Hypertension is a major risk factor for CVD, and it often clusters with other risk factors including central obesity, dyslipidaemia and insulin resistance. The metabolic syndrome (MetS) is a combination of these cardiometabolic risk factors, and it has been associated with an increased risk of type 2 diabetes and atherosclerotic CVD( Reference Beltran-Sanchez, Harhay and Harhay 1 ). The prevalence of the MetS is about 20 % among adult population( Reference Beltran-Sanchez, Harhay and Harhay 1 ).
Lifestyle changes, such as reduction of salt and SFA intake, increase in low-fat dairy product, fruit and vegetable intake, weight reduction and increase in physical activity, play a key role in the prevention and treatment of hypertension and its related disorders( Reference Mancia, De Backer and Dominiczak 2 ). The possible antihypertensive effect of dairy product intake has been linked to the intake of Ca, K and Mg, minerals that exist in rich amount in milk( Reference Massey 3 ), and to bioactive peptides released from milk protein through gastrointestinal digestion, microbial fermentation or enzymatic hydrolysis( Reference Korhonen 4 ).
Recently, three meta-analyses have reported that intake of the milk casein-derived lactotripeptides (LTP) isoleucine–proline–proline (Ile-Pro-Pro) and valine–proline–proline (Val-Pro-Pro) decreases systolic blood pressure (BP) by about 4–5 mmHg and diastolic BP by about 2 mmHg( Reference Cicero, Gerocarni and Laghi 5 – Reference Xu, Qin and Wang 7 ). Another meta-analysis by Pripp( Reference Pripp 8 ) has included clinical trials on both LTP and other peptides derived from food, and has found a lowering effect on systolic/diastolic BP of about 5/2 mmHg. One animal study has suggested that the antihypertensive mechanism of Ile-Pro-Pro and Val-Pro-Pro is competitive inhibition of the angiotensin-converting enzyme (ACE)( Reference Nakamura, Masuda and Takano 9 ). However, contradictory data also exist, as several clinical trials have reported that LTP are without significant influences on BP( Reference Engberink, Schouten and Kok 10 – Reference van Mierlo, Koning and van der Zander 12 ).
The cholesterol-lowering effect of phytosterols (plant sterols and stanols) is well established, as these compounds inhibit the absorption of dietary and endogenous cholesterol and up-regulate intestinal cholesterol efflux transporters( Reference Plat and Mensink 13 ). Daily intake of 2 g of plant sterols or stanols has been reported to reduce total and LDL-cholesterol levels by approximately 10 %( Reference AbuMweiss, Marinangeli and Frohlich 14 ). The efficacy of plant sterols/stanols is affected by subjects' baseline LDL levels, food carrier, and frequency and timing of sterol intake( Reference AbuMweiss, Marinangeli and Frohlich 14 ).
Moreover, two long-term intervention studies have examined the effects of simultaneous intake of LTP and plant sterol esters (Pse) on cardiovascular risk factors in hypertensive and hypercholesterolaemic subjects( Reference Turpeinen, Ikonen and Kivimaki 15 , Reference Turpeinen, Kumpu and Ronnback 16 ). In these studies, the intake of 4·2 mg LTP with 2 g Pse in a low-fat spread had been reported to decrease systolic BP by 4–6 mmHg and LDL-cholesterol concentrations by 0·2–0·3 mmol/l. However, the BP-lowering effect was not consistent in all measurements performed at home, in the office or during ambulatory 24-h recordings( Reference Turpeinen, Ikonen and Kivimaki 15 , Reference Turpeinen, Kumpu and Ronnback 16 ).
Since the evidence about the BP-lowering properties of LTP remains contradictory, we tested the hypothesis whether simultaneous intake of LTP and Pse in fermented milk product lowers BP in subjects with the MetS. For this purpose, two doses of Ile-Pro-Pro and Val-Pro-Pro (5 and 25 mg/d) were studied, and since the concurrent reduction in plasma lipids can be anticipated to provide additional cardiovascular benefits, both products also contained 2 g/d of Pse. For the first time, the long-term effects on haemodynamics were examined in a clinical physiology laboratory in a double-blind, randomised, parallel-group set up.
Methods
Ethical statement
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of Tampere University Hospital (Study code R08012). Written informed consent was obtained from all subjects. The present study is a part of an ongoing investigation, in which haemodynamic measurements are non-invasively recorded (DYNAMIC-study; Clinical Trials registration number NCT01742702), and the recording protocol is also registered in the EU Clinical Trials Register (EudraCT number 2006-002065-39).
