Diabetes mellitus is a complex metabolic disorder characterised by persistent hyperglycaemia resulting from defects in insulin secretion, insulin action or both. The two main types of diabetes mellitus are type 1 (formerly known as insulin-dependent diabetes), and type 2 (formerly known as non-insulin-dependent diabetes). Type 1 diabetes is caused by the autoimmune destruction of the β-cells of the pancreatic islets, whereas type 2 diabetes results from both impaired insulin secretion and resistance to the action of insulin. Diabetes affects around 3% of the adult UK population (Reference Bennett, Dodd and FlatleyBennett et al, 1995). As the prevalence increases with age, the lifetime risk of developing diabetes is around 10% (Reference Neil, Gatling and MatherNeil et al, 1987).
Diabetes is associated with premature mortality, predominantly through atherosclerotic vascular disease (World Health Organization/International Diabetes Federation, 1999). Microvascular complications, which affect the small blood vessels in the eye, kidney and nerves, are associated with considerable morbidity. The economic and social costs of diabetes are enormous, both for health care services and through loss of productivity. In developed countries, 10% or more of the total health budget is spent on the management of diabetes and its complications (Reference JonssonJonsson, 2002; Reference Zimmet, Shaw and AlbertiZimmet et al, 2003).
The prevalence of type 2 diabetes is increased in patients with schizophrenia compared with the general population (Reference Holt, Peveler and ByrneHolt et al, 2004). Although many factors, including genetic and lifestyle issues, contribute to this association, there has been a recent surge in interest in the subject because of the possible link between the use of atypical antipsychotic drugs and the development of diabetes. The increased prevalence of type 2 diabetes among people with schizophrenia has implications for the delivery of care by psychiatrists, as well as for diabetologists and general practitioners. The purpose of this paper is therefore to provide an update on current thinking about diabetes for practising psychiatrists, in order to improve the physical health of their patients. Since type 2 diabetes is the primary cause of concern for patients with schizophrenia, it is the main focus of this review.
METHOD
The current diabetes literature, the National Service Framework for Diabetes, the National Institute for Clinical Excellence guidance on the management of diabetes and literature produced by Diabetes UK were reviewed.
RESULTS
A brief history of diabetes
The oldest description of diabetes as a polyuric state dates back to 1550 bc in ancient Egypt. The word ‘diabetes’, derived from the Greek meaning ‘siphon’ or ‘pass through’, was first used by Aretaeus of Cappadocia in the 2nd century ad when he gave a clinical description of the disease. In the 5th and 6th centuries, Indian physicians recognised the sweet, honey-like taste of urine from polyuric patients, which attracted ants and insects. The Indian descriptions of diabetes mellitus also recognised the distinction between two forms of diabetes: one in older, fatter people, and the other in thin people who rapidly succumbed to their illness.
The sweetness in the urine was rediscovered by the Englishman Thomas Willis, physician to King Charles II in the 17th century, who also noted the importance of lifestyle when he remarked that the prevalence of diabetes was increasing because of ‘good fellowship and gusling down chiefly of unalloyed wine’. A century later, Matthew Dobson in 1776 showed that the urinary sweetness was caused by sugar and was associated with a rise in blood sugar. At the turn of the 18th century, John Rollo was the first person to use the term ‘diabetes mellitus’ (honey) to distinguish the condition from ‘diabetes insipidus’ (tasteless).
Claude Bernard, a French physiologist, made several important advances in the 19th century when he discovered that sugar was stored in the liver in the form of glycogen, and that transfixion of the medulla in conscious rabbits caused hyperglycaemia. Paul Langerhans, also working in the 19th century, discovered the pancreatic islets that now bear his name, although it was Edouard Laguesse in 1893 who suggested that they were the endocrine tissue of the pancreas. In 1889, Joseph von Mering and Oskar Minkowski removed the pancreas from a dog and discovered that the animal developed diabetes, demonstrating the important link between the pancreas and diabetes.
Several workers in different institutions followed this observation by attempting to isolate the blood glucose lowering agent from the pancreas, culminating in the discovery of insulin in 1921 by a team based at the University of Toronto, comprising Frederick Banting, Charles Best, James Collip and J. R. Macleod. In 1922, Leonard Thompson was the first person to receive insulin, thus transforming the management of diabetes forever.
Although insulin has major actions on lipid and protein metabolism, this historical perspective explains why the focus of diabetic management has been the normalisation of blood glucose levels and why the diagnosis of diabetes is based on the finding of chronic hyperglycaemia.
Diagnosis of diabetes
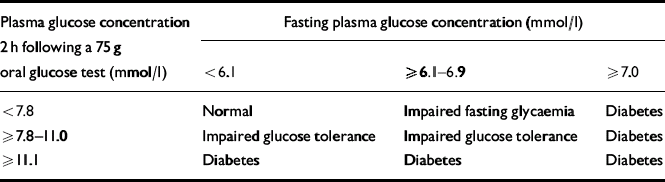
Before the late 1970s, there was no consensus on the diagnostic criteria for diabetes. This led to much confusion and precluded any meaningful comparison of the prevalence of diabetes within or between populations (Reference WestWest, 1975). In 1965 the World Health Organization (WHO) prepared its first expert report on the diagnosis of diabetes, and these diagnostic criteria were subsequently modified and simplified by the WHO and the National Diabetes Group in the USA in 1979, 1980 and 1985. The American Diabetes Association (ADA) then proposed diagnostic criteria that placed a greater emphasis on fasting blood glucose concentration (Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, 1997). In response, the WHO updated its diagnostic criteria (Table 1).
Table 1 1999 World Health Organization diagnostic criteria for diabetes mellitus (World Health Organization, 1999)
| Plasma glucose concentration 2 h following a 75 g oral glucose test (mmol/l) | Fasting plasma glucose concentration (mmol/l) | ||
|---|---|---|---|
| <6.1 | ≥ 6.1-6.9 | ≥ 7.0 | |
| <7.8 | Normal | Impaired fasting glycaemia | Diabetes |
| ≥ 7.8-11.0 | Impaired glucose tolerance | Impaired glucose tolerance | Diabetes |
| ≥ 11.1 | Diabetes | Diabetes | Diabetes |
According to the 1999 WHO criteria (which are also endorsed by Diabetes UK), a diagnosis of diabetes is made if the fasting blood glucose level is 7.0 mmol/l or more, or a random blood glucose test shows a level of 11.1 mmol/l or more. Only one test is required in a patient with classical diabetic symptoms; a supplementary test is required in asymptomatic individuals. The WHO recommends under these circumstances that a 75 g oral glucose tolerance test is performed.
