Introduction
Depression affects approximately 400 million people globally and mental illness is forecasted to be the leading contributor to the global burden of disease by 2030 (Wellcome Global Monitor, 2021). The most prescribed medications for the treatment of depression are selective serotonin reuptake inhibitors (SSRIs) such as escitalopram (Lexapro), fluoxetine (Prozac), and sertraline (Zoloft). A recent comprehensive meta-analysis showed that these drugs are superior to placebo in the treatment of depression (Cipriani et al., Reference Cipriani, Furukawa, Salanti, Chaimani, Atkinson, Ogawa and Geddes2018), although with small effect sizes relative to placebo (<0.3 standardized mean difference) (Hengartner & Plöderl, Reference Hengartner and Plöderl2018). Considering the social and economic costs of depression and that many individuals reject or fail to comply with chronic medication strategies, alternative treatments are needed.
A promising new treatment for depression is psychedelic-assisted therapy (Nutt, Erritzoe, & Carhart-Harris, Reference Nutt, Erritzoe and Carhart-Harris2020). In this paradigm, psychedelics – such as psilocybin and lysergic acid diethylamide (LSD) – are assumed to interact positively with psychotherapeutic processes. During such ‘psychedelic therapy’, patients take a moderate-to-large dose of the psychedelic on one or two occasions with psychological supervision to guide the therapeutic process (Garcia-Romeu & Richards, Reference Garcia-Romeu and Richards2018).
Recently, we conducted a head-to-head comparative trial of escitalopram, a highly selective SSRI and one of the most commonly prescribed antidepressants, v. investigational psilocybin-therapy, for the treatment of depression (Carhart-Harris et al., Reference Carhart-Harris, Giribaldi, Watts, Baker-Jones, Murphy-Beiner, Murphy and Nutt2021). According to the pre-defined primary outcome, the mean of the self-rated 16-item Quick Inventory of Depressive Symptomology (Rush et al., Reference Rush, Trivedi, Ibrahim, Carmody, Arnow, Klein and Keller2003) scale (QIDS-SR-16), the between-treatment difference at the week 6 primary endpoint was not statistically significant. However, both response (70% v. 48%) and remission (57% v. 28%) rates, as scored on the QIDS-SR-16, favored psilocybin. Moreover, on all mental-health-related secondary outcomes, psilocybin therapy was superior by a greater than 95% confidence margin.
Patient expectations can influence therapeutic outcomes (Tambling, Reference Tambling2012). Researchers attempt to address expectancy effects in clinical trials by randomization and experimental ‘blinding’, i.e. by concealing treatment allocation from patients and assessors. However, effective blinding is difficult to achieve in practice (Baethge, Assall, & Baldessarini, Reference Baethge, Assall and Baldessarini2013), particularly in psychedelic trials (Muthukumaraswamy, Forsyth, & Lumley, Reference Muthukumaraswamy, Forsyth and Lumley2021), due to the conspicuous subjective drug effects that enable most patients to deduce their treatment allocation. For example in another recent trial of psilocybin-assisted therapy, 94% of participants correctly guessed their treatment allocation, indicating that blinding was broken (Bogenschutz et al., Reference Bogenschutz, Ross, Bhatt, Baron, Forcehimes, Laska and Worth2022). Based on this consideration, a number of authors have expressed concerns over the methodological soundness of psychedelic trials, arguing that expectancy effects may be biasing the observed results despite the formal blinding procedures (Burke & Blumberger, Reference Burke and Blumberger2021; Muthukumaraswamy et al., Reference Muthukumaraswamy, Forsyth and Lumley2021; Szigeti, Nutt, Carhart-Harris, & Erritzoe, Reference Szigeti, Nutt, Carhart-Harris and Erritzoe2023). In the case of ‘psychedelic microdosing’, where users regularly take small doses of a psychedelic drug without clinical supervision (Polito & Liknaitzky, Reference Polito and Liknaitzky2022), a number of studies, including some that were placebo-controlled (Cavanna et al., Reference Cavanna, Muller, de la Fuente, Zamberlan, Palmucci, Janeckova and Tagliazucchi2022; de Wit, Molla, Bershad, Bremmer, & Lee, Reference de Wit, Molla, Bershad, Bremmer and Lee2022; Szigeti et al., Reference Szigeti, Kartner, Blemings, Rosas, Feilding, Nutt and Erritzoe2021) suggest that positive expectancy may play an important role in driving positive responses highlighting a need to investigate expectancy and related effects in all psychedelic trials.