Study subjects
Altogether, 116 subjects aged 35–62 years with the MetS were recruited through announcements in a local newspaper. The inclusion criteria were waistline ≥ 94 cm for men and ≥ 80 cm for women, systolic BP ≥ 140 mmHg or diastolic BP ≥ 85 mg, and at least one of the following: plasma TAG ≥ 1·7 mmol/l; HDL-cholesterol concentration < 1·03 mmol/l in men and < 1·29 mmol/l in women; fasting glucose ≥ 5·6 mmol/l. The exclusion criteria were use of antihypertensive or lipid-lowering medication, secondary hypertension, unstable coronary artery disease, diabetes, malignancy, milk allergy, smoking, alcohol abuse, pregnancy and lactation.
Of the total study subjects, one discontinued the study during the run-in, and three subjects on placebo and two on 25 mg LTP+2 g Pse discontinued the study during the intervention due to personal reasons; four subjects were excluded because they had failed to report the use of statin (n 1) or metformin (n 3) at study entry, and two subjects due to technical problems during haemodynamic recordings. Altogether, 104 subjects (sixty-three males and forty-one females) were included in the final analysis.
Study design
The present study was randomised, double-blind and placebo-controlled with a parallel design containing two intervention groups on different peptide concentrations and a control group. Separate randomisation lists were used for men and women. During a 4-week run-in period, commercial fermented milk product without the test substances was given twice daily to familiarise the subjects with the trial procedure. After run-in, the subjects received fermented milk product containing (1) 5 mg/d LTP and 2 g/d Pse (LTP5+Pse); (2) 25 mg/d LTP+2 g/d Pse (LTP25+Pse); (3) placebo without added LTP and Pse. The product dose was 125 ml twice daily during the 12-week intervention. The subjects were instructed to maintain their medication, lifestyle and dietary habits constant during the study period and to register the use of the test products to study diaries.
Medications, use of vitamins and other nutrient supplements, and lifestyle habits were reviewed by structured questionnaires. Routine laboratory tests were taken at screening. At weeks 0, 8 and 12, blood samples after approximately 12 h of fasting were collected, and the subjects were weighed. Laboratory measurements included full blood count, C-reactive protein, glucose, creatinine, uric acid, Ca, phosphate (0 and 12 weeks), plasma lipids, Na and K (0, 8 and 12 week). BP was measured at home twice a week during the intervention (see below), and haemodynamic measurements were recorded at weeks 0, 8 and 12.
Study products
All the study products were produced by Valio Limited. The run-in product was a non-fat fermented milk product, different from the test and placebo products, without bioactive LTP and Pse. During the intervention, the fermented milk products contained Ile-Pro-Pro and Val-Pro-Pro (5 or 25 mg/d) and Pse (2 g/d). LTP were added to the test products in the form of peptide powder. The peptides were separated from Lactobacillus helveticus Lc 1936 fermented milk, concentrated using nanofiltration and dried to produce powder. Pse were prepared by esterification of free plant sterols with fatty acids obtained from vegetable oil (Cognis Corporation) and contained β-sitosterol max 80 %, campesterol max 40 %, stigmasterol max 30 %, β-sitostanol max 15 % and campestanol max 5 %. Other sterols and stanols were present at < 5 %. Placebo was a fermented milk product without LTP and Pse. The daily 250 ml dose of both test products contained 3 g fat, 10 g protein and 25 g carbohydrates, and that of placebo contained 1 g fat, 8 g protein and 32 g carbohydrates. The energy content of the products was similar. The daily contents of Ca, K and Mg in the test products were 380, 660 and 41 mg, respectively, and 250, 375 and 28 mg in placebo, respectively.
Laboratory analyses
Blood count was determined using ADVIA 120 or 2120 (Bayer Health Care), and other laboratory values were determined using Cobas Integra 700/800 (F. Hoffmann-LaRoche Limited). Total cholesterol, HDL and TAG levels were measured enzymatically, and LDL level was calculated using the Friedewald equation( Reference Friedewald, Levy and Fredrickson 17 ). Plasma insulin was determined using electrochemiluminescence assay on Cobas e411 analyser (Roche Diagnostics). Plasma renin activity was measured using GammaCoat Plasma Renin Activity assay (Diasorin). Aldosterone concentrations were analysed by active aldosterone RIA (Diagnostics Systems Laboratories, Beckman Coulter). Estimated creatinine-based glomerular filtration rate was calculated using the RULE formula( Reference Rule, Larson and Bergstralh 18 ), as the plasma creatinine values were within the normal range.