Although the diagnostic criteria of the WHO have a slightly different emphasis to those of the ADA, for clinical and epidemiological purposes they are essentially identical, and can be considered to represent an international consensus.
It is instructive to consider why there has been so much debate about the diagnosis of diabetes. Plasma glucose concentrations show a skewed normal distribution in the general population, and the delineation of abnormal from normal thus becomes arbitrary. The WHO and ADA diagnostic criteria reflect the concentration of plasma glucose at which there is an association with the development of microvascular complications, particularly retinopathy (Reference Pettitt, Knowler and LissePettitt et al, 1980). Although the relationship between the exposure to hyperglycaemia and microvascular complications is exponential – allowing for a diagnostic cut-off at the inflection of the curve – the relationship between hyperglycaemia and macrovascular complications is more linear, suggesting that there is no threshold for the development of cardiovascular disease (Fig. 1; Reference Stratton, Adler and NeilStratton et al, 2000). This implies that cardiovascular risk will also increase with increases in blood glucose concentrations across the normal population.
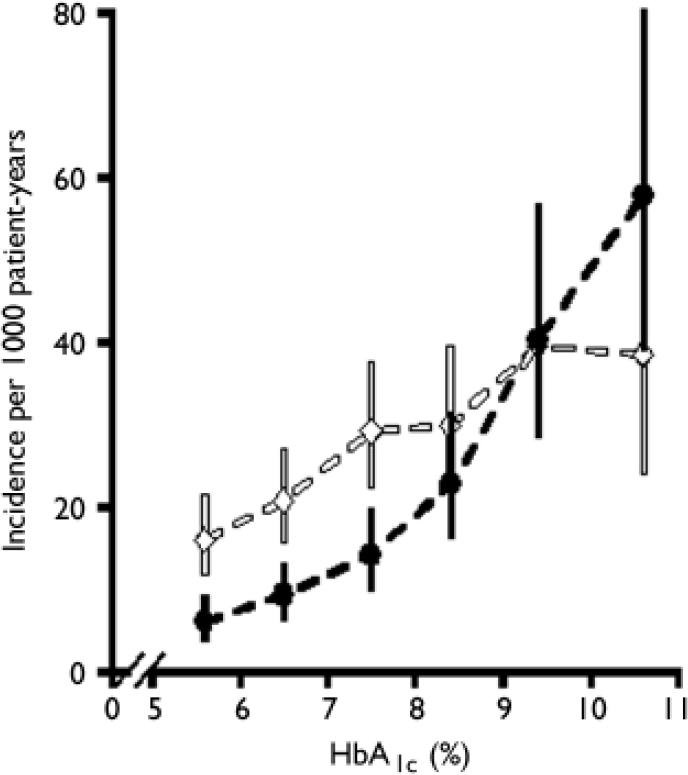
Fig. 1 Relationship between glycosylated haemoglobin (HbA1c) and microvascular disease (black line) and macrovascular complications (myocardial infarction, white line) in 4585 patients with type 2 diabetes (error bars, 95% CI). Reproduced from Stratton et al (Reference Stratton, Adler and Neil2000) BMJ, 321, 405–412 with permission from the BMJ Publishing Group.
The WHO diagnostic criteria also recognise two further categories: impaired fasting glycaemia and impaired glucose tolerance; the latter can only be diagnosed following a 75 g oral glucose tolerance test. Impaired fasting glycaemia and impaired glucose tolerance are not distinct clinical entities, but rather risk factors for future diabetes and cardiovascular disease (DECODE Study Group, 2001). Indeed, the ADA has suggested that the new term ‘pre-diabetes’ be used to describe both these abnormalities (American Diabetes Association, 2002).
A diagnosis of diabetes has important social, legal and medical implications for the patient, and it is therefore essential that any diagnosis is secure. Diabetes should not be diagnosed on the basis of glycosuria, and the blood glucose concentration should be measured on a venous plasma sample in an accredited laboratory (World Health Organization, 1999). At present, diabetes cannot be diagnosed by measuring glycosylated haemoglobin (HbA1c).
Classification of diabetes
The original WHO classification of diabetes introduced in 1980 and revised in 1985 was based on clinical characteristics. The two most common types of diabetes were insulin-dependent diabetes mellitus (IDDM) and non-insulin-dependent diabetes mellitus (NIDDM), and this nomenclature reflected the need for insulin to survive. The WHO classification also recognised malnutrition-related diabetes mellitus, other types of diabetes mellitus associated with specific conditions, and gestational diabetes, which is diabetes diagnosed for the first time during pregnancy. In 1997, the ADA proposed a classification that distinguished the types of diabetes according to aetiology, and this was subsequently endorsed by the WHO (see Appendix) (Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, 1997; World Health Organization, 1999). The terms IDDM and NIDDM were considered to be confusing, with many clinicians categorising insulin-treated patients with NIDDM as having IDDM. The preferred terminology is now type 1 diabetes, which is equivalent to IDDM, and type 2 diabetes, which is equivalent to NIDDM. Malnutrition-related diabetes was omitted from the new classification because its aetiology is uncertain, and it is unclear whether it is a separate type of diabetes.
Type 1 diabetes
Epidemiology
Type 1 diabetes is caused by an absolute deficiency of insulin, and it represents around 10% of all cases of diabetes, affecting approximately 20 million people worldwide (American Diabetes Association, 2001). Although type 1 diabetes affects all age groups, the majority of individuals are diagnosed either at around the age of 4–5 years, or in their teens and early adulthood (Reference Bloom, Hayes and GambleBloom et al, 1975).
The incidence of type 1 diabetes is rising. Across Europe, the average annual increase in the incidence in children under 15 years old is 3.4% (EURODIAB ACE Study Group, 2000), with the steepest rise in those under 5 years old (Reference Karvonen, Pitkaniemi and TuomilehtoKarvonen et al, 1999).
Aetiology
The aetiology of type 1 diabetes remains poorly understood, but it is likely that an environmental factor triggers an autoimmune process in a predisposed individual. Although the genetic susceptibility to type 1 diabetes is inherited, only 12–15% of type 1 diabetes occurs in families. Twin studies have shown that the concordance rate for type 1 diabetes in monozygotic twins is around 20–30% (Reference Barnett, Eff and LeslieBarnett et al, 1981).
Studies indicate that genetic factors do not account entirely for the development of type 1 diabetes, and several environmental triggers, including viral infections, nutritional factors, parental age and low birth weight, have been implicated (Reference Akerblom, Vaarala and HyotyAkerblom et al, 2002).
