Avoidant/restrictive food intake disorder or ARFID is an eating disorder characterised by avoidance or restriction of food that is not caused by food scarcity, cultural or religious practices, or a mental or general medical disorder. The feeding or eating disturbance does not occur exclusively during the course of anorexia nervosa or bulimia nervosa, and there is no evidence of a disturbance in the way in which body weight or shape are experienced (American Psychiatric Association 2013). If there is a concurrent medical or psychiatric illness, ARFID can only be diagnosed if the eating difficulty exceeds that normally associated with the concurrent condition (Mairs Reference Mairs and Nicholls2016).
ARFID can be diagnosed at any age but is usually diagnosed in children and young people who develop significant problems with eating that persist beyond the neophobia stage typical between 2 and 6 years of age (Norris Reference Norris, Spettigue and Katzman2016). The patients typically present to the family doctor or general paediatrician as the first point of contact (Norris Reference Norris, Spettigue and Katzman2016) but these clinicians may find it difficult to distinguish the condition from childhood anorexia or may regard it as a developmental phase that the patient will outgrow.
ARFID may involve an apparent lack of interest in food or eating, avoidance based on the sensory characteristics of food, or concerns about aversive consequences of eating because of previous experience of, for example, a choking episode, food poisoning or significant gastroesophageal reflux symptoms (Box 1) (Nicely Reference Nicely, Lane-Loney and Masciulli2014). However, these features are not necessarily mutually exclusive, and patients can present with more than one.
BOX 1 Diagnostic criteria for avoidant/restrictive food intake disorder (ARFID)
Lack of interest in eating or food; avoidance based on the sensory characteristics of food; concern about/fear of aversive consequences of eating (more than one of these three may be present); associated with one or more of the following:
• significant weight loss (or failure to achieve expected weight gain or faltering growth in children)
• significant nutritional deficiency
• dependence on enteral feeding or oral nutritional supplements
• marked interference with psychosocial functioning.
As a consequence of their eating difficulties, the affected individual experiences persistent failure to meet their nutritional and/or energy needs, which leads to significant weight loss (or failure to gain weight or faltering growth in children), nutritional deficiency, dependence on tube feeding or oral nutritional supplements and marked impairment in psychosocial functioning, such as being unable to eat with others or engage in other age-appropriate social activities (American Psychiatric Association 2013). ARFID may run a chronic course and is not synonymous with ‘picky’ or ‘fussy’ eating, because it places significant burden on the patient and family functioning if not promptly diagnosed and treated.
ARFID was introduced in the DSM-5 eating disorder category as an umbrella term to encapsulate a range of feeding problems previously described in ICD-10 and DSM-IV (World Health Organization 1992; American Psychiatric Association 1994). These include food avoidance emotional disorder and infantile anorexia – diagnoses characterised by apparent lack of interest in eating or food); selective eating, food phobia, extreme picky eating and neophobia, i.e. unwillingness to try new food – avoidance based on the sensory characteristics of food; and emetophobia, functional dysphagia and globus hystericus – concern about aversive consequences of eating (Kurz Reference Kurz, van Dyck and Dremmel2015).
ARFID has also replaced and extended the DSM–IV-TR category ‘Feeding and eating disorders of infancy or early childhood’, as this diagnosis was rarely used in clinical practice or research. It was restricted to children below 6 years of age who presented with a feeding disturbance that caused them to lose or fail to gain weight for at least 1 month but was not caused by another medical or mental disorder (American Psychiatric Association 2000). These restrictive criteria left out a majority of individuals over 6 years old who have eating problems that do not fit into the categories of anorexia nervosa or bulimia nervosa, and yet still placed a substantial emphasis on negative or maladaptive interactions between the child and caregiver (Norris Reference Norris, Spettigue and Katzman2016).
Unfortunately, the condition is underrecognised and underdiagnosed, thus resulting in inadequate commissioning and management. In the UK, most eating disorder services are not commissioned to manage ARFID, even though it is mentioned in NHS England's access and waiting-time standards (NHS England 2015), and this has created a significant gap in access to service and treatment.