Blood pressure measurements in the office and at home, and body weight
Brachial office BP was measured in duplicate using an automated sphygmomanometer (Omron M4; Omron Matsusaka Limited) after 5 min rest in the sitting position at screening and at the end of the intervention( Reference Mancia, De Backer and Dominiczak 2 ). Height and waist and hip circumference were measured to the closest 0·5 cm at screening. Body weight with subject in light clothing was monitored using a digital scale at every visit. After hands-on instructions, home BP (Omron M4) was measured twice a week (one weekday and one weekend day) in the morning approximately 1 h after waking up.
Pulse wave analysis and whole-body impedance cardiography
Haemodynamic recordings using continuous radial pulse wave analysis (SpygmoCor PWMx; Atcor Medical) and whole-body impedance cardiography (CircMonR; JR Medical Limited) were performed in a quiet, temperature-controlled laboratory as reported previously in detail( Reference Tahvanainen, Koskela and Tikkakoski 19 , Reference Tikkakoski, Tahvanainen and Leskinen 20 ). The pulse wave analysis probes in the left arm were at the level of the heart throughout the measurements. For 5 min, the subjects were resting supine on the tilt table, followed by 5 min of head-up tilt to 60°, and then the tilt table was returned to the horizontal position for 5 min. The electrode configuration( Reference Koobi, Kahonen and Iivainen 21 – Reference Koobi, Kaukinen and Turjanmaa 23 ) and the good repeatability and reproducibility of the measurements has been reported( Reference Tahvanainen, Koskela and Tikkakoski 19 ). Caffeine-containing products, smoking, heavy meal for at least 4 h and alcohol for at least 24 h before the recording were to be avoided.
Statistical analyses
With a sample size of thirty-five per group, the present study had a 82 % power for detecting a 7 mmHg difference in systolic BP by using standard deviation 10, α-level 0·05 and power analysis method for two-sample t test( Reference Dupont and Plummer 24 ). Statistical analyses were performed using SPSS for Windows 17.0 (SPSS, Inc.). Home and office BP were given as the mean of two measurements. For home BP, the values from the last week of each period were used. The changes in response to head-up tilt were calculated as mean differences between supine values for 3–5 min and head-up tilt values for 3–5 min, when the signal was most stable. The results are given as crude means with standard deviations or 95 % CI, unless stated otherwise. The normal distribution of variables was checked with the Shapiro–Wilk test before further analyses. The equality of variances among the study groups was tested with Levene's test for homogeneity of variances. Baseline values between the groups were compared using univariate ANOVA, and changes between the groups with corresponding baseline value as a covariate. ANOVA for repeated measurements was used to test (1) interaction of time and group; (2) differences between groups; (3) differences over time in continuous variables. Post hoc comparisons were adjusted with the Bonferroni correction. Adjustment for age was performed when appropriate. To compare within-group haemodynamic response to tilt at 0 and 12 weeks, paired samples t test was utilised. Non-continuous variables were tested using χ2 test. P< 0·05 was considered statistically significant.
Results
Study subjects and compliance
At baseline, there were no significant differences in sex distribution, BMI, office BP, fasting plasma glucose and insulin, lipids, quantitative insulin sensitivity check index (QUICKI)( Reference Katz, Nambi and Mather 25 ) and estimated glomerular filtration rate( Reference Rule, Larson and Bergstralh 18 ) between the groups (Table 1). The average QUICKI index values were clearly within the insulin-resistant range( Reference Katz, Nambi and Mather 25 ). The mean age was 4–5 years higher in the LTP25+Pse group than in the LTP5+Pse and placebo groups. In addition, in females, the waist:height ratio( Reference Lee, Huxley and Wildman 26 ) was higher in the LTP25+Pse than in the placebo group (Table 1). Body weight and fasting plasma glucose, renin activity, aldosterone, K, Na and K:Na ratio remained virtually unchanged during the study period (Table 2).
Table 1 Clinical and metabolic characteristics of the study subjects at baseline (Mean values and standard deviations; n 104)

LTP5+Pse, 5 mg/d lactotripeptides and 2 g/d plant sterol esters; LTP25+Pse, 25 mg/d lactotripeptides and 2 g/d plant sterol esters; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated creatinine-based glomerular filtration rate.