Methods
A trial of escitalopram v. psilocybin
This was a phase 2, investigator-initiated, double-blind, randomized trial in patients with moderate-to-severe major depressive disorder. The core treatment period was six weeks and the trial had two treatment arms. In the ‘psilocybin arm’, patients received two separate doses of 25 mg of investigational drug, COMP360, i.e. psilocybin, three weeks apart plus six weeks of daily placebo capsules. In the ‘escitalopram arm’ patients received two separate doses of 1 mg of psilocybin three weeks apart, which was viewed by the research team as a placebo, plus six weeks of daily oral escitalopram (10 mg/day the first 3 weeks, 20 mg/day for the final 3 weeks) – which was considered the main active component of this arm. Patients were randomly assigned to treatment groups in a 1:1 ratio. All patients received psychological support during the trial period. The trial was registered on ClinicalTrials.gov (NCT03429075), all the patients provided written informed consent and approval was obtained from all relevant regulator bodies, see (Carhart-Harris et al., Reference Carhart-Harris, Giribaldi, Watts, Baker-Jones, Murphy-Beiner, Murphy and Nutt2021) for further details.
Baseline measures
To measure patients' expectations, the following two items were administered one day before both dosing days:
• ‘Please rate the following with regards to the prospect of receiving 6 weeks of daily escitalopram. At the end of the trial, after receiving escitalopram every day for 6 weeks, how much improvement in your mental health do you think will occur?’
• ‘Please rate the following with regard to the prospect of receiving two full strong doses of psilocybin, 3 weeks apart. At the end of the trial, 3 weeks after your second psilocybin dosing session, how much improvement in your mental health do you think will occur?’
Ratings of these items are referred to as ‘escitalopram expectancy’ and ‘psilocybin expectancy’, respectively. These items refer specifically to efficacy-related expectancy, e.g. as opposed to side effects, and will be referred to as expectancy from here on. Here, we use the expectancy measures obtained before the first dosing day, i.e. pre-treatment expectancy. Responses were collected on a 0–100 visual analog scale with anchor points at 0 (‘0% improvement’) and 100 (‘100% improvement’).
In this manuscript, we use ‘received treatment expectancy’ as the expectancy measure, which is the expectancy associated with the actually received treatment for each patient (i.e. escitalopram expectancy when allocated to the escitalopram arm and psilocybin expectancy when allocated to the psilocybin arm). We choose to analyze the data this way because the 25 mg psilocybin used in the current trial induces strong psychological and physical effects that can be reliably recognized and attributed to psilocybin by most patients (Muthukumaraswamy et al., Reference Muthukumaraswamy, Forsyth and Lumley2021), thus, blinding integrity is unlikely to have been maintained (Szigeti et al., Reference Szigeti, Nutt, Carhart-Harris and Erritzoe2023). Similarly, blinding integrity is also often violated in SSRI trials (Scott, Sharpe, & Colagiuri, Reference Scott, Sharpe and Colagiuri2022).
Suggestibility, which is the tendency to comply with suggestions from others (Wagstaff, Reference Wagstaff1991), was assessed with the Short Suggestibility Scale (SSS) (Kotov, Bellman, & Watson, Reference Kotov, Bellman and Watson2004) at baseline. Absorption, which represents a predisposition to experience altered states of consciousness (Ott, Reuter, Hennig, & Vaitl, Reference Ott, Reuter, Hennig and Vaitl2005), was assessed with the Modified Tellegen Absorption Scale (MODTAS) (Jamieson, Reference Jamieson2005) also at baseline. These measures were included here because previous work has indicated that suggestibility and absorption are correlated (Milling, Kirsch, & Burgess, Reference Milling, Kirsch and Burgess2000), and that absorption may be predictive of the nature of psychedelic experiences (Aday, Davis, Mitzkovitz, Bloesch, & Davoli, Reference Aday, Davis, Mitzkovitz, Bloesch and Davoli2021; Haijen et al., Reference Haijen, Kaelen, Roseman, Timmermann, Kettner, Russ and Carhart-Harris2018).
