and Donoghue2014) found no differences in the health parameter of bone strength between pasture-raised and indoor raised broiler chickens, but both broilers with with fixed housing had greater body weights than those on pasture. Liles, Bartlett, and Beckford (Reference Liles, Bartlett and Beckford2015) found little difference in stress levels of broilers raised indoors compared to those raised on pasture. In addition, chickens raised outside are exposed to temperature extremes, disease, parasite infestations and predation (Sossidou et al., Reference Sossidou, Dal Bosco, Elson and Fontes2011).
Moving poultry through vegetable fields in a pastured system would satisfy the livestock living conditions parameter of the USDA organic certification requirement (7 CFR Part 205 Subpart C 205.239). Despite the potential benefits of reintegrating livestock, food safety risks are presented if raw manure comes in contact with food crops. Research that adds to the growing body of knowledge on food safety risks associated with animal-crop integration is necessary for food safety policy, farm management, and food system stakeholder decision-making.
Given the increasing interest in, yet lack of research on, integrated vegetable-poultry systems, we designed a study to evaluate the impacts of an integrated vegetable-poultry system on soil health, food safety risks, and vegetable and chicken yield. More specifically, we tested two chicken-integration systems, vs a control vegetable cropping system with no chickens. We evaluated three hypotheses: i) integrated vegetable-chicken systems would improve soil health by increasing soil microbial biomass, microbial activity, and permanganate oxidizable carbon (C) which measures a portion of soil organic carbon thought to be easily accessible to soil microbes, and alter soil microbial function measured as a catabolic response to a suite of C-substrates (via Ecolog®), ii) Summer and autumn integration of chickens would provide a reasonable amount of time for the safe production of spring vegetables, and iii) Summer and autumn integration of chickens would increase yield and quality of spring grown vegetables. In addition, we observed growth and feed efficiency of chickens grown outdoors in movable coops with diverse forage options.
Materials and methods
The study was carried out at the Iowa State University Horticulture Research Station in Ames, Iowa (latitude 42.106778, longitude − 93.589583) on certified organic land during the 2017, 2018, and 2019 growing seasons. The soils are derived from Wisconsonan glacial till, primarily Clarion loam (fine-loamy, mixed, superactive, mesic Typic Hapludoll). The mean annual temperature for the area is 9.5 ± 1°C, mean annual precipitation is 895.3 ± 206.1 mm (Table 1).
Table 1. Total monthly rainfall (mm) and mean daily average air temperature (°C) at the research site

Experimental design
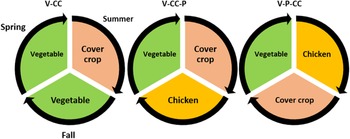
Treatments included three crop rotations (n = 3), replicated four times (n = 4) using a randomized complete block design. Each experimental plot was 4.5 m × 7.5 m in area. The rotations/treatments were vegetable-cover crop (V-CC), vegetable-poultry-cover crop (V-P-CC), and vegetable-cover crop-poultry (V-CC-P) (Fig. 1). Each of the previously mentioned rotations were assigned to one plot for the three-year duration of the study. Each crop rotation treatment was completed within the cropping season and repeated for three consecutive years. Vegetable crops were different each year to demonstrate a typical organic vegetable enterprise where there is ideally a three or more-year separation between crops within the same family. In 2017, the V-P-CC treatment was Broccoli (Brassica oleraceae var. Italica cv. ‘Belstar’, Seedway Hall, NY), Red Ranger chicken (RRC, Welp Hatchery, Bancroft, IA), and cereal rye (Secale cereale VNS, Albert Lea Seeds, Albert Lea, MN). Vegetable-cover crop-poultry (V-CC-P) was broccoli, a cover crop mixture of crimson clover (Trifolium incanatum, Green Cover Seed, Bladen, NE) and oats (Avena sativa, Albert Lea Seeds, Albert Lea, MN), and RRC. Vegetable-cover crop (V-CC) followed the same pattern as V-CC-P but instead of RRC, romaine lettuce (Lactuca sativa cv. ‘Holon’ Johnny's Seeds, Winslow, ME) was planted. In 2018 and 2019, rotations followed the same sequence except chickens were Imperial (IC, Moyer's Chicks, Quakertown, PA) and vegetable crops comprised of lettuce in V-CC-P and V-P-CC and pepper (Capsicum annuum) in V-CC in 2018 and spinach (Spinacea oleracea) as a spring crop in all treatments and carrot (Daucus carota) as an autumn crop in V-CC in 2019 (Table 2). Lettuce in 2018 and spinach in 2019 both followed the previous integration of chickens and are described here in the materials and methods. Additional vegetable and cover crop culture is described in Appendix I and Table A1.

Figure 1. Treatment rotations implemented for 2017, 2018, and 2019 growing seasons at the Iowa State University Horticulture Research Station, Ames, IA. V-CC, vegetable-cover crop rotation; V-CC-P, vegetable-cover crop-poultry rotation; V-P-CC, vegetable-poultry-cover crop rotation.
Table 2. Description of the three treatment rotations and corresponding field activities carried out at the Iowa State University Department of Horticulture greenhouses and Horticulture Research Station Ames, IA in 2017, 2018, and 2019