Mean value was significantly different from that of the placebo group: * P= 0·048, ** P= 0·038.
† Mean value was significantly different from that of the LTP5+Pse group (P= 0·009).
‡ Age as covariate.
§ χ2 test.
∥ Waist:height ratio( Reference Lee, Huxley and Wildman 26 ).
¶ Insulin-sensitivity QUICKI index( Reference Katz, Nambi and Mather 25 ).
‡‡ RULE formula( Reference Rule, Larson and Bergstralh 18 ).
Table 2 Body weight and plasma glucose, electrolytes, renin and aldosterone during the study period (Mean values and standard deviations)

LTP5+Pse, 5 mg/d lactotripeptides and 2 g/d plant sterol esters; LTP25+Pse, 25 mg/d lactotripeptides and 2 g/d plant sterol esters.
* Age as covariate.
The subjects presented with the following diagnoses; however, all of the clinical conditions listed below were stable and well controlled or in remission: allergy (n 21); arthrosis (n 2); asthma (n 1); benign prostate hyperplasia (n 1); depression (n 11); epilepsy (n 1); fibromyalgia (n 1); gastro-oesophageal reflux (n 1); Gilbert's syndrome (n 2); glaucoma (n 1); gout (n 4); hypothyroidism (n 8); paroxysmal atrial fibrillation (n 1); restless legs (n 1); rheumatoid arthritis (n 1); systemic lupus erythematosus (n 1). The medications used by the subjects are tabulated in online Supplementary Digital Content 1.
The mean duration of the intervention period was 84 (sd 4), 83 (sd 4) and 82 (sd 5) d in the LTP5+Pse, LTP25+Pse and placebo groups, respectively. Compliance was excellent, as only one subject (LTP5+Pse group) consumed < 80 % of the test products. Although the subjects were instructed to maintain their normal lifestyle and dietary habits, some subjects reported minor modifications that are shown in online Supplementary Digital Content 2.
Home blood pressure measurements
Mean values of home BP measurements at weeks 0, 8 and 12 are presented in Table 3. Neither of the treatments influenced BP, as there were no significant group × time interactions in systolic and diastolic BP during the study period. Home diastolic BP was lower in the LTP5+Pse group than in the LTP25+Pse group (P= 0·009); however, neither of these groups differed from placebo. Home systolic and diastolic BP were slightly reduced in all groups from baseline to week 12 (Table 3).
Table 3 Home systolic (SBP) and diastolic blood pressure (DBP) during the intervention (Mean values, standard deviations and 95 % confidence intervals)
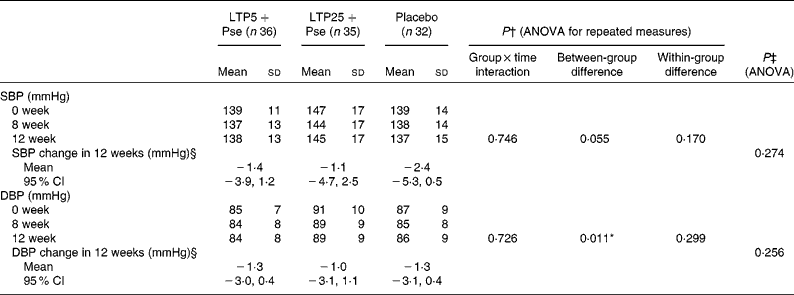
LTP5+Pse, 5 mg/d lactotripeptides and 2 g/d plant sterol esters; LTP25+Pse, 25 mg/d lactotripeptides and 2 g/d plant sterol esters.
* The LTP25+Pse group differed significantly from LTP5+Pse group (P= 0·009) in post hoc comparisons (Bonferroni corrections applied).
† Age as covariate.
‡ Age and baseline measure as covariates.
§ SBP and DBP change in 12 weeks is expressed as means and 95 percent confidence intervals.
Haemodynamic measurements in the laboratory
The haemodynamic baseline data, captured during the 15-min recording protocol, are shown in Fig. 1. ANOVA for repeated measurements (age as covariate) showed a significant group × time interaction in radial systolic (P= 0·009) and diastolic (P= 0·004) BP and systemic vascular resistance index (SVRI) (P= 0·001), but not in cardiac index (P= 0·186), during the 15-min protocol at baseline. Thus, functional haemodynamic regulation was not corresponding in all study groups, and the clearest difference was that the LTP5+Pse group showed lower supine, but not upright, DBP and SVRI than the placebo group.