Outcome measures
Pre-defined primary outcome was the change in the mean sum score of the self-rated Quick Inventory of Depressive Symptomology Scale (QIDS-SR-16) (Rush et al., Reference Rush, Trivedi, Ibrahim, Carmody, Arnow, Klein and Keller2003) at the six-week primary endpoint, while secondary outcomes included the clinician-rated Beck Depression Inventory (BDI) (Beck, Ward, Mendelson, Mock, & Erbaugh, Reference Beck, Ward, Mendelson, Mock and Erbaugh1961), the Hamilton Depression Rating Scale (HAM-D) (Hamilton, Reference Hamilton1960) and the Montgomery-Åsberg Depression Rating Scale (MADRS) (Montgomery & Asberg, Reference Montgomery and Asberg1979). Here we re-analyzed these outcomes together with other mood-related secondary outcomes, specifically the self-rated State-Trait Anxiety Inventory-Trait (STAI-T) (Spielberger, Reference Spielberger1983) and the Warwick-Edinburgh Mental Well-being Scale (WEMWBS) (Tennant et al., Reference Tennant, Hiller, Fishwick, Platt, Joseph, Weich and Stewart-Brown2007).
Statistical models
We used linear mixed modeling to assess baseline differences. The first model had expectancy as the dependent variable, patient ID as random effect, and expectancy type (i.e. whether expectancy measure corresponds to escitalopram or psilocybin expectancy) as fixed effect. In a second model, we also added treatment allocation and its interaction with expectancy type as fixed effects to investigate potential between arm differences. Note that in these expectancy models each patient contributed two rows of data: one for escitalopram expectancy and one for psilocybin expectancy. Next, we constructed similar models for suggestibility/absorption, where suggestibility/absorption was the dependent variable and treatment allocation was the independent variable, see online Supplementary Table S1 for model formulas.
Linear mixed modeling was used to assess the ‘within arm’ association between outcomes and expectation/suggestibility/absorption, separately, for the two treatment arms. In these models, the dependent variable was score on one of six mood/well-being related outcomes (HAM-D, BDI, MADRS, QIDS-SR-16, STAI-T, or WEMWBS), four of which are widely used depression symptom severity scales (HAM-D, BDI, MADRS, QIDS-SR-16), patient ID as random effect, timepoint, one of the baseline covariates (expectancy/suggestibility/absorption) and it's interaction with timepoint as fixed effects, see online Supplementary Tables S2 and S3 for model formula.
Linear mixed modeling was also used to construct between-arms models adjusted for either expectancy or suggestibility. As before, the dependent variable was score on one of the mental-health-related outcomes (HAM-D, BDI, MADRS, QIDS-SR-16, STAI-T, or WEMWBS), with patient ID as a random effect, and timepoint, treatment allocation, expectancy/suggestibility, and their interactions as fixed effects, and see online Supplementary Table S4 and S5 for model formulas.
In all models, the pre-treatment covariates, i.e. expectancy, suggestibility, and absorption, were normalized by subtracting the median and then dividing by the standard deviation. Consequently, all results should be understood as representative at the median level of the covariate and estimates represent the change associated with an increase of 1 standard deviation. We choose to normalize the data at the median instead of the mean to protect against extreme values; however, we note here that normalizing at the mean yields the same qualitative results. In both the within- and between-arms models expectancy is defined as the ‘received treatment expectancy’, i.e., escitalopram expectancy for patients in the escitalopram arm and psilocybin expectancy for patients in the psilocybin arm, see Baseline measures for details. To control for multiple comparisons, we adjusted the p values with the Bonferroni method (Sedgwick, Reference Sedgwick2012). Throughout the manuscript, we report these adjusted p values, while all unadjusted p values can be found in the online Supplementary materials. For all models, the normality of the residuals was checked visually from QQ-plots. All models were constructed in R (v4.1.2) using the lme4 (v1.1-27.1) and lmerTest (v3.1-3) packages.