This article aims to highlight the existence of ARFID, improve confidence in diagnosis of the condition and offer preliminary suggestions for its treatment, given that there is at present no evidence-based treatment guideline available. Also, as there is currently a paucity of published research, we hope that this article will generate interest in research into this condition. The case vignettes in the article are fictitious but based on our clinical experience.
Epidemiology
ARFID is equally common in males and females in infancy and early childhood but has a male predominance in middle childhood, when it is often comorbid with autism (Box 2) (Nicely Reference Nicely, Lane-Loney and Masciulli2014). Using a self-report measure, a preliminary community-based study of ARFID showed a point prevalence of 3.2% in a Swiss school-based sample of 1444 children aged 8–13 years (Kurz Reference Kurz, van Dyck and Dremmel2015). In a tertiary setting, the prevalence rates ranged from 5 to 23% (Norris Reference Norris, Spettigue and Katzman2016). Current prevalence rates have been calculated using retrospective data to assign patients to a diagnosis of ARFID. It is not unlikely that the prevalence may change when ARFID diagnosis becomes more stable in terms of recognition (e.g. through prospective surveillance and population studies).
A large multi-site retrospective chart review of 700 adolescents with restrictive eating disorders showed that individuals with ARFID were younger, had a longer duration of illness before presentation and were more likely to be male than those with anorexia nervosa or bulimia nervosa. Additionally, those with ARFID had higher incidence of a coexisting anxiety and/or medical condition but a lower incidence of a coexisting depression (Forman Reference Forman, McKenzie and Hehn2014).
BOX 2 Case vignette 1: ARFID associated with autism
Jamie is a 10-year-old boy with a diagnosis of autism and sensory processing difficulties. His mother describes him as a temperamental child, difficult to settle when upset and insisting on sameness of routine. He has always been a fussy eater, preferring meals of white colour (white chocolate mousse, bread and pasta) and a particular brand of pasta. He often experiences constipation.
Jamie experienced an episode of gastrointestinal upset (severe diarrhoea and vomiting) that caused him significant distress and necessitated a 2-week admission to hospital. Following this traumatic experience, Jamie developed a fear of vomiting and began to avoid eating his previously favourite food, causing him to rapidly lose significant amounts of weight. This impaired his ability to function at home and in school as he became lethargic and susceptible to minor infections. He denied having any body image disturbance or fears relating to weight gain.
He required enteral feeding through a nasogastric tube to ensure adequate calorie intake and was belatedly referred to the local eating disorder service (consisting of a child psychiatrist, dietitian, psychologist and occupational therapist) for assessment and treatment of food refusal. He was diagnosed with ARFID. Jamie's parents and paediatrician expressed surprise at this diagnosis as they were unfamiliar with the existence of ARFID. The family was offered psychoeducation and family therapy and he was gradually weaned off tube feeding through graded exposure and hierarchical introduction of meals.
Clinical presentation
The clinical presentation varies and may be dependent on individual risk factors such as temperament, environment and genetic predisposition (Aviram Reference Aviram, Atzaba-Poria and Pike2015). Avoidance due to fear of aversive consequences can arise at any age, whereas avoidance due to lack of interest in food or to the food's sensory characteristics most commonly develops in early childhood and is generally stable into adulthood (American Psychiatric Association 2013).
ARFID occurs more commonly in children than in adults, and there is often a long delay between onset and clinical presentation (Micali Reference Micali, Simonoff and Elberling2011). Infants with ARFID may present with lethargy, distress or being too agitated to feed, and mealtimes become an unpleasant time for both the child and their caregiver. The older children and adolescents may present with more generalised emotional difficulties that do not meet diagnostic criteria for anxiety or depression.
Owing to the effects of starvation and inadequate nutrition, the individual may feel cold or experience irritability, poor concentration or tiredness. These may be evident at home and in school, affecting interpersonal relationships and academic engagement. Teenagers may avoid having school lunch because they are embarrassed by their selective or restrictive attitude to meals.