Type 2 diabetes
Epidemiology
Type 2 diabetes is a heterogeneous disorder that results from an interaction between a genetic predisposition and environmental factors. It accounts for around 90% of all cases of diabetes. There are approximately 1.4 million people with diagnosed type 2 diabetes in the UK, with a further 1 million having undiagnosed type 2 diabetes (Reference Bennett, Dodd and FlatleyBennett et al, 1995). The incidence of diabetes increases with age, with most cases being diagnosed after the age of 40 years. This equates to a lifetime risk of developing diabetes of 1 in 10 (Reference Neil, Gatling and MatherNeil et al, 1987).
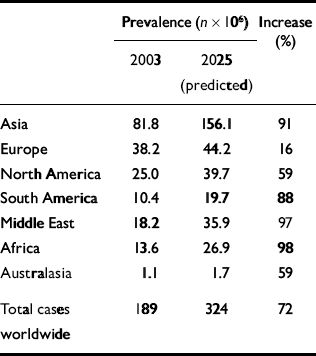
The prevalence of diabetes is increasing rapidly. The World Health Organization (2003) has predicted that by 2030 the number of adults with diabetes will have almost doubled worldwide, from 177 million in 2000 to 370 million. Using the WHO's data, Zimmet et al (Reference Zimmet, Shaw and Alberti2003) have compared current and predicted prevalence for certain regions for the years 2003 and 2025 (Table 2). A marked geographical variation in the prevalence of type 2 diabetes exists (Reference ZimmetZimmet, 1982). The highest rates are found in the Pima Native Americans of Arizona and in the inhabitants of the South Pacific island of Nauru, where approximately half the adult population have diabetes. In contrast, in the rural communities of China and Chile, the prevalence is less than 1%. In general, rates of type 2 diabetes are higher in urban populations than in rural communities (Reference ZimmetZimmet, 1982).
Table 2 Worldwide prevalence of diabetes
| Prevalence (n × 106) | Increase (%) | ||
|---|---|---|---|
| 2003 | 2025 (predicted) | ||
| Asia | 81.8 | 156.1 | 91 |
| Europe | 38.2 | 44.2 | 16 |
| North America | 25.0 | 39.7 | 59 |
| South America | 10.4 | 19.7 | 88 |
| Middle East | 18.2 | 35.9 | 97 |
| Africa | 13.6 | 26.9 | 98 |
| Australasia | 1.1 | 1.7 | 59 |
| Total cases | 189 | 324 | 72 |
| worldwide | |||
Regional and ethnic differences in the prevalence of type 2 diabetes reflect not only differences in environment, but also differences in genetic susceptibility. For example, in the UK people from the Indian subcontinent living in Southall have a rate of diabetes four times higher than the local White population (Reference Mather and KeenMather & Keen, 1985).
Genetic predisposition. The heritability of type 2 diabetes is greater than for type 1 diabetes, and is estimated to account for 40–80% of total disease susceptibility. Many patients will have a family history of diabetes (Reference Thomas, Balkau and Vauzelle KervroedanThomas et al, 1994), and twin studies show a high concordance rate (60–90%) in monozygotic twins (Reference Barnett, Eff and LeslieBarnett et al, 1981). A maternal history of diabetes confers a higher risk of type 2 diabetes in the offspring than a paternal history of diabetes (Reference Thomas, Balkau and Vauzelle KervroedanThomas et al, 1994), possibly through an effect of maternal hyperglycaemia during pregnancy (Reference Pettitt, Nelson and SaadPettitt et al, 1993).
Type 2 diabetes is a polygenic disorder, and it is clear that no single major locus explains its inheritance. There appear to be many candidate genes involved in controlling insulin secretion and action, and these are all likely to play a part in the development of type 2 diabetes.
Environmental factors. The most important environmental risk factors for diabetes are obesity and physical inactivity. The massive explosion in obesity rates worldwide has largely been responsible for the increase in diabetes, and it is estimated that up to 80% of all new cases of diabetes can be attributed to obesity (Reference LeanLean, 2000). In the UK, the average body mass index (BMI) of a person with type 2 diabetes is 30.0 kg/m2 (Reference JonssonJonsson, 2002); in the USA, 67% of those with type 2 diabetes have a BMI of more than 27 kg/m2 and 46% have a BMI of more than 30 kg/m2 (National Task Force on the Prevention and Treatment of Obesity, 2000). The risk of developing type 2 diabetes increases across the normal range of BMI, such that the risk in a middle-aged woman whose BMI is over 35 kg/m2 is 93.2 times greater than in a woman whose BMI is below 22.5 kg/m2 (Fig. 2; Reference Colditz, Willett and RotnitzkyColditz et al, 1995).