Fig. 1 Radial systolic blood pressure (a), radial diastolic blood pressure (b), systemic vascular resistance index (SVRI) (c) and cardiac index (CI) (d) during haemodynamic recordings performed at baseline. All subjects underwent the 15-min recordings during which the 5-min head-up tilt was performed between 5 and 10 min. Values are means, with their standard errors represented by vertical bars. (a) P= 0·009 (group × time interaction), (b) P= 0·004 (group × time interaction) and (c) P= 0·001 (group × time interaction). ○, Placebo; ▲, LTP5+Pse (5 mg/d lactotripeptides and 2 g/d plant sterol esters); ■, LTP25+Pse (25 mg/d lactotripeptides and 2 g/d plant sterol esters).
Changes in the haemodynamic variables during the 15-min recording protocol from week 0 to week 12 are presented in Fig. 2. ANOVA for repeated measurements (with age as covariate) showed no significant group × time interactions, or differences between the groups or within the groups, in the changes in radial systolic and diastolic BP, SVRI or cardiac index. Similarly, no BP-lowering effects of LTP+Pse diets were observed at week 8 (data not shown).

Fig. 2 Changes in radial systolic blood pressure (a), radial diastolic blood pressure (b), systemic vascular resistance index (SVRI) (c) and cardiac index (CI) (d) during the 15-min recording protocol as calculated from the first recording (at 0 weeks) to the third recording (after 12 weeks of intervention). The 5-min head-up tilt was performed between 5 and 10 min. Values are means, with their standard errors represented by vertical bars. ○, Placebo; ▲, LTP5+Pse (5 mg/d lactotripeptides and 2 g/d plant sterol esters); ■, LTP25+Pse (25 mg/d lactotripeptides and 2 g/d plant sterol esters).
The cardiovascular response to head-up tilt at baseline and after 12 weeks of intervention is shown in Fig. 3. Within groups, there were no significant differences in the systolic BP or SVRI response to head-up tilt at weeks 0 and 12; however, in the LTP5+Pse and placebo group, the cardiac index response to head-up tilt showed a greater reduction at week 12 than at baseline (P= 0·008 and P= 0·036, respectively). In addition, the diastolic BP response to head-up tilt decreased in the LTP5+Pse group (P= 0·046). The increase in SVRI during head-up tilt was lower in the LTP25+Pse group than in the LTP5+Pse group both at baseline and at week 12 (P< 0·01), showing consistent functional differences in haemodynamics between these groups.

Fig. 3 Haemodynamic responses in the 5 mg/d lactotripeptides and 2 g/d plant sterol esters (LTP5+Pse), 25 mg/d lactotripeptides and 2 g/d plant sterol esters (LTP25+Pse) and placebo groups to upright posture at baseline (□) and after 12 weeks of intervention (![]() ): radial systolic blood pressure (BP) (a), radial diastolic BP (b), systemic vascular resistance index (SVRI) (c) and cardiac index (d). Values are means, with their standard errors represented by vertical bars. Within groups, the haemodynamic responses at weeks 0 and 12 did not differ, apart from the accentuated cardiac index decline in the LTP5+Pse and placebo groups at week 12 (P= 0·008 and P= 0·036, respectively), and the diastolic BP decline in the LTP5+Pse group (P= 0·046). Paired-samples t test applied in within-group comparisons, ANCOVA with age as covariate applied in between-group comparisons, with Bonferroni corrections in post hoc comparisons. * Mean value was significantly different from that of the placebo group (P< 0·05). Mean value was significantly different from that of the LTP5+Pse group: † P< 0·05, †† P< 0·01. Mean value was significantly different from that at baseline for the same group: ‡ P< 0·05.
): radial systolic blood pressure (BP) (a), radial diastolic BP (b), systemic vascular resistance index (SVRI) (c) and cardiac index (d). Values are means, with their standard errors represented by vertical bars. Within groups, the haemodynamic responses at weeks 0 and 12 did not differ, apart from the accentuated cardiac index decline in the LTP5+Pse and placebo groups at week 12 (P= 0·008 and P= 0·036, respectively), and the diastolic BP decline in the LTP5+Pse group (P= 0·046). Paired-samples t test applied in within-group comparisons, ANCOVA with age as covariate applied in between-group comparisons, with Bonferroni corrections in post hoc comparisons. * Mean value was significantly different from that of the placebo group (P< 0·05). Mean value was significantly different from that of the LTP5+Pse group: † P< 0·05, †† P< 0·01. Mean value was significantly different from that at baseline for the same group: ‡ P< 0·05.