V-P-CC, vegetable-poultry-cover crop; V-CC-P, vegetable-cover crop-poultry, V-CC, vegetable-cover crop-vegetable.
a 2017 = Brassica oleraceae, 2018 = Lactuca sativa and Capsicum annuum, 2019 = Spinacia oleracea.
b 2017 = Red ranger chicken (RRC), 2018 and 2019 = imperial chickens (IC).
c Mixture of Trifolium incanatum and Avena sativa.
d Secale cereal.
e 2017 = Lactuca Sativa, 2019 = Daucus carota.
Lettuce and spinach production
In 2018, five romaine lettuce cultivars [Lactuca sativa cv ‘Coastal star’, ‘Paris Island’, ‘Jericho’ (Johnny's Seeds, Winslow, ME), ‘Greene Towers’, and ‘Freckles’ (High Mowing Organic Seeds, Walcott, VT)] were transplanted on 24 April 2018 to V-P-CC and V-CC-P treatments. Spinach [Spinacea oleracea cv ‘Corvair’, ‘Acadia’ (Johnny's Seeds, Winslow, ME), ‘Regiment’, ‘Butterflay’, ‘Renegade’ (High Mowing Organic Seeds, Walcott, VT)] was direct seeded to all plots on 16 April 2019. Transplant production was carried out in the Department of Horticulture greenhouses at Iowa State University, Ames, IA, and sown into 72 cell trays using an organic potting mix (Beautiful Land Products, West Branch, IA). Transplants were fertilized as needed using an organic 2N-4P-1K liquid fertilizer derived from hydrolyzed fish (Neptune's Harvest organic fertilizer, Gloucester, MA) at a rate of 7.8 ml L−1. Before planting, field plots were fertilized with granular 4N-6P-4K (Sustane Natural Fertilizer Inc. Cannon Falls, MN), and rates were determined based on soil test results and reported nutrient requirements of the particular crop. Each plot consisted of five vegetable beds 7.5 m long and spaced 1 m apart. In-row spacing between seedings within the bed was 30 cm. Two rows of lettuce transplants per bed were hand-planted at the Horticulture Research Station with 30 cm between rows and plants. Twenty-five lettuce plants per row totaled 50 lettuce plants per bed. Crops were irrigated using drip irrigation to provide 2.5 cm of water each week Organic 2–4-1 liquid fertilizer was applied using an injector (Dosatron, Clearwater, Florida) during crop growth at a rate of 200 ml L−1. Plants were monitored and sprayed as needed with DiPel Pro (Valent BioSciences Corp., Osage, IA; Bacillus thuringiensis v kurstaki) to manage lepidopteran insect pests. Spinach was directly seeded (four rows per bed with 15 cm between rows) using a Jang seeder (Jang Automation Co., Ltd. Beobwon-ro, Songpa-gu, Seoul, Korea). Lettuce harvest began on 29 May 2018 and continued weekly until 14 June 2018. Lettuce was counted and graded for marketable yield based on number and weight. Heads that had bolted or displayed tip burn were deemed unmarketable. Head length and head diameter were recorded by pulling five marketable heads from each treatment and measuring from the top of the head to the cut end and by taking two measurements at the widest point of the head. Spinach harvest started on 29 May 2019 followed by harvests on 6 and 11 June 2019 by harvesting a 1.5 m section of one center row of each of the five beds. Total and marketable yield and dry weight of spinach were recorded. Dry weight was determined according to methods described by Demir et al. (Reference Demir, Sahin, Kadioglu, Pilbeam and Gunes2010). For the sake of evaluating the effect of Chicken integration, yields of the different cultivars are pooled for a total yield per plot, and results of cultivar evaluations are not presented here.
Chicken integration and measurements
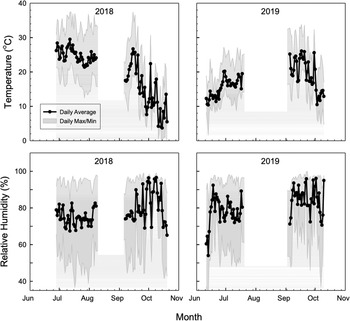
To protect chickens from predators, an electric fence was erected around the perimeter of the field each year after the spring vegetable harvest. Chickens were integrated into the V-P-CC rotation on 10 July 2017, 28 June 2018, and 11 June 2019, and removed on 30 August 2017, 8 August 2018, and 18 July 2019. Chickens were integrated into the V-CC-P rotation on 15, 7, and 6 of September 2017, 2018, and 2019, respectively, and removed on 8 November 2017, 20 October 2018, and 31 October 2019. Chickens were housed in 1.5 by 1.2 m floorless movable coops (Fig. 2) to allow them to forage on vegetables or cover crop residue. One pen per replication was used and housed 9–10 birds on average, n = 40 birds per treatment. This stocking density provided each chicken with 0.6 m of pen space common to pastured poultry systems (Liles, Bartlett, and Beckford, Reference Liles, Bartlett and Beckford2015). Broiler chicken types RRC and IC were chosen for characteristics such as a longer hock, larger leg and thigh, narrow breast, and slower growth that are thought to be more ideal for an outdoor rearing system compared to their conventional Cornish cross counterparts. Chicks were purchased on the day of the hatch from their respective hatcheries and brooded for three to four weeks at the Iowa State University Poultry Research Farm (Ames, IA), after which they were moved to the experimental field plots. Temperature and relative humidity (RH) inside the chicken houses were recorded (HOBO MX2300, ONSET, Bourne, MA) in 2018 and 2019 (Fig. 3). Chickens were provided a complete balanced ration of starter, grower, and finisher (Natures Grown Organics, Westby, WI). Chicken coops were moved daily to allow access to fresh plant material. To calculate feed conversion ratio (FCR) and average daily gain (ADG), feed consumed and body weights were recorded three times at 2-week intervals while chickens were on the plots. Feed disappearance was collected by weighing feed leftovers and calculating disappearance based on feed added. In 2017, all chickens from the pen were weighed and the weights were averaged to record a whole pen weight. In 2018 and 2019, 4–5 chickens were randomly selected from the pen for weighing to determine an average pen weight. Birds were removed from plots after foraging for 51 and 54 d in 2017, 41 and 43 d in 2018, and 37 and 55 days in 2019 for V-P-CC and V-CC-P, respectively. If health issues prevented birds from meeting their quality-of-life needs, the chicken was removed from the study. Euthanasia was performed either by the Iowa State Lab Animal Resources (LAR) team or protocol personnel according to the approved Institutional Animal Care and Use Committee (IACUC) protocol. One chicken in the summer, one in the autumn of 2017, and one chicken in summer of 2018 did not complete the study.

Figure 2. Mobile chicken coop (112.8 m2) shown with 10 chickens. Coops were utilized in poultry-integrated treatments (vegetable-cover crop-poultry and vegetable-poultry-cover crop rotation) at the Iowa State University Horticulture Research Station, Ames, IA in 2017, 2018, and 2019.

Figure 3. Air temperature and relative humidity collected from inside the chicken coop utilized for vegetable-poultry-cover crop and vegetable-cover crop-poultry rotations at the Horticulture Research Station, Ames, IA. Placed at the time of chicken integration in summer and autumn 2018 and 2019.
Soil sampling and routine soil measurements
On 3 March 2017 before the start of the study, a baseline soil sample of the whole study site was collected (Table 3). Additional soil samples were collected four times throughout the season (at planting, after harvest, mid-summer, and end of the season) and analyzed for chemical, physical, and biological properties. Mid-summer sampling coincided with the removal of chickens from V-P-CC rotation and the establishment of summer cover crops in V-CC-P and V-CC rotations. The end of season/autumn corresponded with the removal of chickens from V-CC-P. At each sampling, five 0–15 cm cores (2.2 cm diameter) were collected and composited. Soil samples were analyzed for nitrate (NO3-N), ammonium (NH4-N), phosphorus (P), potassium (K), calcium (Ca), sodium (Na), Zinc (Zn), sulfur (S), magnesium (Mg), pH, organic matter (OM), and cation exchange capacity (CEC) (Solum Laboratories, Ames, IA). In 2017, pre-season and post-harvest samples were not analyzed for Na, S, or Zn. Soil samples after harvest, mid-summer, and end of season 2018 and 2019 were also analyzed for micronutrients boron (B), iron (Fe), copper (Cu), and manganese (Mn). The methods and machines these measurements were analyzed on can be found in the Appendix (Table A2).
Table 3. Selected soil properties sampled from the whole study field at the Horticulture Research Station Ames, IA on 3 March 2017 before the start of the study at 0–15 cm