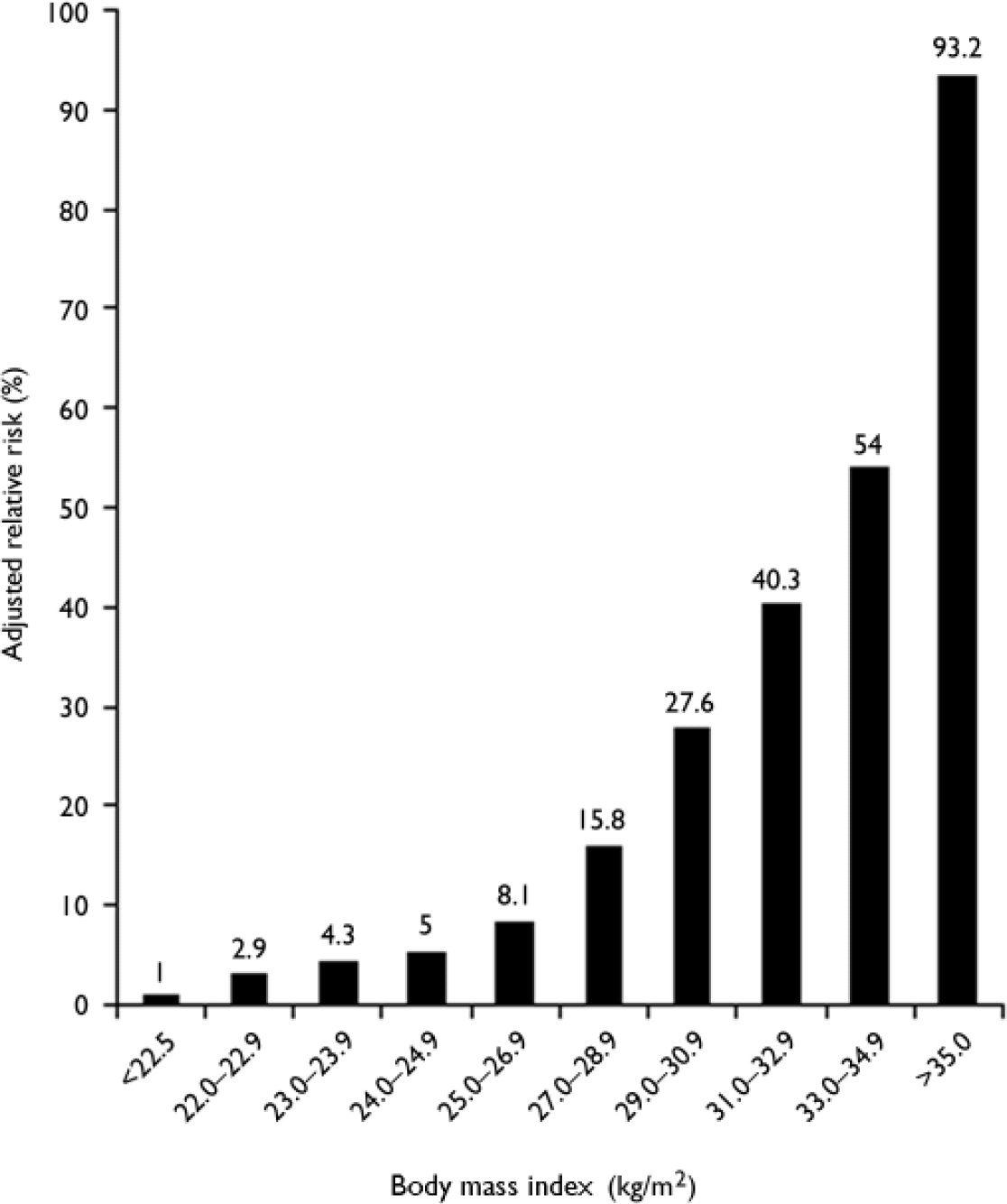
Fig. 2 Risk of developing diabetes according to body mass index (BMI) in 114 281 women participants in the US Nurses’ Health Study. Data from Colditz et al (Reference Colditz, Willett and Rotnitzky1995).
In addition to total adiposity, the distribution of fat is important. For any given level of obesity, the more visceral fat an individual has, the greater the risk of developing diabetes (Reference Carey, Walters and ColditzCarey et al, 1997). Physical inactivity is also associated with an increased risk of diabetes (Reference Hu, Sigal and Rich EdwardsHu et al, 1999). In the US Nurses’ Health Study, those in the top quintile for exercise (equivalent to more than 200 min of exercise per week) had a 46% lower risk of diabetes compared with those in the lowest quintile (less than 20 min of exercise). Although some of the difference can be explained by differences in adiposity, after adjusting for BMI those in the most active quintile still had a 26% reduction in risk of diabetes.
The intrauterine environment is also considered to be important in the development of type 2 diabetes. There are now over 48 reports linking poor foetal growth with impaired glucose metabolism in later life (Reference Newsome, Shiell and FallNewsome et al, 2003). In most of these studies, birth weight has an inverse relationship with plasma glucose and insulin concentrations, the prevalence of type 2 diabetes and measures of insulin resistance and secretion. Birth weight is a crude measure of foetal growth, and it appears that adult glucose intolerance is more closely associated with thinness at birth, as assessed by the birth ponderal index (weight/length3) (Reference Phillips, Barker and HalesPhillips et al, 1994).
Pathogenesis
Under normal physiological conditions, plasma glucose concentrations are maintained within a narrow range, despite wide fluctuations in supply and demand, through a tightly regulated and dynamic interaction between tissue sensitivity to insulin (especially in the liver) and insulin secretion (Reference DeFronzoDeFronzo, 1988). In type 2 diabetes these mechanisms break down, with the consequence that the two main pathophysiological defects in type 2 diabetes are impaired insulin secretion through a dysfunction of the pancreatic β-cell, and impaired insulin action through insulin resistance.
Insulin resistance. Insulin resistance is now regarded as being synonymous with a reduced rate of whole-body insulin-mediated glucose disposal in insulin-sensitive tissues (Reference DeFronzo and FerranniniDeFronzo & Ferrannini, 1991; Reference Moller and FlierMoller & Flier, 1991). This definition is too narrow, and insulin resistance may be better defined as existing when normal insulin concentrations fail to produce a normal biological response (Reference KahnKahn, 1978). The main advantage of the latter definition is that it does not restrict consideration of insulin action to a solitary aspect of intermediary metabolism.
The importance of insulin actions on other aspects of intermediary metabolism, including lipid and protein metabolism, has now been appreciated. In 1988, Reaven described syndrome X (now commonly known as the metabolic syndrome or insulin resistance syndrome) as the association between several cardiovascular risk factors, including hypertension, dyslipidaemia and glucose tolerance, and he proposed that insulin resistance was the underlying cause (Reference ReavenReaven, 1988). Since his original description, it has been recognised that a significant proportion of patients with the metabolic syndrome do not have insulin resistance (Reference Ford, Giles and DietzFord et al, 2002). The definition of the metabolic syndrome has therefore been modified: most notably, central obesity has been added as a core feature. This is important, as it is increasingly recognised that central obesity is fundamental to the origin of this disorder (Reference Maison, Byrne and HalesMaison et al, 2001).
The metabolic syndrome is thought to affect just under a quarter of American adults (Reference Ford, Giles and DietzFord et al, 2002), and it is known to be a significant risk factor for type 2 diabetes and cardiovascular disease. However, one major problem with the concept of the metabolic syndrome has been the lack of specific diagnostic criteria or an easy measure of insulin resistance. Fortunately, the publication of diagnostic criteria, by the National Cholesterol Education Program and the WHO (Table 3), has largely rectified this problem (Reference Alberti and ZimmetAlberti & Zimmet, 1998; Expert Panel on Detection, Evaluation, and Treatment of High Cholesterol in Adults, 2001). These criteria have facilitated epidemiological research as well as improving identification of individuals at risk of diabetes and cardiovascular disease.