Plasma lipids and lipoproteins
The changes in plasma lipid concentrations from baseline to week 12 are presented in Table 4. There was a numerical but not statistically significant tendency towards lower plasma LDL-cholesterol concentration after the intervention in the test groups (P= 0·066). When the two test groups with similar daily doses of Pse were analysed together as one group, the test treatment decreased LDL-cholesterol concentration compared with placebo (P= 0·024). Plasma total cholesterol, HDL-cholesterol and TAG concentrations were virtually unchanged in all study groups.
Table 4 Changes in plasma lipid concentrations during the study (Mean values and 95 % confidence intervals; n 104)
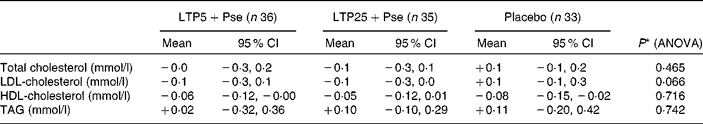
LTP5+Pse, 5 mg/d lactotripeptides and 2 g/d plant sterol esters; LTP25+Pse, 25 mg/d lactotripeptides and 2 g/d plant sterol esters.
* Age and baseline value as covariates.
Discussion
At present, the evidence about the putative beneficial effect of LTP on BP remains contradictory( Reference Xu, Qin and Wang 7 , Reference Engberink, Schouten and Kok 10 – Reference van Mierlo, Koning and van der Zander 12 ). Therefore, we conducted for the first time a clinical trial utilising detailed haemodynamic measurements to examine the possible BP-lowering efficacy of two doses of LTP (5 v. 25 mg daily) in a long-term, 12-week intervention. Since simultaneous reduction in plasma lipids can be anticipated to provide additional cardiovascular benefits, both of the LTP-products were fortified with Pse (daily dose 2 g). However, the present results showed that intake of Ile-Pro-Pro and Val-Pro-Pro with Pse in fermented milk product was without significant antihypertensive effect; however, a borderline lipid-lowering effect was observed in 104 subjects with the MetS. Of note, the results on BP were consistent in measurements performed both at home and in the laboratory. The study also uncovered a significant haemodynamic difference in the SVRI response to head-up tilt between the LTP5+Pse and LTP25+Pse groups both at baseline and after 12 weeks of intervention. Thus, these two groups showed persistent functional cardiovascular differences in the supine to upright regulation of peripheral vascular resistance.
Previously, four meta-analyses including Asian and Caucasian subjects in 12–19 placebo-controlled trials, with interventions lasting from 4 to 21 weeks, and daily peptide doses ranging from 2 to 52·5 mg, have reported that products containing bioactive peptides may reduce BP by about 4–5/2 (systolic/diastolic) mmHg( Reference Cicero, Gerocarni and Laghi 5 – Reference Pripp 8 ). Based on these previous studies, our 12-week intervention with doses of 5 and 25 mg of LTP should have been sufficient for detecting a lowering effect on BP, if present. Of note, LTP have been suggested to show more pronounced BP-lowering effect in hypertensive subjects than in pre-hypertensive subjects( Reference Xu, Qin and Wang 7 ). The present study population had a clearly elevated BP with a mean office BP of 163/100 mmHg at screening, and a mean home BP of 142/88 mmHg in the beginning of the intervention.
Corresponding to the present results, Dutch intervention studies have shown no significant decreases in BP after intake of 4·6–14 mg/d of Ile-Pro-Pro and Val-Pro-Pro for 4–8 weeks( Reference Engberink, Schouten and Kok 10 – Reference van Mierlo, Koning and van der Zander 12 ). Furthermore, the BP-lowering effect of LTP may be influenced by ethnic factors. In the meta-analysis by Cicero et al. ( Reference Cicero, Gerocarni and Laghi 5 ), the effect of LTP is more pronounced in Asian subjects (systolic BP − 6·93 mmHg; diastolic BP − 3·98 mmHg) than in European subjects (systolic BP − 1·17 mmHg; diastolic BP − 0·52), and is not related to age, baseline BP, dose of LTP or length of the treatment. One possibility is that differences in background diet may explain the variable responses to intake of LTP.