Assessment
History
It is essential to conduct a comprehensive biopsychosocial assessment and to obtain collateral information when possible (Box 3). Therapeutic alliance should be established from the outset by working in collaboration with the patient/family to set treatment goals. The clinician needs to determine the nature, duration and causes of the avoidant or restrictive behaviour. Then they should determine the consequences of this behaviour on the person's physical health and functioning, and whether they meet any of the exclusion criteria. It is important to assess factors that serve to maintain ARFID as well as resources available to the patient and their family.
BOX 3 Key areas to assess in diagnosing avoidant/restrictive food intake disorder (ARFID)
Nature of avoidant/restrictive behaviour
Is the person managing to eat an adequate age-appropriate amount and range of foods?
Duration
Has the behaviour been present for at least a month?
Cause (more than one may be present)
• Lack of interest in food:
• Is the person failing to recognise hunger cues/lack of appetite?
• Is the eating disturbance related to mood/emotions?
• Does the person show a high level of arousal/inattention/distractibility?
• Preference for or avoidance of certain sensory characteristics of foods (taste, texture, appearance/colour, brand)
• Fear of experiencing distress or discomfort, e.g. choking/vomiting or worry that harm may be caused
Consequences
• Weight loss, failure to follow weight/height centiles?
• Symptoms/signs of nutritional deficiency or malnutrition (e.g. tiredness, poor concentration)?
• Does the person rely on oral nutritional supplements or tube feeding?
• Does their eating behaviour significantly affect their education/occupation, social development or family functioning?
Presence of any exclusion criteria
• Does the person have any current concerns about their weight/shape? Would they be worried about putting on weight?
• Can the eating behaviour be attributed to another condition or medication?
• Is the behaviour part of cultural/religious practice (e.g. fasting), or is there a lack of available food?
Other useful information
• Developmental history (including history of feeding and eating)
• Family context (mealtimes, family relationship with food and eating)
• Presence of any sensory sensitivities?
• Ascertainment of coexisting mental disorders, e.g. anxiety, obsessive–compulsive disorder
Some screening tools for identification of children and adolescents with ARFID are currently available in specialist eating disorder centres. These include the Eating Disturbances in Youth–Questionnaire (EDY-Q) (Hilbert Reference Hilbert and van Dyck2016) and Pica, ARFID, and Rumination Disorder Interview (PARDI) (Bryant-Waugh Reference Bryant-Waugh and Cooke2017).
Examination
Calculate body mass index (BMI) in adults. In children and adolescents, as well as calculating percentage weight-for-height, plot their height and weight on a growth chart and, if possible, compare the data with previous values to identify faltering growth or lack of appropriate weight gain. Also assess their pubertal phase to determine whether there is any delay (which, if present, may warrant referral to a paediatrician or endocrinologist). Examine for presence of any signs of malnutrition or medical conditions.
Investigations
Blood tests and imaging can be used to screen for medical causes of weight loss (e.g. coeliac disease, Crohn's disease or ulcerative colitis), gastrointestinal discomfort and vomiting, and any nutritional deficiencies (Box 4). These tests may include full blood count, urea and electrolytes, magnesium, vitamin D, calcium, vitamin B12 and folate levels. In most cases, diagnosis of ARFID can be made from a comprehensive history but in rare cases, more extensive investigations, such as barium swallow, endoscopy or brain imaging, are needed to exclude anatomical or physiological conditions (Box 5). A swallowing assessment by a speech and language therapist may also be useful. However, there is a risk that patients can be over-investigated.
BOX 4 Case vignette 3: childhood adversity, neurodevelopmental disorder and bowel disease
Tom is a 12-year-old boy who currently lives with his grandparents, having been placed on the Child Protection Register and taken from his biological parents by the local Children's Services Department. He has neurodevelopmental disorders, namely autism spectrum disorder and ADHD. He was not on stimulant medication for his ADHD.