Equivalence testing
In null hypothesis significance testing (NHST), the null hypothesis is either rejected or not, but the null hypothesis itself cannot be confirmed (Lakens, McLatchie, Isager, Scheel, & Dienes, Reference Lakens, McLatchie, Isager, Scheel and Dienes2020). Equivalence testing allows for an inference to be made on whether the null hypothesis can be accepted, i.e. results of this test can provide evidence to infer an absence of an effect. Specifically, equivalence testing can provide evidence that the true effect is smaller than a pre-specified equivalence bound, also known as ‘smallest effect size of interest’ or ‘region of practical equivalence’. If an equivalence test yields significant results, it means that we can reject the hypothesis that the true effect is as extreme or more extreme than the chosen equivalence bound (Lakens et al., Reference Lakens, McLatchie, Isager, Scheel and Dienes2020). In contrast, a non-significant equivalence test means that effects as large as the equivalence bound cannot be ruled out.
We used the ‘two one-sided t tests’ (TOST) equivalence test procedure as implemented by the parameters package (https://rdrr.io/cran/parameters/) to further examine results where the null hypothesis was not rejected. We choose the equivalence bound to be 0.5 standardized mean difference (s.m.d.) because it corresponds to a suggested criterion for inferring minimum clinically important difference across a range of medical conditions (Norman, Sloan, & Wyrwich, Reference Norman, Sloan and Wyrwich2003).
Data and code sharing
The manuscript's repository (https://github.com/szb37/psilodep2) contains a conda computational environment, the data used, and analysis scripts to reproduce all figures and major statistical findings presented here.
Results
Pre-treatment between-group differences
In the full sample, we found a significant difference between the pre-trial efficacy-related expectancy for escitalopram v. psilocybin (est ± s.e.: 25.8 ± 3.5; p < 0.001***), with estimated means of 54% (psilocybin) v. 28.2% (escitalopram) – on a scale of expecting 0–100% mental-health improvements, see Methods for details. There were no significant effects associated with treatment allocation (est ± s.e.: −3.2 ± 5.7; p = 0.580), nor with its interaction with expectancy type (est ± s.e.: 9.3 ± 6.9; p = 0.183), implying that, irrespective of group allocation, the sample had uniformly higher expectancy for psilocybin therapy. Changes in expectancy between the first and the second session are described in the online Supplementary materials. Briefly, no significant changes were observed for either escitalopram or psilocybin expectancy after the first and before the second psilocybin (1 or 25 mg) dosing session.