Table 3 Metabolic syndrome: National Cholesterol Education Program (NCEP) Adult Treatment Program III and World Health Organization (WHO) diagnostic criteria
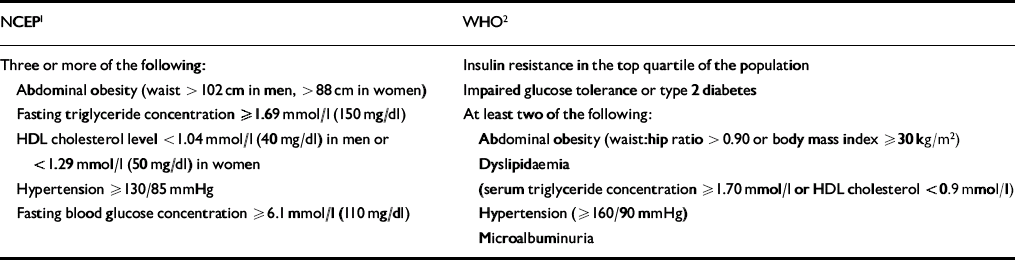
| NCEP1 | WHO2 |
|---|---|
| Three or more of the following: | Insulin resistance in the top quartile of the population |
| Abdominal obesity (waist > 102 cm in men, > 88 cm in women) | Impaired glucose tolerance or type 2 diabetes |
| Fasting triglyceride concentration ≥1.69 mmol/l (150 mg/dl) | At least two of the following: |
| HDL cholesterol level <1.04 mmol/l (40 mg/dl) in men or <1.29 mmol/l (50 mg/dl) in women | Abdominal obesity (waist:hip ratio >0.90 or body mass index ≥30 kg/m2) |
| Dyslipidaemia | |
| Hypertension ≥130/85 mmHg | (serum triglyceride concentration ≥1.70 mmol/l or HDL cholesterol <0.9 mmol/l) |
| Fasting blood glucose concentration ≥6.1 mmol/l (110 mg/dl) | Hypertension (≥160/90 mmHg) |
| Microalbuminuria |
Beta-cell dysfunction. In response to glucose, insulin is secreted in a characteristic biphasic pattern: an acute first phase lasting a few minutes, followed by a sustained second phase. The major β-cell abnormalities in type 2 diabetes are a marked reduction in first-phase insulin secretion and, in established diabetes, an attenuated second phase.
Abnormalities in β-cell function are found early in the natural history of type 2 diabetes and in first-degree relatives of people with type 2 diabetes (Reference Pratley and WeyerPratley & Weyer, 2001), suggesting that they are an integral component of the pathogenesis of type 2 diabetes.
In the UK Prospective Diabetes Study, which was a large epidemiological study of newly diagnosed patients with type 2 diabetes, long-term increases in fasting plasma glucose levels were accompanied by a progressive decline in β -cell function of around 4% per year (UK Prospective Diabetes Study Group, 1995). By the time of diagnosis, mean β-cell function was already less than 50%, and further deterioration of β-cell function was not prevented by any of the diabetic therapies used in the study.
Extrapolation of the observed rate of β-cell decline suggests that the loss of β-cell function begins around 12 years prior to diagnosis (Reference HolmanHolman, 1998; Fig. 3). This progressive loss of β-cell function explains why patients require escalations in the number and dosage of oral hypoglycaemic agents with time, and why eventually they become refractory to the oral treatments and require insulin.
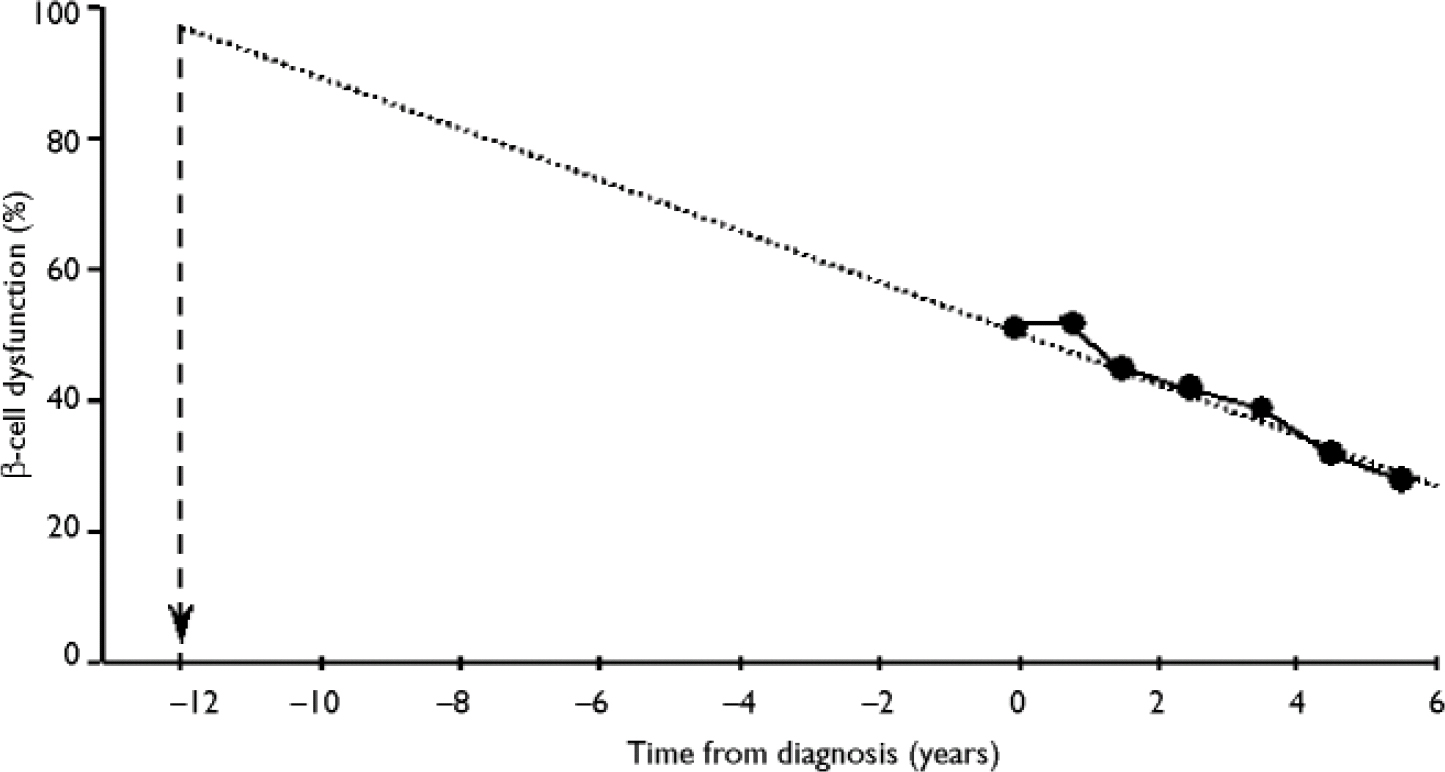
Fig. 3 Extrapolation of the time of deterioration of β-cell dysfunction. From Holman (Reference Holman1998) with permission from Elsevier.