Soil labile carbon, microbial biomass, and microbial functional diversity
Analysis of labile C and microbial functional diversity was performed on samples collected at the end of the season in 2017, 2018, and 2019. The permanganate oxidizable carbon (POXC) method was used to measure the soil organic carbon (SOC) pool responsive to management practices (Weil et al., Reference Weil, Islam, Stine, Gruver and Samson-Le-big2003; Culman, Freeman, and Snapp, Reference Culman, Freeman and Snapp2012). Analysis of microbial biomass C (MBC) was performed on end-of-the-season soil samples collected in 2017 and 2018. Microbial biomass C was determined using a chloroform fumigation extraction method modified from (Vance, Brookes, and Jenkinson, Reference Vance, Brookes and Jenkinson1987).
Microbial functional diversity was assessed using a community-level physiological profile (CLPP) by Sole-C-source utilization of culturable heterotrophic soil microbes characterized by Biolog-EcoPlate (BIOLOG Inc., CA, USA) with methods described by Nair and Ngouajio (Reference Nair and Ngouajio2012). Analysis of functional diversity included substrate richness and average well color development (AWCD). Substrate richness is the number of substrates utilized by soil microbes in each sample and is a count of the positive optical density (OD) measurements. The average well color development is a combined measure of the diversity and abundance of soil microbes calculated from each sample on days 1–7 using the following equation:
Presence and absence of soil pathogens
To determine the food safety risks of planting vegetable crops after chicken integration soil samples were collected on 6 November 2018 and 8 April and 5 November 2019 and analyzed for the presence and absence of E. coli 0157: H7 and Salmonella spp. using an ELFA method (Enzyme Linked Fluorescent Assay; SPR and VIDA bioMérieux SA Chemin de l'Orme 69280 Marcy-l'Etoile – France; Razzuoli et al., Reference Razzuoli, Vencia, Fedele, Mignone, Lazzara, Rubini, Vito, Porcario, Bozzetta and Ferrari2018). Additionally, spinach samples were collected on 5 June 2019 and the surface was tested for the presence of E. coli 0157: H7 and Salmonella spp. A 1000-g spinach sample was collected for each treatment plot from three replications.
Statistical analysis
Soil data was analyzed using proc mixed (SAS). LSMEANS ADJUST = TUKEY was used to determine the significance of treatment and year × treatment interactive effects between rotations at a given sampling time (α = 0.05). Vegetable yield and quality data from the lettuce and spinach crops were analyzed using proc glimmix to determine the effect of rotation on lettuce and spinach yields. Only these two crops were examined in this paper as these crops followed the integration of chickens. Yields of individual cultivars were pooled to analyze the total yield for each rotation.
Soil microbial substrate use data (Ecolog® plates) were analyzed using principal components analysis in R (v3.4.3) using the vegan package (Oksanen, Reference Oksanen, Blanchet, Kindt, Legendre, Minchin, O'hara, Simpson, Solymos, Stevens, Wagner and Oksanen2013). A correlation matrix was used in prcomp function. For ease of interpretation, the 31 C substrates (Table 4) were grouped into five categories or types based on chemical class – amines/amides, amino acids, carbohydrates, carboxylic acids, and polymers. Principal components (PC) one, two, and three were visualized and ANOVAs run on each PC using aov function because these were the three most important PCs (93% of total variance). In addition, all PCs were analyzed using manova function to determine any treatment effects on overall microbial catabolism.
Table 4. Biolog EcoplateTM carbon source guild groupings

Results
Soil nutrients and chemical/physical properties
There were no significant differences among treatments in any of the measured soil properties at planting in 2017 and 2019, after harvest in 2017, and end of season 2018 (Table 5).
Table 5. Soil macro and micronutrients, and selected soil properties at four sampling dates over three years for each rotation

Collected at 0–15 cm at the Iowa State University Research Station Ames. IA.
† At planting = planting of spring vegetable; after harvest = immediately after harvest of spring planted vegetable; mid-summer = at the time of removal of chickens from V-P-CC rotation and the establishment of summer cover crops in V-CC-P and V-CC rotations. Rotations: V-CC = vegetable-cover crop, V-CC-P = vegetable-cover crop-poultry, V-P-CC = vegetable-poultry-cover crop.
* Values with different letters are significantly different P < 0.05.
−Indicates that this variable was not tested at a particular sampling time.
Not surprisingly, nitrogen, especially NO3–N was the most dynamic nutrient analyzed and fluctuated with the integration of chickens. The response variable NO3–N was significant for treatment (P = 0.0237, 0.0073 and, 0.0045 at planting, after summer chicken removal, and after autumn chicken removal, respectively). NO3–N was also significant for the year (P < 0.0001 all sampling times) and the interaction of treatment and year (P < 0.0001) at planting and end of the season. Most of the variation in NO3–N at planting can be attributed to year as NO3–N was higher in V-P-CC in 2018 as compared to both V-CC and V-CC-P (P = 0.001 and <0.001, respectively). After removal of chickens from V-P-CC in 2018 and 2019 V-P-CC was on average 2 times higher in NO3–N as compared to V-CC and V-CC-P, respectively. After the removal of chickens from V-CC-P in the autumn of 2019, V-CC-P was 2.1 and 1.8 times higher in NO3-N as compared to V-CC and V-P-CC, respectively. When looking at the response variable P, there was a significant year effect at all sampling times (P < 0.0001) and a significant treatment by year interaction after autumn chicken removal only (P = 0.0152). On average, P increased from 2017 to 2019. Mean P across all treatments were 53.7, 84.9, and 96.9 mg kg−1 soil for 2017, 2018, and 2019, respectively, a 45% change from 2017 to 2018 and 13.2% change from 2018 to 2019.
Permanganate oxidizable carbon, microbial biomass, and microbial functional diversity
Both POXC and MBC did not show any significant interactions between treatments and time of sampling. Permanganate oxidizable C showed high intra-annual variability, and some differences between years (Fig. 4). However, there was no main effect of chicken integration on POXC. Microbial biomass carbon (MBC), however was more dynamic (Fig. 5) and increased from summer 2017 to autumn 2018 from 219.75 to 303.23 mg C kg−1. Integrating chickens increased MBC by 25%, on average between both treatments across all sampling dates, compared to the V-CC control (P = 0.042).