Tom's developmental history is that of a child with significant global developmental delay, especially in speech and social communication. He experienced physical and emotional neglect from his biological parents, who had been diagnosed with substance use disorder.
Tom displayed profound rigidity about what he ate and how he ate his meals (for instance he did not like food items on his plate touching). He had a restricted diet from an early age and did not eat fruit or vegetables. He had severe sensory sensitivities relating to taste and texture of food as well as a dislike of wearing wool, long-sleeved shirts and trousers. Mealtimes were incredibly difficult for the household as he would not eat and would sometimes hide food or throw it away. School mealtimes were also challenging as he would not eat his lunch in front of others, even when presented with his preferred meal. He failed to meet his projected weight and height.
Tom was initially referred by his GP to the paediatrician owing to concerns about the boy's restrictive food intake and resultant faltering growth. A CAMHS referral was made when he experienced significant anxiety, appetite loss and rapid weight loss as he struggled with the transition to secondary school. His grandmother stated that his eating had reduced over the past month – he was eating smaller portions and less frequently than previously. The GP informed the family that Tom's anxiety about transition to secondary school may have contributed to the reduced food intake but she felt that a dual referral to the paediatrician and CAMHS was warranted given his dramatic weight loss.
During Tom's initial assessment in CAMHS he denied any concerns with body image. He identified as being low weight and said he would like to increase his weight. He said that he experienced fatigue and recent gastrointestinal symptoms (abdominal pain, loose stool and loss of appetite for his usual food), and revealed a history of chronic constipation with overflow.
Tom appeared emaciated, pale and tired. His weight was well below the third centile for his age and his percentage median BMI was less than 70% weight-for-height. His blood tests showed evidence of anaemia (low haematocrit, haemoglobin and red blood cells) and inflammatory process (increased erythrocyte sedimentation rate and C-reactive protein). Based on his presentation and blood results, CAMHS referred the boy to a paediatric gastroenterologist to help rule out any organic disease such as an inflammatory bowel disease. Following assessments by the gastroenterologist, a diagnosis of Crohn's disease was made. Despite this, it was agreed that Tom's historical features still met the diagnostic criteria for ARFID.
CAMHS worked closely with the gastroenterology team and supported Tom and his family with psychological therapies tailored to his needs. He had several sessions of CBT to address his generalised anxiety and specific anxieties about food. He practised mindfulness techniques, systematic desensitisation and gradual reintroduction to feared foods (using a hierarchy of food challenges and associated contingent reinforcements). His caregivers attended family-based treatment sessions as well as having support from the CAMHS clinical psychologist, who had expertise in management of children who have experienced adversity in childhood.
BOX 5 Case vignette 2: ARFID and separation anxiety
Molly is a 14-year-old girl who was referred by her consultant paediatrician to a CAMHS eating disorder clinic following many months of battling with anxiety relating to food, and poor weight gain. Her anxiety increased after she choked on her sandwich during a school trip. She was traumatised and embarrassed by the incident. She became more self-conscious and also began to avoid solid foods because of fear of choking.
Molly was investigated by the paediatric gastroenterologist for swallowing difficulties and for Addison's disease, as she also complained of tiredness and dizziness. As no medical cause was identified, her paediatrician felt that her condition could be of a psychological nature.
In addition, Molly had always struggled with separation anxiety. She worried excessively about her mother's safety, even at night, and therefore refused to sleep in her own bed. She was unable to attend school, where her mother was a part-time dinner lady, on the days that the mother was not at work. She could not bear to be away from home for a sleep-over at a friend's house.
Developmentally, from the time of weaning, Molly showed a clear dislike of meat and of certain food textures. She struggled with the sound of other people eating, saying that it made her feel nauseous, so she had to wear headphones during mealtimes. Her mother reported that Molly chewed her food repeatedly before swallowing. Other aspects of her developmental history did not indicate presence of autism spectrum disorder.