Results from experimental studies have suggested that LTP may have competitive ACE inhibiting activity( Reference Lehtinen, Jauhiainen and Kankuri 27 ); however, this has not been confirmed in human subjects( Reference Usinger, Ibsen and Linneberg 28 ). ACE inhibitor drugs that are used in the treatment of CVD have been reported to increase plasma renin activity, reduce plasma aldosterone concentration( Reference Azizi, Chatellier and Guyene 29 – Reference MacFadyen, Elliott and Meredith 31 ) and elevate plasma K concentration( Reference Alderman, Piller and Ford 32 , Reference de Denus, Tardif and White 33 ). During the present study, there were no significant differences in plasma renin activity, plasma aldosterone and K concentrations, and plasma K:Na ratio between the groups. These findings, together with the absence of changes in BP, indicate that the possible ACE inhibiting activity of the study products in vivo was not sufficiently strong to elicit detectable changes in the aforementioned variables.
Also, other modes of antihypertensive action for LTP, such as inhibition of the sympathetic nervous system and an effect on cardiac output, have been suggested( Reference Usinger, Ibsen and Linneberg 28 , Reference Cicero, Rosticci and Gerocarni 34 ). Intake of Val-Pro-Pro and Ile-Pro-Pro slightly improved variables of cardiac flow (stroke volume and stroke index) and contractility (acceleration index and velocity index), but was without effect on variables of fluid dynamics or vascular resistance in subjects with high-normal BP or mild hypertension( Reference Cicero, Rosticci and Gerocarni 34 ). The present study showed no effect of LTP on systemic vascular resistance and cardiac index, which is in agreement with the results reported by Cicero et al. ( Reference Cicero, Rosticci and Gerocarni 34 ). One previous study utilising a tilt table test has suggested that LTP intake may have a mild lowering effect on sympathetic activity, since plasma noradrenaline response to head-up tilt was reduced after 8 weeks of intervention, although no significant changes were seen in BP and heart rate( Reference Usinger, Ibsen and Linneberg 28 ). In the present study, LTP treatment was without influence on radial BP either in supine position or during head-up tilt.
In the present investigation, a simultaneous intake of LTP and Pse was used. In experimental studies, combined treatment with LTP and Pse had been found to attenuate the elevation of BP in spontaneously hypertensive rats( Reference Jakala, Pere and Lehtinen 35 ). However, the antihypertensive effect of combined LTP+Pse intake is less marked than the effect of LTP treatment alone( Reference Jakala, Pere and Lehtinen 35 ), while Pse treatment alone did not affect BP( Reference Ehlers, Kivimaki and Siltari 36 ). Further, plant sterols have even been reported to increase BP in salt-loaded stroke-prone spontaneously hypertensive rats( Reference Ogawa, Yamamoto and Kamisako 37 ). Based on these experimental results, it can be speculated whether the simultaneous administration of Pse attenuated the possible BP-lowering effect of LTP in the present study, and whether an even higher dose of LTP would have resulted in the lowering of BP. Nevertheless, in previous human studies, the simultaneous intake of LTP and Pse for 10 weeks had been shown to decrease systolic BP at home measurements, but not in the office or during 24-h ambulatory recordings( Reference Turpeinen, Ikonen and Kivimaki 15 , Reference Turpeinen, Kumpu and Ronnback 16 ). In addition, a recent study has suggested an acute BP-lowering effect 8 h post-dose of fermented milk product containing 25 mg LTP and 2 g Pse in subjects with mild hypertension( Reference Turpeinen, Ehlers and Kivimaki 38 ).
The present cholesterol-lowering effect of 2 g daily Pse intake remained modest when compared with a previous meta-analysis reporting a mean reduction of 0·31 mmol/l in plasma LDL-cholesterol concentration( Reference AbuMweiss, Marinangeli and Frohlich 14 ). Earlier, the efficacy of plant stanols and sterols has been studied in different food matrices including low-fat milk products( Reference AbuMweiss, Marinangeli and Frohlich 14 ), and the beneficial effects have been confirmed in both normocholesterolaemic and hypercholesterolaemic subjects( Reference Hendriks, Weststrate and van Vliet 39 ), as well as in type 2 diabetic patients( Reference Baker, Baker and Coleman 40 ). However, the efficacy of phytosterols in subjects with the MetS is contradictory, since negative results have been reported after intake of Pse-enriched milk, breakfast cereal and margarine( Reference Hernandez-Mijares, Banuls and Jover 41 , Reference Ooi, Watts and Barrett 42 ), while consumption of plant stanol ester yogurt drink has been shown to lower non-HDL-cholesterol in the MetS subjects( Reference Plat, Brufau and Dallinga-Thie 43 ). In particular, reduced intestinal cholesterol absorption observed in the MetS subjects may interfere with the mechanism of action and efficacy of the plant sterols( Reference Hernandez-Mijares, Banuls and Jover 41 ). It should also be noted that seasonal variation of blood lipid levels in hypercholesterolaemic subjects, with a characteristic peak of total and LDL-cholesterol in fall/winter and trough in spring/summer( Reference Ockene, Chiriboga and Stanek 44 ) may have attenuated the cholesterol-lowering effect of the present study, half of which was conducted in fall. We observed a slight numeric increase in the mean values of total and LDL-cholesterol of the placebo group.