Figure 4. Soil permanganate oxidizable carbon, measured from soil samples collected in autumn after removal of chickens from V-CC-P for years 2017, 2018, 2019. V-CC, Vegetable-cover crop; V-CC-P, vegetable-cover crop – poultry; V-P-CC, vegetable-poultry-cover crop. Letters indicate statistical significance at P ≤ 0.05. Error bars indicate standard error.

Figure 5. Microbial biomass carbon (MBC) measured from soils collected – after harvest (summer) and end of season (autumn) for years 2017 and 2018. V-CC, vegetable-cover crop; V-CC-P, vegetable-cover crop – poultry; V-P-CC, vegetable-poultry-cover crop. Letters indicate statistical significance at P ≤ 0.05. Error bars indicate standard error.
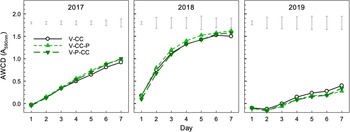
Average well color development (AWCD) of Biolog-EcoPlateTMC substrates over a 7-d period is an indicator of microbial activity. While there were significant differences in AWCD dynamics by date (Fig. 6), this was not of interest. We wanted to test if there were treatment differences and did not find any differences amongst treatments for AWCD nor dynamics. Multivariate analyses of the substrate usage using PCA also emphasized the strong effect of year of sampling on microbial substrate usage (Fig. 7). The MANOVA of all PCs also showed a strong effect of year (P < 0.001), but no treatment effect likely owing to overwhelming effect of year. Examining nuanced treatment effects on individual PCs 1 to 3 (94% of total variation), however, did show treatment effects on PC2 (P = 0.020). Vegetable-poultry-cover crop (V-P-CC) soils were significantly different than V-CC (P = 0.028) at PC2, which corresponds to carbohydrates and amino acids respectively. Vegetable-cover crop (V-CC) preferentially used carbohydrates, compared to V-P-CC, which corresponded to greater amino acid usage.

Figure 6. Average well color development (AWCD) during Biolog-EcoPlate™ incubation. Measured from soil samples collected in autumn after removal of chickens from V-CC-P for years 2017, 2018, 2019. V-CC, vegetable-cover crop; V-CC-P, vegetable-cover crop – poultry; V-P-CC, vegetable-poultry-cover crop. Floating error bars represent Fisher's least significant differences.

Figure 7. Principal component analysis (PCA) of substrate use via Biolog-EcoPlate™. Data separated by treatments and three years (2017, 2018, 2019). Vectors are simplified with substrate classes, and visualized as (a) PC1 and PC2, and (b) PC2 and PC3. Percentage of variation explained in parentheses of PC. V-CC, vegetable-cover crop; V-CC-P, vegetable-cover crop – poultry; V-P-CC, vegetable-poultry-cover crop.
Presence and absence of soil pathogens
Twelve soil samples (one from each rotation replication) were analyzed for the presence of E. coli O157:H7 and Salmonella spp. on 2 November 2018, 8 April, and 5 November 2019. All soil samples were negative for Salmonella spp. for all days and treatments except one V-CC sample on 2 November 2018 (Table 6). For E.coli, all soil samples from 2 November 2018 were negative while on 8 April 2019, all samples were confirmed positive. On 5 November 2019, one sample from V-P-CC, and two samples from V-CC were positive (Table 6). All spinach samples from 5 June 2019 were negative for E. coli O157:H7 and Salmonella spp. (Table 6).
Table 6. Soil and spinach samples collected from all rotations at the Iowa State University Horticulture Research Station Ames, IA and analyzed for presence of E. coli O157:H7 and Salmonella spp. using the standard testing protocols for mini Vidas ECPT® and SPT®

Rotations are V-CC, vegetable-cover crop; V-CC-P, vegetable-cover crop-poultry; V-P-CC, vegetable-poultry-cover crop; ND, None Detected.
Lettuce and spinach yield
Lettuce heads grown in V-P-CC and V-CC-P rotations in 2018 following the 2017 chicken integration were not different in total weight, total number, dry weight, or head length of lettuce heads (Table 7). The marketable weight of lettuce heads and head diameter was higher for V-CC-P as compared to V-P-CC. There was no difference among treatments in spinach total and marketable yield, or dry weight in 2019 (Table 8) following chicken integration in 2018.
Table 7. Total weight (kg), total number, marketable weight (kg) and marketable number, dry weight (g), head length (cm), and head diameter (cm) from lettuce harvested in June 2018 from chicken integrated rotations located at the Iowa State Horticulture Research Station, Ames IA

V-P-CC, vegetable-poultry-cover crop; V-CC-P, vegetable-cover crop-poultry; ±, standard deviation from the mean.
Values with the same letters are not statistically different at P < 0.05.
Each plot included five beds of lettuce with two rows of 25 plants totaling 50 plants per bed.
a Head dry weight, length and diameter is the average of five marketable heads.
Table 8. Total, marketable, and dry weight (kg) of spinach harvested from all rotations V-P-CC. V-CC-P, and V-CC from May to June 2019 at the Iowa State University Horticulture Research Station Ames, IA

Rotations are V-CC, vegetable-cover crop; V-CC-P, vegetable-cover crop-poultry; V-P-CC, vegetable-poultry-cover crop Data was collected from a 1.5 m section of one center row of each of the five beds totaling 7.5 m harvested; ±, standard deviation from the mean.
Chicken FCR and ADG
Feed conversion ratios of slow growing broiler chickens of both V-P-CC and V-CC-P treatments were calculated based on the weight of starter, grower, and finisher feed consumed and the weight of chickens. Feed conversion ratios ranged from 2.43 for V-P-CC in 2018 to3.25 for V-CC-P in 2017 and 2019 measured at 70–75 days. Our FCR values reported are similar to those reported for FCR of chickens on pasture in the literature (2.79–3.06 at 65–82 d of age; Gordon and Forbes, Reference Gordon and Forbes2002; Mikulski et al., Reference Mikulski, Celej, Jankowski, Majewska and Mikulska2011). Mean separation comparing birds raised in the summer compared to autumn showed that FCR tended to be lower for birds in V-P-CC compared to V-CC-P (Table 9) indicating better conversion of feed to meat in summer. Average Daily Gain varied from 0.07 to 0.09 kg per day (Table 9).
Table 9. Feed conversion (FCR) and average daily gain (ADG) for chickens integrated into V-CC-P and V-P-CC rotations in the summer and fall for 2017, 2018, 2019 at the Iowa State University Horticulture Research Station Ames, IA