During her assessment, Molly presented as a petite girl who made good eye contact and did not appear depressed. She stated that she would like to be able to try eating like her friends at school and to gain weight. She would also like to be less anxious.
Molly was diagnosed with ARFID. She and the family were offered psychoeducation regarding the diagnosis and treatment. She was helped by the occupational therapist to address her sensory problems. The psychiatrist prescribed sertraline for her anxiety disorder and the clinical psychologist offered weekly sessions of CBT. These CBT sessions consisted of psychoeducation, cognitive restructuring, systematic desensitisation, and exposure and response prevention. The team also worked closely with Molly's school to apply for an education, health and care plan (ECP) to enable provision of support during mealtimes at school.
Aetiology
Table 1 summarises common contributory factors in ARFID. These fall into three categories: predisposing, precipitating and perpetuating.
TABLE 1 Common contributors to avoidant/restrictive food intake disorder (ARFID)
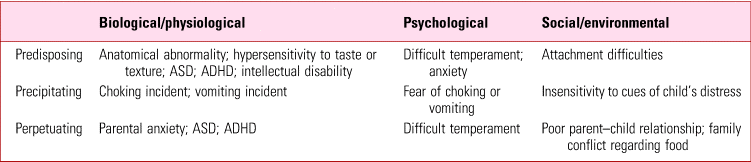
ADHD, attention-deficit hyperactivity disorder; ASD, autism spectrum disorder.
Predisposing factors
Neurodevelopmental disorders such as autism spectrum disorder (ASD), attention-deficit hyperactivity disorder (ADHD) and intellectual disability underlie some presentations. About 80% of children with developmental delays are reported to experience some type of feeding problem, compared with 25–45% of normally developing children (Bryant-Waugh Reference Bryant-Waugh, Markham and Kriepe2010). The sensory sensitivities often present in ASD may affect the individual's ability to eat certain foods, as they find the appearance, texture or smell unpleasant, and may also mean that they restrict themselves to eating a particular brand of food.
It is also plausible that the alexithymia (inability to identify and express or describe one's feelings) seen in ASD may contribute, with restrictive eating used as a means of internalising distress. ADHD can affect eating behaviour because of distractibility, lack of interest in eating and high levels of arousal at mealtimes.
A large proportion of children with eating difficulties have an associated primary medical condition, most commonly a gastrointestinal and neurological disorder, as well as food allergies (Nicely Reference Nicely, Lane-Loney and Masciulli2014). In some children, failure to thrive occurs in the context of abuse, neglect or attachment disorders (American Psychiatric Association 2013).
Some features of ARFID closely resemble anxiety disorders (e.g. choking/vomiting phobia) and are likely to have a similar aetiology (Mairs Reference Mairs and Nicholls2016). A familial or maternal history of anxiety and eating disorders increases rates of feeding disturbances in children (Nicely Reference Nicely, Lane-Loney and Masciulli2014).
Precipitating factors
Having experienced or witnessed a traumatic episode of abdominal discomfort, vomiting, choking or gagging on a particular food item, some children become fearful of eating similar food and completely avoid its consumption.
Perpetuating factors
Parental frustration and feeding the child their preferred foods (or avoiding presenting novel foods) in response to the child's refusal are known to contribute to children's feeding problems (Mitchell Reference Mitchell, Farrow and Haycraft2013).
Comorbidity and differential diagnosis
As mentioned above, ARFID can present alongside neurodevelopmental disorders such as ASD, ADHD and intellectual disability, and it may be present in children with attachment difficulties (Zucker Reference Zucker, Copeland and Franz2015).
Anxiety disorders such as emetophobia (fear of vomiting) and social phobia may coexist with ARFID. When the eating problem has become the primary focus in such presentations, diagnosis of ARFID would be more appropriate. An obsessive fear of vomiting and rituals to prevent this can be seen as part of an obsessive–compulsive disorder, especially in the presence of other obsessions and compulsions (American Psychiatric Association 2013).