We found no significant between group differences with respect to baseline trait suggestibility (est ± s.e.: −2.5 ± 2.8; p = 0.368), absorption (est ± s.e.: 4.4 ± 4; p = 0.272), or any of the absorption related subscales, see online Supplementary Table S1 for details and Fig. 1 for boxplots.
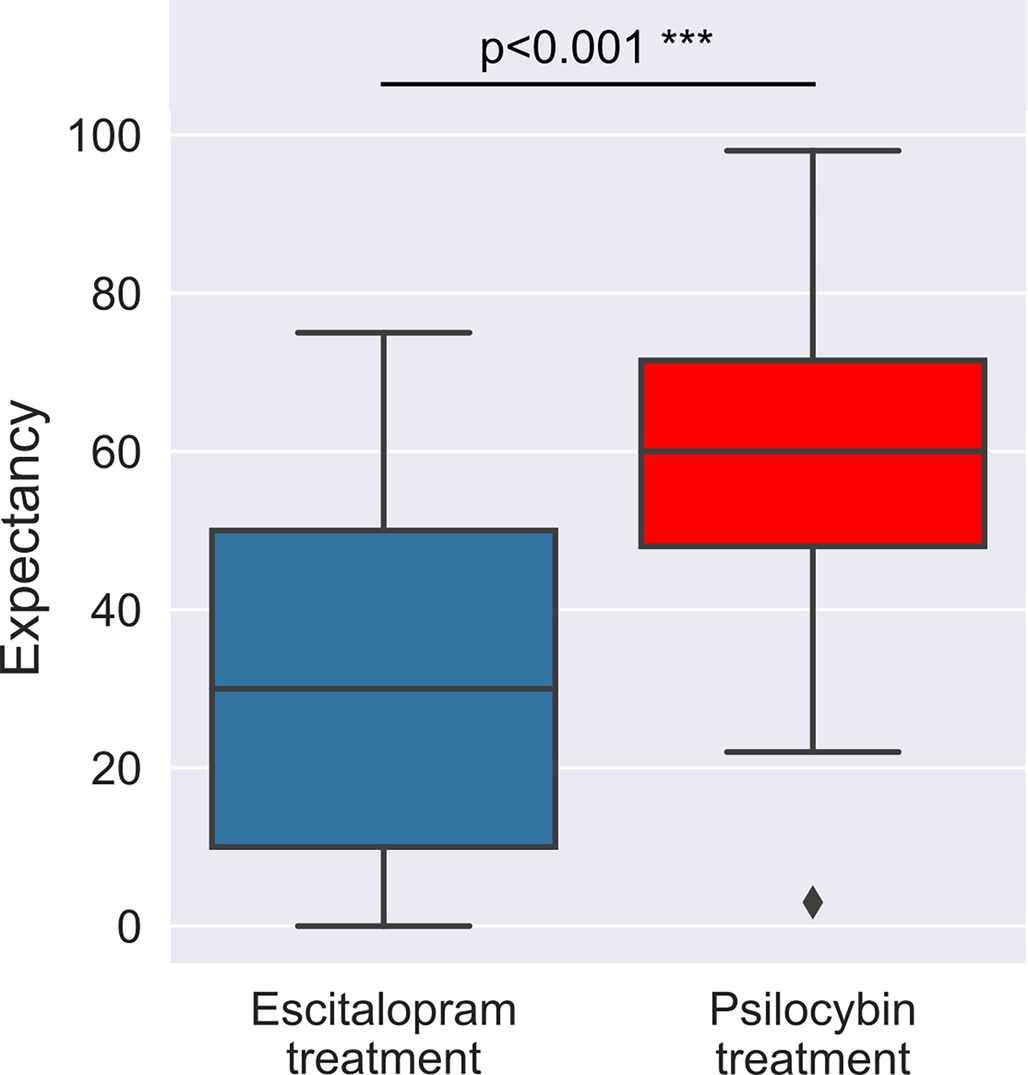
Figure 1. Boxplots of the observed expectancy scores at baseline. A substantial difference was found between escitalopram and psilocybin expectancy with estimated means of 54 (psilocybin) v. 28.2 (escitalopram). An expectancy score of 0 for either treatment implied an expectation of no improvement in mental health, whereas a score of 100 would have implied 100% improvement, see Methods for details. This expectancy imbalance was equally present in both treatment arms, see online Supplementary Table S1 for details.
Within-treatment-arm association between expectancy and therapeutic outcomes
In the escitalopram arm, we found a significant interaction between expectancy and timepoint when predicting outcomes on the HAM-D (est. ± s.e.: −3.91 ± 0.9, adj. p = 0.001**), BDI (est. ± s.e.: −5.47 ± 1.61, adj. p = 0.013*), MADRS (est. ± s.e.: −4.87 ± 1.52, adj. p = 0.022*) and STAI-T (est. ± s.e.: −5.2 ± 1.68, adj. p = 0.028*) scales, but not on the QIDS-SR-16 (est. ± s.e.: −2.46 ± 0.98, adj. p = 0.115) and WEMWBS (est. ± s.e.: 2.95 ± 1.76, adj. p = 0.641) scales, see online Supplementary Table S2 for details. These findings suggest that on the HAM-D, BDI, MADRS, and STAI-T scales, there is a positive association between pre-treatment expectations for escitalopram and improved outcomes in the escitalopram arm. Specifically, on the HAM-D scale, each standard deviation (~22 points on the expectancy scale) increase in expectancy is associated with 3.91 points reduction in depression scores, etc.
Conversely, in the psilocybin arm, we found no significant interaction between expectancy and timepoint when predicting outcomes on any of the scales (HAM-D est. ± s.e.: 1.16 ± 1.05, adj. p = 1; BDI est. ± s.e.: 1.14 ± 2.4, adj. p = 1; MADRS est. ± s.e.: 1.69 ± 1.81, adj. p = 1; QIDS-SR-16 est. ± s.e.: 0.56 ± 1.25, adj. p = 1; STAI-T est. ± s.e.: 0.64 ± 2.66, adj. p = 1; WEMWBS est. ± s.e.: −2.96 ± 2.44, adj. p = 1), suggesting a lack of association between pre-treatment expectancy and therapeutic outcomes, see online Supplementary Table S3 for details. Equivalence testing the expectancy × timepoint interaction term yielded non-significant results on all scales, suggesting that we cannot rule out a true effect as large as the minimum important difference, see Equivalence testing and online Supplementary Table S6 for details. Figure 2 shows the expectancy v. outcomes regression lines for both treatment arms.