V-CC-P, vegetable-cover crop-poultry; V-P-CC, vegetable-poultry-cover crop, ±, standard deviation.
* Calculated using the average feed consumed over the average final live weight.
□ Calculated based on total weight gained from the first day on the plot divided by the number of days on the plot. 2017 = red ranger chickens (Welp hatchery, Bancroft IA) and 2018 and 2019 = Imperial (Moyer's Chicks, Quakertown, PA.
Values with the same letters are not statistically significant at P ≤ 0.05.
Discussion
This study examined soil health, food safety, vegetable yield and quality, and poultry feed efficiency in a chicken-vegetable crop integrated system. The hypotheses were that soil health would improve under chicken integration and that integrating chickens in the autumn would provide enough residual N for the vegetable crop the following spring. Yields of vegetables grown following chicken integration would be higher. Integrating chickens with a long rotation would provide little food safety risks if vegetables were not grown directly after chickens.
Hypothesis (i) impacts on soil health
Chicken manure as a fertility source can provide all 13 essential plant nutrients typically derived from soil (Chastain, Camberato, and Skewes, Reference Chastain, Camberato and Skewes1999). Levels of NO3–N were higher in soil samples directly after chicken removal, especially after removal of chickens in the summer of 2018 and 2019, as well as removal of chickens in the autumn of 2019. We observed elevated levels of NO3–N at planting in 2018 but this was the only year we saw high levels of residual N from the previous year. whereas expected and as measured in other studies increases in N were present within poultry-integrated systems (Miao et al., Reference Miao, Glatz, Ru, Wyatt and Rodda2005; Hilimire, Gliessman, and Muramoto, Reference Hilimire, Gliessman and Muramoto2012). Nitrogen levels also varied based on season and production system, a phenomenon also measured by Rudisill et al. (Reference Rudisill, Bordelon, Turco and Hoagland2015). Chickens were on plots for 54 days in the autumn of 2017 but only 43 days in the autumn of 2018, which may have contributed to the high residual nitrate in the spring of 2018 but not the spring of 2019. If growers are to implement integrated vegetable crop systems similar to this study where chickens follow vegetables, additional research to determine optimum stocking density and time to provide adequate residual N is needed.
Although increased Phosphorus is common following the application of manure (Rudisill et al., Reference Rudisill, Bordelon, Turco and Hoagland2015), there was no significant difference among all three treatments in levels of P. P increased each year in all treatments regardless of chicken integration. This increase in P was likely due to the application of poultry-derived pre-plant fertilizer used at the beginning of each season in all treatments. Although poultry manure can increase soil fertility, the nutrient composition of the poultry manure is largely affected by the diet and age of the animals (Lorimor, Powers, and Sutton, Reference Lorimor, Powers and Sutton2004) therefore, applying poultry manure by the integration of animals may give inconsistent results. Growers should use soil tests each year to determine P requirements when using an integrated system to avoid over application of P.
Statistically significant differences in percent organic matter were only observed in 2017 at mid-summer (3.6, 3.2, and 3.1% for V-CC, V-CC-P, and V-P-CC, respectively) and end-of-season sampling (3.2, 3.4, and 3.1% V-CC, V-CC-P, and V-P-CC, respectively). Permanganate oxidozable carbon has been used as an indicator of the SOC fractions likely to be affected by management practices. Cover crops and animal manures have improved active carbon (Blair and Crocker, Reference Blair and Crocker2000; Rudisill et al., Reference Rudisill, Bordelon, Turco and Hoagland2015; Butler, Bates, and Eichler Inwood, Reference Butler, Bates and Eichler Inwood2016). POXC increased from 2017 to 2018 across all treatments but was not different from 2018 to 2019. SOC can be influenced by crop and cover crops, rotation, and tillage practices (Jagadamma et al., Reference Jagadamma, Essington, Xu and Yin2019), but increases in SOM can be slow, and incremental. For example, it took 15 years to observe appreciable increases in organic matter under the application of dairy manure (Verlinden et al., Reference Verlinden, McDonald, Kotcon and Childs2017). Our study examined soil sample cores of 0–15 cm but short-term changes in soil organic matter (SOM) may be more readily observed in the 0–5 cm depth (Acosta-Martínez, Zobeck, and Allen, Reference Acosta-Martínez, Zobeck and Allen2004). Additionally, our system, as well as many other intensive and diversified organic vegetable farms, use frequent tillage to turn over crops, prepare beds, and to cultivate, which may have led to the breakdown of soil carbon more quickly than it could be generated. Intensive tillage negatively affects measures of soil health (Butler, Bates, and Eichler Inwood, Reference Butler, Bates and Eichler Inwood2016), and could have reduced the benefits observed from integrating chickens. Total C was 40% higher under a perennial pasture integrated system compared to a continuous cotton cropping system (Acosta-Martínez, Zobeck, and Allen, Reference Acosta-Martínez, Zobeck and Allen2004).
Soil microbes are the mediators for critical nutrient cycling and are sensitive to changes in management practices and can be observed before other soil health parameters are detected (Adeli et al., Reference Adeli, Tewolde, Sistani and Rowe2010). Poultry litter as well as diverse cropping systems has increased MBC (Acosta-Martínez, Zobeck, and Allen, Reference Acosta-Martínez, Zobeck and Allen2004; Adeli et al., Reference Adeli, Tewolde, Sistani and Rowe2010; Nair and Ngouajio, Reference Nair and Ngouajio2012). Microbial biomass C increased from summer 2017 to autumn 2018 for all treatments (219.7 to 303.2, a 32% change). Treatment differences occurred only in the autumn 2018 sampling time. Inconsistent results in MBC may be due to an environmental effect or that crop type is more important on the effect of microbiological and biochemical soil properties (Acosta-Martínez, Zobeck, and Allen, Reference Acosta-Martínez, Zobeck and Allen2004). Minimal changes in MBC may also be due to the complete removal of vegetation when chickens were in place resulting in a reduction in substrates for microbial growth (Bucher and Lanyon, Reference Bucher and Lanyon2005; Acosta-Martínez et al., Reference Acosta-Martínez, Bell, Morris, Zak and Allen2010). After seven years of crop-livestock integration, MBC was higher than continuous cotton (Acosta-Martínez, Zobeck, and Allen, Reference Acosta-Martínez, Zobeck and Allen2010).