Although the mechanisms underlying the β-cell dysfunction remain unclear, they are likely to be multifactorial. As well as genetic factors (Reference Pratley and WeyerPratley & Weyer, 2001), a number of environmental factors (including early-life malnutrition and obesity) and hyperglycaemia and hyperlipidaemia per se may all accelerate the decline in β-cell function (Reference Hales, Barker and ClarkHales et al, 1991; Reference Prentki, Joly and El AssaadPrentki et al, 2002).
There has been much discussion about the relative roles of insulin resistance and β-cell function in the natural history of diabetes, since both components occur early in the disease process. One model for the development of diabetes is shown in Fig. 4. As insulin sensitivity falls, the β-cell responds by increasing insulin secretion to compensate and to maintain glucose concentrations within the normal range. The maximal insulin secretory capacity is eventually reached and, beyond that point, insulin secretion declines. The blood glucose concentrations rise, initially in the postprandial period, as the individual develops impaired glucose tolerance before the onset of frank diabetes.
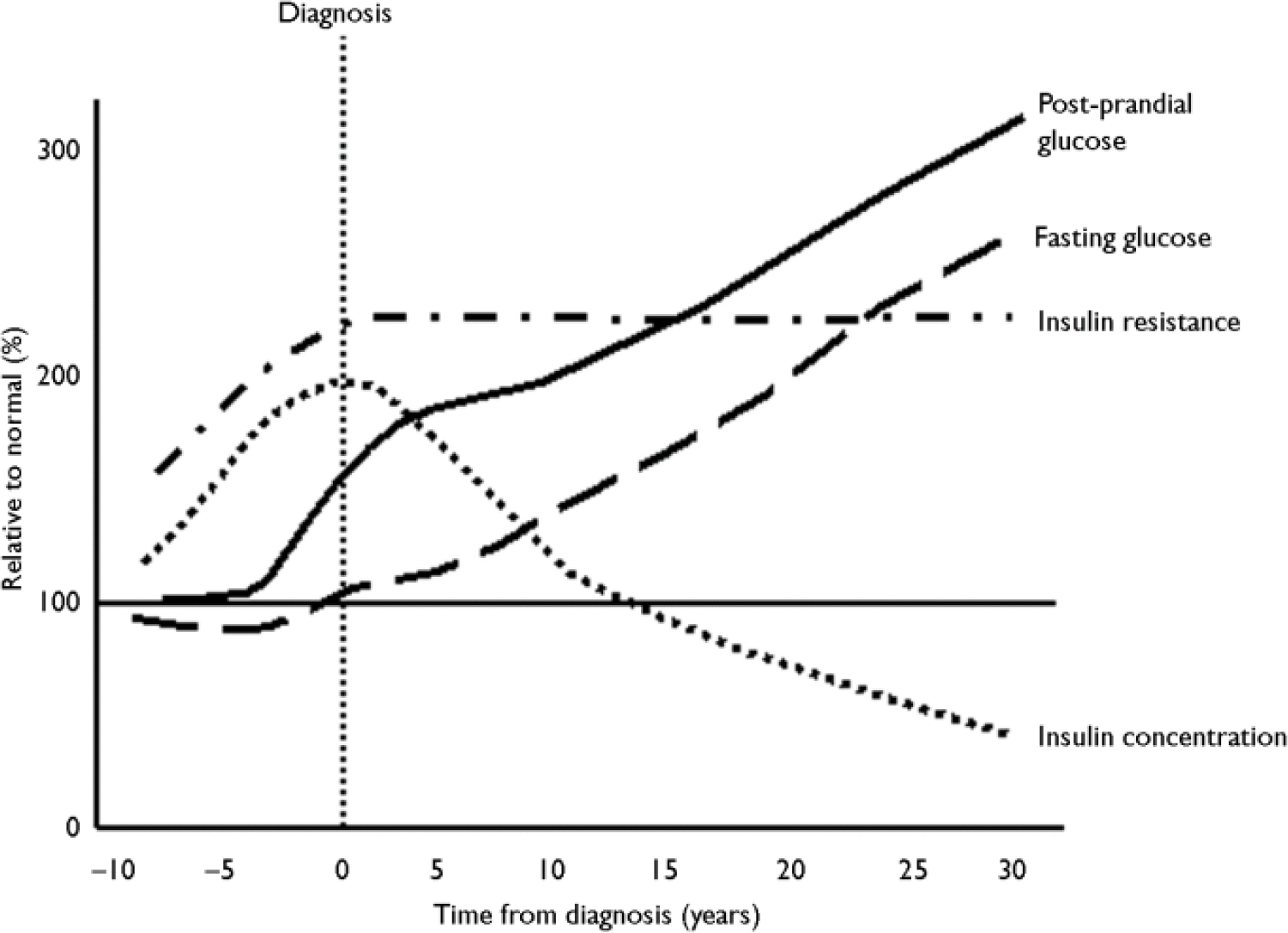
Fig. 4 Natural history of insulin resistance and insulin secretion in type 2 diabetes.
This process may, however, be reversible. Although around 2–5% of individuals with impaired glucose tolerance will progress to diabetes each year, many revert to normal (Reference Saad, Knowler and PettittSaad et al, 1988), and since there is good evidence to show that lifestyle intervention at this stage can reduce incident diabetes, people falling into these categories should be managed actively with advice about their diet and exercise (Reference Pan, Li and HuPan et al, 1997; Reference Tuomilehto, Lindstrom and ErikssonTuomilehto et al, 2001; Reference Knowler, Barrett Connor and FowlerKnowler et al, 2002).
Drug-induced diabetes
Many drugs can worsen or precipitate hyperglycaemia through a number of mechanisms. These include drugs that are directly toxic to the β-cell, such as cyclosporin and pentamidine, or drugs that worsen insulin resistance, such as glucocorticoids and high-dose thiazide diuretics. These effects are often reversible, but other treatments should be used whenever possible. Where no alternative exists, careful monitoring of glycaemic control and treatment of the diabetes can mitigate the effect of these drugs.