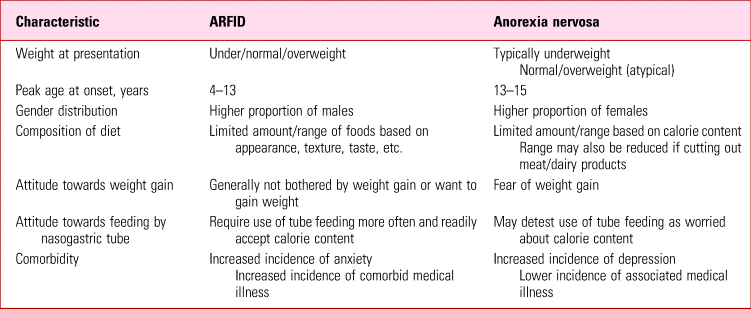
At initial presentation, especially when the person is underweight, it may be difficult to distinguish ARFID from anorexia nervosa, as there may be a seeming overlap of symptoms (Table 2). Some patients with anorexia nervosa deny intentional weight loss and fear of becoming fat but these will become more apparent as they start to gain weight with treatment. If the clinical picture is uncertain, it may be prudent to make a provisional diagnosis of anorexia nervosa. Final diagnosis may be resolved over time through an increasingly positive therapeutic relationship, with progress reviews, asking the individual about concerns relating to body shape or weight, ensuring close monitoring and obtaining collateral information from caregivers.
TABLE 2 A comparison of avoidant/restrictive food intake disorder (ARFID) and anorexia nervosa

Treatment
General principles
The treatment of ARFID is individualised and based on the clinical presentation. For pragmatic reasons, it may be sensible to approach treatment in two phases: an acute and a maintenance phase. The acute phase would focus on weight restoration (getting the patient well), whereas the maintenance phase should involve preventing relapse (keeping the patient well). Treatment can be delivered in an out-patient or in-patient unit. However, medically unstable individuals need to be admitted to general hospital for stabilisation before continuing with out-patient care.
The consensus is that treatment should be multidisciplinary and based on what factors are thought to be driving the distress and eating disturbances. Convening a multidisciplinary meeting may be essential in complex cases to ensure a unity of message between all parties. A comprehensive treatment plan is constructed in partnership with the patient/family. With children and young people, it is also essential to work closely with the school, to ensure a consistent approach. This requires parental consent.
Given the potential chronic course of most eating disorders, we would advocate using stepped care and chronic disease management models to manage ARFID. This approach should include opportunity for continued psychoeducation and self-management to empower the patient/family and to enhance self-efficacy. Validated outcome measures might be used to monitor progress and to support clinical decision-making.
Having identified the nature and severity of the problem, use biopsychosocial formulation to identify targets for intervention, particularly any maintaining factors. Identify priorities for treatment (i.e. what would have the biggest impact) and, with the patient, set goals and an end-point for treatment. The end-point often only needs to be a ‘good enough’ diet to optimise physical health and level of functioning.
It is not unusual for patients to present with significant low weight and this can cause a degree of anxiety in clinicians. But it is important to remember that these individuals have adjusted to being chronically underweight and weight restoration should be done slowly. Ongoing clinical reviews should include evaluation of functional impairment and quality of life.
Biological and pharmacological management
Medical conditions are managed by the paediatrician or general practitioner, working in collaboration with the psychiatrist and other allied professionals. A decision should be made about who will carry out any blood monitoring. For deficiencies identified on dietary review or blood tests, a general multivitamin and/or specific medication (such as ergocalciferol for vitamin D deficiency) should be prescribed. If the person is not able to increase the amount they eat, oral nutritional supplements may be prescribed in conjunction with other treatment.
The paediatrician supports the multidisciplinary team by providing expertise in ensuring stability of physical health and monitoring growth and pubertal development. The dietitian assesses nutritional needs and provides meal plans, and the occupational therapist provides sensory assessment, meal coaching and parent empowerment. The speech and language therapist assesses oral motor problems and swallow safety. The role of the psychiatrist within the team includes biopsychosocial formulation of the patient's presentation, assessment for comorbid psychiatric conditions and guidance on appropriate use of psychotropic medications.