Catabolic activity increased after incubation regardless of rotation. Although there were no differences in the number of different substrates utilized (richness) or the uniformity at which they were utilized (eveness) this does not provide information about specific substrates utilized (Zak et al., Reference Zak, Willig, Moorhead and Wildman1994). The multivariate approach of principal component analysis can be used to cluster utilized substrates with treatments to get an idea of the substrate catabolic activity of a community (Zak et al., Reference Zak, Willig, Moorhead and Wildman1994). There were some differences in substrate utilization based on rotation and communities. Vegetable-cover crop (V-CC) preferentially used carbohydrates, compared to V-P-CC, which corresponded to greater amino acid usage. Vegetable-cover crop-poultry (V-CC-P) plots were cover cropped with oats and crimson clover, V-P-CC with cereal rye. The choice of cover crop species can be a strong determinant of the microbial functional diversity of an agroecosystem (Kim et al., Reference Kim, Zabaloy, Guan and Villamil2020), other research points to the organic amendment being stronger (Nair and Ngouajio, Reference Nair and Ngouajio2012) as well as the combination of both (Bucher and Lanyon, Reference Bucher and Lanyon2005; Gu et al., Reference Gu, Guo, Cai, Zhang, Wu, Li, Zhang and Yang2018). Other possible factors could include pre-plant fertility sources of manure origin used in all rotations.
Hypothesis (ii) food safety risks
E. coli 0157:H7 and Salmonella spp. are common perpetrators of foodborne illness. Found in the GI tract of animals, their manure is a common vector. E. coli 0157:H7 and Salmonella spp. can become present at harmful levels in the soil when raw manure is applied (Ingham et al., Reference Ingham, Losinski, Andrews, Breuer, Breuer, Wood and Wright2004). E. coli O157:H7 and Salmonella spp. sampling was negative for all plots but one V-CC plot in 2018. All plots sampled at spring planting 2019 were positive for E. coli O157:H7. Only select plots were positive for E. coli O157:H7 at the end of the season in autumn 2019. Spinach samples had no pathogens detected when the surfaces of spinach samples were tested in the spring of 2019. While the source of the E. coli O157:H7 is not known it was shown to be present prior to the poultry integration. Seasonal variations in E. coli O157:H7 can be due to wild animal migration, irrigation water contamination, or contamination from personnel, equipment, or tools (Solomon, Yaron, and Matthews, Reference Solomon, Yaron and Matthews2002).
Food Safety Modernization Act Produce Safety Rule (FSMA PSR) regulations encourage the use of applying only treated or composted manure and waiting a sufficient amount of time before planting or harvesting from fields where manure has been applied. Application of biological soil amendments of animal origin (BSAAO) (manure or compost) must be done in a way to minimize contact with the edible portion of covered produce (21 CFR subpart F. 112.56). Currently, there is no recognized required waiting time between the application of BSAAO and harvest of produce, thus any produce that comes into contact with raw BSAAO must be discarded and seen as adulterated (FDA 2015: FSMA PSR 21 CFR subpart I 112.83). The National Organic Program (NOP) regulations require at least 120 days between application of non-composted manure and harvesting when edible portions of organic crops come in contact with soil (USDA 2000: NOP 7 CFR 205.203). Studies that examined the application of fresh manure to fruit and vegetable plots have found indigenous E. coli persisted after ≤120 days of application (Ingham et al., Reference Ingham, Losinski, Andrews, Breuer, Breuer, Wood and Wright2004; Ingham et al., Reference Ingham, Fanslau, Engel, Breuer, Breuer, Wright, Reith-Rozelle and Zhu2005). Although E. coli O157:H7 was detected in all plots in the spring of 2019 and select replications of plots in the autumn of 2019 and Salmonella spp. being found in one plot in 2018, no pathogens were detected on the spinach crop when leaf surfaces were tested. Further research in this area to determine better mitigation strategies in the midst of positive soil tests is needed, although testing is not required by growers.
Hypothesis (iii) vegetable yield and poultry growth
In 2018, lettuce harvested off V-CC-P had higher marketable yields in number (38% >) and weight (37% >) and larger head diameter (22% >) than those grown in V-P-CC plots. Due to the high levels of residual N in the V-CC-P plots at planting in 2018 fertilizer rates were reduced to compensate. The residual N may still have been more readily available and contributed to the higher marketable yields. In 2019 there was no difference in spinach yield for any of the rotations.
Feed conversion ratios for pastured broilers have been reported between 2.79 at 65 d of age (Mikulski et al., Reference Mikulski, Celej, Jankowski, Majewska and Mikulska2011) and 3.06 at 82 d of age (Gordon and Forbes, Reference Gordon and Forbes2002). Our results are similar to those reported (2.43 to 3.25 at 70–75 d of age). Summer-integrated birds had lower FCR than autumn-integrated birds. Higher FCR in the autumn may be due to cold temperatures and cold stress (Campo, Prieto, and Dávila, Reference Campo, Prieto and Dávila2008). High FCR could be attributed to the difference in quality of available forages (oats and crimson clover for autumn-integrated chickens, vegetable residues for summer-integrated chickens). Legumes over grasses may be preferred (Woo-Ming et al., Reference Woo-Ming, Arsi, Moyle, Gaunsalis, Owens, Clark, Fanatico, Upadhyay, Donoghue and Donoghue2018), but there is little research on comparison of vegetable residues. High FCR in the Red Ranger or Imperial chickens in a pasture-type system may also be due to less feed consumption overall. Lorenz, Kany, and Grashorn (Reference Lorenz, Kany and Grashorn2013) found that slower-growing chickens on pasture had less feed in their crops and gizzard, but higher pasture contents than fast-growing chickens. Forage intake reduces feed efficiency in chickens, as their ability to digest and ferment fibers in an appreciable way is reduced compared to other monogastric animals (Tufarelli, Ragni, and Laudadio, Reference Tufarelli, Ragni and Laudadio2018). Chickens raised outside are exposed to heat, cold, and predation that could alter the success of the system. Continued evaluation of chicken-vegetable crop systems is needed to determine what factors promote success. Studies should compare the FCR of birds on different vegetable and cover crop forages at different integration times. Additionally, studies should further assess the stress levels of chickens raised in pastured conditions such as those reported by Campo, Prieto, and Dávila (Reference Campo, Prieto and Dávila2008), Liles, Bartlett, and Beckford (Reference Liles, Bartlett and Beckford2015)).
Conclusions