In addition to hypertension and dyslipidaemia, central obesity and insulin resistance have been associated with the MetS( Reference Beltran-Sanchez, Harhay and Harhay 1 ). During the present intervention, BP, body weight and plasma glucose remained unaltered in all study groups. We did not investigate the change in plasma insulin at the end of the study. However, the absence of alterations in the above cardiometabolic risk factors suggests that insulin sensitivity did not significantly change in the groups during the study.
The particular strength of the present study is the randomised, double-blind, placebo-controlled design. In addition to home BP measurements, we utilised detailed cardiovascular reactivity test for determining haemodynamic information in both supine position and during orthostatic challenge. Furthermore, we used two doses (5 and 25 mg) of LTP and 12-week intervention. Compliance was excellent, and according to the unchanged body weight, the subjects were able to maintain their habitual lifestyle and dietary pattern during the intervention. Characteristically of studies examining the antihypertensive influences of various treatments, BP and SVRI were numerically lower after the 12-week follow-up also in the placebo group( Reference Gould, Mann and Davies 45 ).
The present study has some limitations. There were differences between the groups in the demographic characteristics at baseline, in spite of the randomisation protocol. Age was higher in the LTP25+Pse group than in the LTP5+Pse and placebo groups, and in female subjects, waist:height ratio was higher in the LTP25+Pse group than in the placebo group. In addition, the results showed persistent functional differences in upright haemodynamics between the groups, since the increase in SVRI during orthostatic challenge remained lower in the LTP25+Pse group than in the LTP5+Pse group throughout the study. A cross-over protocol, instead of the present parallel design, would have reduced possible confounding resulting from the divergent cardiovascular regulation in the study groups. However, the differences in the control of peripheral vascular resistance during head-up tilt were difficult to anticipate, as there is very little previous information about dietary influences on upright cardiovascular regulation. As the present parallel study included two intervention groups and a control group during a relatively long-term period (12 weeks), a corresponding cross-over design with each subject receiving all treatments would have been more strenuous to carry out (36 weeks altogether), and this might also have reduced compliance. Finally, consumption of the study products was monitored by the use of study diaries, and compliance was calculated from the registrations of the study subjects. In order to really ensure the accurate intake of the study products, an intervention with a laborious controlled feeding protocol should have been used( Reference Appel, Moore and Obarzanek 46 ).
In conclusion, the present study showed no antihypertensive effect of LTP and Pse intake in fermented milk product at home or in the laboratory, but a mild lipid-lowering effect in subjects with the MetS.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114515002032
Acknowledgements
The authors are grateful to Reeta Kulmala, Research Nurse, and Paula Erkkilä, Research Nurse, for invaluable assistance.
The present study was supported by Valio Limited, Helsinki, Finland, and the Finnish Foundation for Cardiovascular Research, Paavo Nurmi Foundation, Pirkanmaa Regional Fund of the Finnish Cultural Foundation, Competitive Research Funding of the Pirkanmaa Hospital District, Tampere Tuberculosis Foundation and the Sigrid Jusélius Foundation.
T. M. and A. M. T. are the present employees of Valio Limited, and S. L. and R. K. are the former employees of Valio Limited. Fermented milk products, which were delivered to the participants in all three study groups, were received from commercial source Valio Limited. Other authors have no conflicts of interest to declare.
The authors' contributions are as follows: M. K., T. M., A. M. T., H. V., R. K. and I. H. P. formulated the research questions; M. K., T. M., A. M. T., H. V., R. K. and I. H. P. designed the study; A. J. T., A. T., S. L., O. N. and I. H. P. carried out the study; E. J. H., A. J. T., K. N. and I. H. P. analysed the data; E. J. H., A. M. T., H. V. and I. H. P. wrote the article.