Pharmacological treatment could be used as an adjunct and is targeted towards managing anxiety using selective serotonin reuptake inhibitors or atypical antipsychotics. Sant'Anna et al (Reference Sant'Anna, Hammes and Porporino2014) observed in their retrospective chart review of children treated with cyproheptadine, an antihistamine, that the medication helped to stimulate appetite when psychological and nutritional supports were unsuccessful.
Psychosocial intervention
This should involve utilising evidence-based psychological treatments such as family-based treatment, cognitive–behavioural therapy (CBT) and parent training designed for families with young children diagnosed with ARFID (Lukens Reference Lukens and Silverman2014; Lock Reference Lock2015). The family-based treatment model has been extended to make it suitable for individuals with ARFID (Fitzpatrick Reference Fitzpatrick, Forsberg, Colborn, Loeb, LeGrange and Lock2015). Training parents to implement at home with their child techniques that have been initiated in clinic, as well as modifying their own behaviours in response to their child's distress, can be an important part of treatment, as can working closely with caregivers to enable pleasurable mealtimes.
There are currently no Level 1 or Grade A (e.g., multisite randomised controlled trials) studies guiding the treatment of ARFID and most interventions have been based on case studies and a few randomised controlled trials. Bryant-Waugh (Reference Bryant-Waugh2013) used a behavioural approach in the treatment of a 13-year-old with a lack of interest in food and very selective eating, and Thomas et al (Reference Thomas, Brigham and Eddy2017) used an adapted cognitive–behavioural approach, CBT-AR, with a 13-year-old with ARFID who had experienced a choking incident. Sharp et al (Reference Sharp, Stubbs and Adams2016), in a pilot trial, developed an intensive manual-based behavioural feeding intervention for chronic severe food refusal in children aged between 13 and 72 months. They also incorporated parent training similar to that in family-based treatment for anorexia nervosa as a component of their intervention. Their results showed high caregiver satisfaction and acceptability of the intervention and evidence of increased oral food intake in the children.
Although case studies may not be generalisable, they help to illustrate the various ways that ARFID could present and be managed. With regard to CBT-AR, the potential challenge for clinical practice is that the intervention has been designed for use in individuals who are over 10 years of age, are medically stable and do not have severe developmental disabilities or reliance on tube feeding. The manual-based behavioural intervention developed by Sharp and colleagues focused primarily on young children with paediatric feeding disorder and may not be applicable to older children or adolescents.
Dietary management
With the support of the dietitian, the aim is to create a meal plan that includes regular meals and snacks throughout the day to prevent the loss of hunger signals and reduce anxiety about what or when the next meal will be (Mairs Reference Mairs and Nicholls2016). A list of the types of food and texture the individual prefers is used to identify foods with similar characteristics that may allow opportunities to increase the range of foods accepted. Weaning from tube feeding could be facilitated by behavioural or psychological interventions, for example reducing the frequency of tube feeding to manipulate appetite. It is essential to explain to caregivers that there may be an initial weight loss as the child gradually moves onto oral feeding.
If the individual presents with low interest in food, poor appetite, or high arousal or distractibility at mealtimes, behavioural interventions such as improved routine regarding meals and behaviour-based rewards, and increasing portion size/caloric content of food can be used (Mammel Reference Mammel and Ornstein2017). Treatment of inattention and regulation of arousal levels can also be considered.
If the presentation is related to sensory food avoidance, large, more frequent portions of preferred foods should be given, with repeated small exposures to one or two new items at a time and gradually increased proportions of the exposure foods, once accepted. The new foods are based on a hierarchy developed by the patient and their parents/caregivers, and anxiety management, gradual exposure and response prevention, and rewards for tasting new foods and accepting increasing portions are used (Mammel Reference Mammel and Ornstein2017).