The goal of organic vegetable production is to build healthy soils and to strive for on farm nutrient cycling. Organic vegetable production systems require diversity to be resilient. Integrating chickens along with other typical organic practices, such as cover crops and crop rotation, has the potential to improve soil health indicators such as microbial biomass, with little-to-no effect on productivity of vegetables. An optimal rotation that includes chickens would offer residual nitrogen, eliminate off-farm fertilizer sources, and minimize food safety risks while providing additional sources of income. Integrating chickens in the autumn for at least 54 days or increasing chicken stocking density has promised to provide growers residual nitrogen to a cash crop the following spring. However, poultry feed is often an off-farm input and should be considered when determining the true N input of this system. Chickens reared on cover crops and vegetable crop residues in the summer and early autumn appear to be able to withstand heat and have similar efficiency to chickens raised on more typical pasture systems. Economic analyses on the economic viability of pastured poultry integration are needed as the profit margin for pasture poultry production is thin, with small scale growers often only netting $0.57–0.98 per pound above variable costs (Painter et al., Reference Painter, Myhre, Bary, Cogger and Jemmett2015; Dasgupta and Bryant, Reference Dasgupta and Bryant2016).
Integrating chickens comes with food safety risks as chickens can introduce harmful pathogens to the soil especially E. coli and Salmonella spp. The FDA is currently working on assessing the risk between the timing of manure application and vegetable harvest. In the meantime, growers are encouraged to follow the NOP guidelines for manure application. The recommendation is to wait 120 days or more from the time of application to harvest. A 120-day or more wait period from animal removal to harvest would be comparable. Plots without chickens can become contaminated from plots with chickens from windblown feces and growers can take additional precautions such as providing distance between plots where animals are housed and those that do not. Additional precautions to reduce the risk of contamination include not growing produce that comes in contact with manured soil and using physical barriers between the soil and the crop.
Integration of chickens into vegetable cropping systems has the ability to increase soil health indicators and soil NO3–N, but food safety measures must be taken so that raw consumed produce does not become adulterated. Additional research on vegetable–animal integration is required to determine maximum benefit to the producer, the animals, and the environment. Long-term studies are needed to gain insight into carbon and nutrient cycling. An optimal vegetable-poultry integrated system would build soil health, promote the health of chickens, and minimize P accumulation and death of soil cover (as this could affect microbial biomass growth). Diversifying vegetable production operations with chicken integration could be one avenue to improving diversity and resilience on organic vegetable farms. Although economic feasibility and food safety considerations should be examined further, integrated systems should also be evaluated by the environmental and social services provided, such as reduction of energy used to transport fertilizer from manufacturer to farm, reduction of nutrient leaching, increased farm biodiversity, and farms that build and involve the local community.
Acknowledgements
Thank you to all that provided guidance and insight; the staff at the Horticulture Research Station, Cameron Hall, at the ISU Poultry Science Farm, and ISU LAR on-call veterinarians.
Funding statement
This work was supported by the North Central Sustainable Agriculture and Research and Education Service (NC-SARE) [GNC-17-236]. 120 BAE, University of Minnesota|1390 Eckles Avenue | St. Paul, MN.
Competing interests
None.
Appendix I: Details of each treatment rotation used in the study by rotation are described below.
Vegetable-cover crop (V-CC)
2017
On 3 March spring of 2017 in the Department of Horticulture greenhouses at Iowa State University, Ames, IA. Broccoli (Brassica oleraceae var. Italica cv. ‘Belstar’, Seedway Hall, NY) was sown into 72 cell trays using an organic potting mix (Beautiful Land Products, West Branch, IA). After emergence, broccoli plants were thinned to one seedling per cell. Broccoli transplants were fertilized as needed using an organic 2N-4P-1K liquid fertilizer derived from hydrolyzed fish (Neptune's Harvest organic fertilizer, Gloucester, MA) Broccoli transplants were grown for six weeks. Six-week-old broccoli transplants hand planted on 17 April 2017 at the Horticulture Research Station into 4.5 × 7.5 m plots. Plots were set up with five beds each. Bed length was 7.5 m long with 30 cm between plants and 1 m between beds. After planting broccoli was fertilized with 2N-4P-1K every other week through a fertilizer injector (Dosatron, Clearwater, Florida). Plants were monitored and sprayed as needed with DiPel Pro (Bacillus thuringiensis v kurstaki, Valent BioSciences Corp., Osage, IAs) to protect against cabbage looper (Trichoplusia ni) and imported cabbageworm (Pieris rapae). June was unseasonably warm and broccoli did not perform well. Broccoli was harvested on 1 July 2017 by cutting broccoli heads at the stem to leave a 3.5–10 cm stalk. All broccoli was deemed as unmarketable due to insufficient head size or discoloration. After harvest, On 11 July 2017, V-CC rotations were planted to a cover crop mixture of crimson clover (Trifolium incarnatum, Green Cover Seed, Bladen, NE) and oats (Avena sativa, Albert Lea Seeds, Albert Lea, MN) at a rate of 112 and 33.5 kg ha−1, respectively by broadcasting by hand and lightly raking to incorporate. Overhead sprinkler irrigation was used. On 12 September 2017, crimson clover and oat biomass were collected by placing a 25 × 25 cm quadrat four times randomly throughout the plot and cutting all aboveground growth within the quadrat. Biomass was placed in a 67°C oven to dry down to a constant weight. On 17 August 2017, romaine lettuce (Lactuca sativa cv. ‘Holon’ Johnny's Seeds, Winslow, ME) was seeded in 72 cell flats using the same materials and methods as previously mentioned for broccoli transplants. On 13, September 2017 V-CC plots were tilled, fertilizer was hand broadcasted and incorporated. Lettuce was transplanted into beds of double rows five rows per plot. Plants were spaced 30 cm apart in all directions with plants in the opposite row staggered. Lettuce heads were harvested on 9 November 2017. The lettuce did not reach marketable size but was graded for quality (data not presented).
2018
On 16 March 2018, five cultivars of pepper (Capsicum annuum cv. ‘Sweet Chocolate’, ‘Milena’, ‘King of the North’, ‘California Wonder’, and ‘Golden California Wonder’) were seeded in 288 cell trays using an organic medium (Beautiful land products, West Branch, IA) in the Department of Horticulture greenhouses (pepper seedlings were later repotted into 50 cell flats). Peppers were transplanted into V-CC plots on 16 May 2018 in single rows, which were 1 m apart. Spacing between plants within a row was 46 cm between plants. Three days after transplanting crimson clover (134 kg ha−1) was seeded between rows of peppers and mowed regularly to suppress weeds. Peppers were irrigated through drip irrigation and weeded (within the row) and scouted regularly following recommended organic production practices. Peppers were harvested weekly starting on 17 July 2018 and continued until 26 September 2018. Peppers were graded for marketability based on the size and presence/absence of abiotic and biotic disorders (data not presented). Aboveground biomass was collected from the cover crop between the rows using methods explained previously.
2019