Hyperglycaemia has been reported with both conventional and atypical antipsychotic drugs. These drugs are not directly toxic to the β-cells (Reference Sowell, Mukhopadhyay and CavazzoniSowell et al, 2002) but are associated with significant weight gain (Reference Allison and CaseyAllison & Casey, 2001), so the underlying mechanism has been assumed to be through an increase in insulin resistance (Reference Sowell, Mukhopadhyay and CavazzoniSowell et al, 2002). However, since there is no direct relationship between weight gain and the development of diabetes, other mechanisms are thought to be involved (Reference Jin, Meyer and JesteJin et al, 2002). This is discussed in greater detail by Haddad (Reference Haddad2004, this supplement) and Bushe & Leonard (2004, this supplement).
Clinical features of type 2 diabetes
Type 2 diabetes usually affects overweight individuals, and most cases are diagnosed in those over 40 years old (Reference JonssonJonsson, 2002). However, the demographics of this disease are changing, and it is now becoming increasingly common in children and young adults (American Diabetes Association, 2000).
Type 2 diabetes has a gradual and insidious onset, with nearly a third of cases being identified as an incidental finding (1988) or in the coronary care unit (Reference Malmberg, Ryden and EfendicMalmberg et al, 1995). The diagnosis is often delayed, and some degree of hyperglycaemia may have been present for more than 20 years before the diagnosis is confirmed (Reference Liu, Molyneaux and ChuaLiu et al, 2002). This accounts for the so-called ‘missing million’ people with undiagnosed diabetes in the UK. The Isle of Ely Diabetes Project was undertaken to determine the prevalence of undiagnosed glucose intolerance in this community (Reference Williams, Wareham and BrownWilliams et al, 1995). Of the 1122 participants in the study, 4.5% were found to have undiagnosed diabetes, and 16.8% were found to have impaired glucose tolerance. This high prevalence of undiagnosed diabetes in the general population should be borne in mind when interpreting epidemiological data assessing the effects of antipsychotic medications on the development of diabetes.
Around half of patients with type 2 diabetes are diagnosed as a result of the typical diabetic symptoms of polyuria, nocturia, thirst, tiredness and blurred vision; a further 16% of patients are diagnosed after presenting with an infection (UK Prospective Diabetes Study Group, 1988). Microvascular diabetic complications are frequently present at the time of diagnosis, but account for only 2% of all presentations.
Prevention of diabetes
One of the most exciting areas in diabetes at present is a focus on the possibility that type 2 diabetes can be prevented, or at least its onset delayed. Interest in this area has been rekindled by the publication of studies showing that both lifestyle and pharmacological interventions can reduce incident diabetes.
Lifestyle studies
The first randomised study to show that lifestyle interventions could reduce diabetes was the Da Qing Impaired Glucose Tolerance and Diabetes Study published in 1997 (Reference Pan, Li and HuPan et al, 1997). Individuals with impaired glucose tolerance who were randomised to receive a dietary intervention, an exercise intervention, or a combination of both, had a nearly 50% reduction in risk of diabetes over 6 years compared with a control group.
More recently, the results of the Finnish Diabetes Prevention Study (Reference Tuomilehto, Lindstrom and ErikssonTuomilehto et al, 2001) and the US Diabetes Prevention Program (Reference Knowler, Barrett Connor and FowlerKnowler et al, 2002) have been published. In the Finnish study, 523 overweight individuals aged 40–65 years with impaired glucose tolerance were randomly assigned to a control or lifestyle intervention group and were followed for a mean of 3.2 years. The control group was given general verbal and written information about a healthy diet and exercise; the intervention group received individualised dietary counselling, aimed at reducing weight by 5%, reducing total fat and saturated fat intake (to less than 30% and 10% of total energy intake, respectively) and increasing intake of fibre, given in frequent face-to-face sessions with a dietician. The intervention group members were encouraged to undertake 30 min of aerobic exercise and resistance training per day through individually designed programmes. The proportion of individuals who progressed to diabetes was reduced from 7.8% per annum in the control group to 3.2% per annum in the intervention group – a substantial risk reduction of 58%. The rate of diabetes was found to be related to the number of targets achieved; there were no cases of diabetes during the study in those who achieved four or five of the targets.
The Diabetes Prevention Program compared the effectiveness of intensive lifestyle modifications similar to those used in the Finnish study with metformin (850 mg twice daily) treatment in preventing type 2 diabetes (Reference Knowler, Barrett Connor and FowlerKnowler et al, 2002). Originally, a third group received the thiazolidinedione troglitazone, but this arm of the study was stopped after the drug was withdrawn from the market. A major strength of the Diabetes Prevention Program was its size and diversity: 3234 people with impaired glucose tolerance took part, of whom 45% had non-White European backgrounds and 68% were women. After an average follow-up period of 2.8 years, the incidence of diabetes in the lifestyle intervention group was reduced by 58%; metformin reduced incident diabetes by 31%. Since none of the trial participants received both the lifestyle intervention and metformin, it is interesting to speculate whether further benefits might accrue from combining these interventions. A remarkable observation in both the Finnish and the American studies was that the impressive reductions in diabetes were achieved with only moderate reductions in weight.
Unfortunately, it is unlikely that the lifestyle intervention used in these studies could ever be replicated outside a research setting. Public health policies are therefore urgently required that will encourage people to adopt a healthy lifestyle and prevent the development of impaired glucose tolerance and diabetes. Primary prevention strategies should target individuals at especially high risk of developing type 2 diabetes, including those with prediabetes, first-degree relatives of people with type 2 diabetes, women with a history of gestational diabetes, individuals with other features of the metabolic syndrome (Reference Zimmet, Shaw and AlbertiZimmet et al, 2003) and, I suggest, those with severe mental illness.
Pharmacological intervention
In addition to the Diabetes Prevention Program, there have been several other pharmacological intervention studies. The STOP-NIDDM trial was designed to evaluate the effect of acarbose, an α-glucosidase inhibitor, in reducing the risk of type 2 diabetes (Reference Chiasson, Josse and GomisChiasson et al, 2002). After a mean follow-up of 3.3 years, a 25% relative risk reduction in progression to diabetes was observed in the acarbose-treated group compared with the placebo group, with an absolute risk reduction in the acarbose-treated group of 9%.
In the TRIPOD study (Reference Buchanan, Xiang and PetersBuchanan et al, 2002), 236 Hispanic women with previous gestational diabetes were randomized to receive either placebo or troglitazone. After a median follow-up of 30 months, troglitazone treatment was associated with a 56% relative reduction in incident diabetes. Furthermore, a beneficial effect of the drug was seen during the 8-month wash-out period immediately after the end of the trial, suggesting that troglitazone might affect the natural history of glucose intolerance, thereby preventing diabetes in some people.