To treat fear-based food avoidance, both cognitive (especially in older children) and behavioural approaches such as those mentioned above for sensory food avoidance may be successful. It may also be worth assessing whether any adjustments can be made to the mealtime environment if this is overstimulating for the individual.
Barriers to treatment
ARFID is a heterogeneous condition with varied presentation in different individuals, making diagnosis and referral difficult. It therefore tends to be managed across generic CAMHS, specialist eating disorder services and paediatric services. There may not always be a coordinated response between professionals, partly owing to a lack of knowledge about the condition and the need for multifaceted approach to treatment (Norris Reference Norris, Spettigue and Katzman2016).
Parents or caregivers may feel judged and may delay seeking professional help (Zucker Reference Zucker, Copeland and Franz2015). Given its unfamiliarity to clinicians and service commissioners, ARFID is neither included in the current National Institute for Health and Care Excellence (NICE) guidance for eating disorders, nor is it seen as a priority, compared with anorexia nervosa or bulimia nervosa, in established eating disorder services.
Ideally, we need to work towards joint service planning for feeding and eating disorders across physical and mental healthcare in order to better meet the needs of this complex patient group. Services providing care for these patients can start to develop more specific care packages based on the treatment recommendations in this article.
Complications and prognosis
The complications of ARFID are largely the same as those of other eating disorders. Cognitive functioning is affected by being at a low weight, and the physical consequences of delayed gastric emptying and constipation act as maintaining factors for ARFID, because of gastrointestinal discomfort when eating. Dependence on enteral feeding is more likely to occur with ARFID, owing to professionals’ lack of knowledge about diagnosis and treatment of the disorder and the absence of concerns about calorie content of food or weight gain (Ornstein Reference Ornstein, Essayli and Nicely2017).
During treatment, ARFID develops into anorexia nervosa in a significant minority of cases (Mairs Reference Mairs and Nicholls2016) as, for example, the patient starts to express weight/shape concerns as they gain weight. Therefore, it is important to keep reassessing the diagnosis, particularly if the young person is not progressing in treatment as expected.
Conclusions
ARFID is not uncommon; it occurs with varied presentations across the lifespan and can place significant stress on the affected individual and their family. There is a paucity of research into this condition because of its relatively recent inclusion in DSM-5. It is extremely important for evidence-based treatment guidelines and validated outcome measures to be developed to support therapists, parents and patients in coping with this often debilitating disorder. Clinicians should continue to advocate for increased commissioning of services for patients with eating disorders that do not come under the broad category of anorexia nervosa or bulimia nervosa.
MCQs
Select the single best option for each question stem
1 Which of the following is consistent with a diagnosis of ARFID?
a presence of body-image disturbance
b intense fear of gaining weight or becoming fat
c lack of available food
d culturally sanctioned eating practices
e dependence on enteral feeding.
2 Which of the following is a known risk factor for developing ARFID?
a dieting behaviour
b low mood
c experience of emotional or physical neglect
d overeating
e history of bullying about weight.
3 As regards the management of ARFID:
a it is best managed by a single clinician
b if the person is underweight, weight restoration should occur as quickly as possible
c there is no role for medication
d the paediatrician and psychiatrist would work collaboratively to manage the person's physical health
e there is an expectation that the person will eat a normal range of diet at the start of treatment.
4 As regards psychological treatments for ARFID:
a there is a large body of evidence to guide clinical practice
b gradual exposure and response prevention is used to help introduce new foods to the diet
c only cognitive approaches are recommended in treating fear-based food avoidance
d no specific approaches have been developed for psychological treatment of ARFID
e the person is likely to need psychological support related to distress about weight gain.
5 Comparing ARFID with other eating disorders:
a the physical complications resulting from starvation are similar
b patients with ARFID are more likely to binge and purge
c ARFID has lower incidence of coexisting medical illness
d patients with ARFID worry excessively about calorie intake when on tube feed
e CBT is unhelpful in management of ARFID.
MCQ answers
1 e 2 c 3 d 4 b 5 a




eLetters
No eLetters have been published for this article.