In 2019, preplant fertilizer was applied using 4-6-4 (Sustane Natural Fertilizer Inc. Cannon Falls, MN) and plots were tilled. On 1directlyl 2019, spinach [Spinacea oleracea cv. ‘Corvair’, ‘Acadia’ (Johnny's Seeds, Winslow, ME), ‘Regiment’, ‘Butterflay’, ‘Renegade’ (High Mowing Organic Seeds, Walcott, VT)] was direct seeded using a Jang seeder (Jang Automation Co., Ltd. Beobwon-ro, Songpa-gu, Seoul, Korea). Beds had four rows spaced 15 cm apart. The crop was irrigated using drip irrigation and hand weeded as needed throughout the growing season. Spinach harvest started 29 May 2019 followed by harvests on 6, June and 11, June 2019 by harvesting a 1.5 m section of one center row of each of the five beds. The total and marketable yield of spinach was recorded along with the dry weight of spinach. Spinach was deemed unmarketable if it was yellowing or starting to bolt. Most spinach graded as unmarketable was placed in the bolting or yellowing category in the final harvest. Dry weight was recorded by drying down all marketable spinach from 1.5 m section was dried to constant weight at 67°C and weighed for determination of dry weight. After spinach harvest cover crops were seeded as in 2017. Oats and crimson clover did not establish well and it was reseeded with buckwheat on 7 August 2019. Carrots (Daucus carota cv. ‘Miami’, ‘Nantes Fancy’, ‘Napoli’, ‘Negovia’, ‘Yaya’) were directly seeded after the destruction of the summer cover crop on 7 August 2019 using the same methods as for spinach. All rows of carrots were harvested on 30 October 2019. Carrots tops were removed and then graded based on marketability. Carrots were deemed unmarketable if they fell into the categories of forked, cracked, damaged by rodents, or small. Five marketable carrots were pulled to determine the average length and shoulder diameter. The same five carrots were set aside and sliced put through a juicer and the juice were analyzed for brix. The juice was filtered through cheesecloth and three readings were collected and averaged.
Vegetable-cover crop-poultry (V-CC-P)
2017
The V-CC-P rotation followed the same sequence as in V-CC previously mentioned with the production of broccoli followed by the establishment of summer cover crop. On 15 September 2017, red ranger chickens (RRC, Welp Hatchery Bancroft, IA) were placed on the cover crop in V-CC-P using plots using 1.5. × 1.2 m floorless movable coops to allow them to forage where they remained for ten weeks and were removed on 8 November 2017.
2018
On 8 March, five cultivars of organic romaine lettuce [‘Freckles', ‘Green Towers’ (High Mowing Organic Seeds, Walcott, VT), ‘Jericho’, ‘Coastal Star’, and ‘Paris Island’ (Johnny's Seeds, Winslow, ME)] were seeded in 72 cell trays using methods previously described for broccoli production in 2017. Field plots were prepared for transplanting by applying N using 4N-6P-4K (Sustane Natural Fertilizer Inc. Cannon Falls, MN) and plots were tilled, and lettuce was transplanted. Plot establishment and management followed methods previously described for broccoli in 2017. Lettuce harvest began on 29 May 2018 and continued once a week until 14 June 2018. Lettuce was counted and graded for marketable yield based on number and weight bolted heads, number, and weight of heads with tip burn. Head length and head diameter were recorded by pulling five marketable heads from each treatment and measuring from the top of the head to the cut end and by taking two measurements at the widest point of the head.
Summer cover crop was established as previously mentioned for V-CC rotations. Chickens were integrated into the standing cover crop mixture of oats and crimson clover on 7 September 2018 and removed on 20 October 2018. Imperial chickens (IC, Moyer's, Quakertown, PA) were used in place of RRC. Three replications of V-CC-P coops housed ten chickens and one housed 11.
2019
The V-CC-P rotation followed the same sequence as in V-CC previously mentioned with the production of spinach followed by the establishment of summer cover crop. Chickens (IC) were integrated into the standing cover crop mixture of buckwheat on 6 September 2019 and removed on 31 October 2019.
Vegetable-poultry-cover crop (V-P-CC)
2017
The V-P-CC rotation followed the same methods of broccoli establishment, production, and harvest as previously mentioned for V-CC and V-CC-P. On 10 July 2017 chickens (RRC) were placed on plots. Chickens were 37 days old. Due to mortalities during brooding only 38 of 40 chicks were available for the study. Ten birds were placed in reps one and four, nine were placed in reps two and three. Chickens remained on plots for nine weeks and were removed on 30 August 2017. After chicken removal plots were tilled and cereal rye (Secale cereal, cv Variety not stated, Albert Lea Seed, Albert Lea MN) was hand broadcast seeded at 112 kg ha−1.
2018
On 19 April, 2018 rye biomass from V-P-CC plots was collected. Rye biomass was dried weighed and ground to 1 mm using a Thomas Wiley Laboratory Mill (Thomas Scientific, Philadelphia PA). The biomass from all four replications was combined to form one composite sample, which was sent to Ward Laboratories (Kearney, NE) for analysis of total C and N.
Lettuce production was carried out as mentioned previously for V-CC-P in 2018. On 28 June 2018, 3-week-old imperial chickens were introduced into V-P-CC plots. Coops housed ten chickens in rep one, eight in rep two, and nine chickens in reps three and four. Chickens were removed on 8 August 2018 followed by fall cover crop as previously mentioned in 2017.
2019
In 2019, rye biomass was collected on 15 April 2019 using methods previously described. Spinach production was carried out as mentioned previously for V-CC-P in 2019. On 11 June 2019, 4-week-old chickens (IC) were introduced into plots and coops housed 10 birds in each replication. Chickens were removed on 18 July 2019 followed by fall cover crop as previously mentioned in 2018.
Cover crop biomass & carbon and nitrogen contents
Each year V-CC-P and V-CC rotations were seeded to oats and crimson clover, seeding rate of 112 and 33.5 kg ha−1, respectively. Seeds were broadcast by hand and raked into the soil. Overhead irrigation was used to ensure proper germination and establishment of cover crops. In 2018, when peppers were grown in the V-CC rotation, crimson clover was inter-seeded between the rows at 134 kg ha−1. In 2019, Oats and crimson clover did not establish well and V-CC-P rotations were reseeded with buckwheat on 6 August 2019. In all years, cereal rye was hand broadcasted and incorporated at 112 kg ha−1 in V-P-CC rotation. Aboveground cover crop biomass for oats and crimson clover was collected from V-CC plots in 2017, V-CC-P in 2018, and from both V-CC-P and V-CC in 2019 by placing four 25 × 25 cm quadrats randomly throughout the plot and cutting all above-ground growth within the quadrat. Cereal rye biomass was collected at the start of each season in 2018 and 2019 using the methods previously mentioned. Cover crops were dried and ground to 1 mm using a Thomas Wiley Laboratory Mill (Thomas Scientific, Philadelphia PA) and analyzed for total C and N (Ward Laboratories Kearney, NE; Table A1).
Table A1. Total C and N and C:N of cover crops used in the rotations.

Table A2. Soil analysis methods.