The results of the XENDOS study have recently been published (Reference Torgerson, Hauptman and BoldrinTorgerson et al, 2004). In this study, the use of orlistat plus lifestyle interventions reduced the risk of developing type 2 diabetes by 37% compared with lifestyle interventions alone. Furthermore, improvements in other cardiovascular risk factors, including blood pressure and lipid profile, were also achieved.
Screening for diabetes
The high prevalence of undiagnosed diabetes and the proportion of patients with evidence of complications at diagnosis undoubtedly create a strong imperative for screening. Many possible screening methods have been shown to be feasible, acceptable and accurate (Reference Wareham and GriffinWareham & Griffin, 2001), but there is still debate about whom to screen. Universal screening for diabetes is currently impractical because of the burden that would be placed upon general practitioners (Reference Wylie, Hungin and NeelyWylie et al, 2002). There is justification, however, for screening high-risk groups in whom undiagnosed diabetes is especially prevalent. Patients with schizophrenia fall within this high-risk category, and therefore should be considered for screening for diabetes. This is discussed in greater detail by Gough & Peveler (Reference Gough and Peveler2004, this supplement).
Complications of type 2 diabetes
Since the introduction of effective treatment that allows patients with diabetes to live through the acute metabolic consequences of the illness, it has become apparent that diabetes is associated with a number of chronic microvascular complications, which affect the eyes, kidneys and nervous system, and macrovascular complications, which lead to an increased risk of myocardial infarction, stroke and peripheral vascular disease.
Microvascular complications
Microvascular complications are frequently present at diagnosis in patients with type 2 diabetes. Retinopathy and cataracts each affect around 15% of individuals with type 2 diabetes and are frequently present at diagnosis (UK Prospective Diabetes Study Group, 1988; American Diabetes Association, 2001). Maculopathy – a retinopathy affecting the macula and therefore the central vision – is the most common cause of sight-threatening retinopathy in type 2 diabetes; proliferative retinopathy – the most common cause of sight-threatening retinopathy in type 1 diabetes – is less common.
The risk of developing nephropathy is lower in type 2 diabetes than in type 1 diabetes because of the generally later onset of the former disorder. The prevalence of microalbuminuria – a higher than normal albumin excretion that cannot be detected by standard urine reagent strips and a sign of early diabetic nephropathy – is around 25–30%. Approximately 5–13% of patients with type 2 diabetes have frank proteinuria (American Diabetes Association, 2001). Although the individual risk of developing end-stage renal failure is lower in type 2 diabetes, patients with this form of diabetes requiring renal replacement therapy outnumber patients with type 1 diabetes because of the much greater prevalence of type 2 diabetes.
Neuropathy affects 20–50% of patients with type 2 diabetes, and its sequelae, such as foot ulceration and amputation, cause considerable morbidity and mortality (Reference Boulton and MalikBoulton & Malik, 1998). Fifteen per cent of people with type 2 diabetes develop foot ulcers, of whom 5–15% require foot amputations.
Macrovascular disease
Diabetes confers a two-fold to four-fold increased risk of myocardial infarction and stroke in men, and up to a ten-fold increased risk in pre-menopausal women (2001). Mortality following a myocardial infarction is far greater in people with diabetes. Furthermore, 60–75% of all people with diabetes die from cardiovascular disease (Reference Stamler, Vaccaro and NeatonStamler et al, 1993). These statistics, coupled with the observation that people with diabetes who have not had a previous myocardial infarction share the same risk of having a myocardial infarction as non-diabetic patients who have had a myocardial infarction (Reference Haffner, Lehto and RonnemaaHaffner et al, 1998), have begun to shift the focus of management of diabetes from glucose control to a greater emphasis on arterial risk-factor management.
Hyperglycaemia per se is insufficient to explain the increased prevalence of cardiovascular disease in diabetes, and it is my belief that increased prevalence of metabolic syndrome features in people with diabetes largely explains the excess cardiovascular disease in these patients (Reference Stratton, Adler and NeilStratton et al, 2000). In the Prospective Cardiovascular Münster Study, for example, 49% of individuals with diabetes had hypertension, 24% had a low level of high-density lipoprotein cholesterol and 37% had hypertrigylceridaemia, compared with values of 31%, 16% and 21%, respectively, in people without diabetes (Reference Assmann and SchulteAssmann & Schulte, 1988).
DISCUSSION
Diabetes is already a costly disease in individual, social and economic terms, and the global burden of diabetes is increasing. Diabetes is associated with premature morbidity and mortality, and it should never be considered to be a ‘mild’ condition. Many people with diabetes have asymptomatic disease, and it is hoped that, with improved screening procedures and better treatments, the long-term outlook for these individuals will be greatly improved.
APPENDIX
The 1999 World Health Organization classification of diabetes
Type 1 diabetes
Immune-mediated
Idiopathic
Type 2 diabetes
Equivalent to non-insulin-dependent
diabetes
Type 3 diabetes (other specific types)
Diabetes secondary to pancreatic disease
-
• chronic pancreatitis
-
• haemochromatosis
-
• pancreatic surgery
Diabetes secondary to endocrine disease
-
• acromegaly
-
• Cushing's syndrome
-
• phaeochromocytoma
Diabetes secondary to drugs and chemicals
-
• glucocorticoids
-
• diuretics
-
• neuroleptics
-
• β-blockers
Diabetes secondary to genetic abnormalities
-
• maturity onset diabetes of the young (MODY)
-
• glucokinase mutations
-
• hepatic nuclear factor mutations
-
• insulin promoter factor 1 mutations
-
-
• leprechaunism
-
• cystic fibrosis
-
• Wolfram syndrome (diabetes insipidus, diabetes mellitus, optic atrophy and deafness DIDMOAD)
-
• lipoatrophy
-
-
Type 4 diabetes
Gestational diabetes
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Diabetes is associated with significant morbidity and premature mortality.
-
▪ The prevalence of undiagnosed impaired glucose tolerance and diabetes is high, suggesting that active screening in high-risk groups would be advantageous.
-
▪ Lifestyle modification and pharmacotherapy can prevent or delay the development of type 2 diabetes.
LIMITATIONS
-
▪ Most of the studies described in this article are in people without mental illness.
-
▪ An holistic approach, which is not solely focused on blood glucose, is needed to prevent cardiovascular disease in people with diabetes.
-
▪ A greater understanding of the molecular mechanisms that cause insulin resistance and pancreatic β-cell failure is needed.










eLetters
No eLetters have been published for this article.