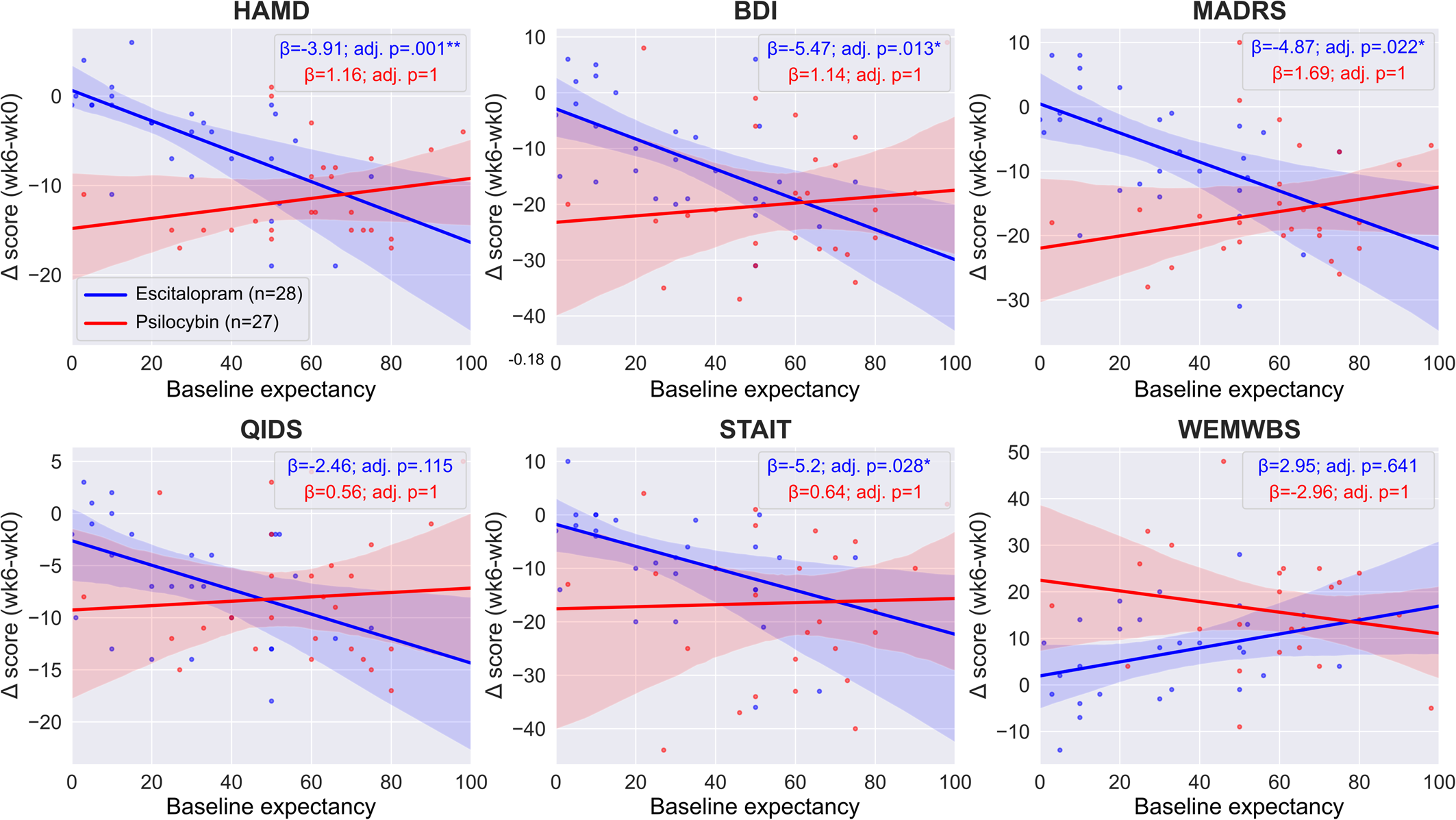
Figure 2. Regression lines of pre-treatment efficacy expectations v. clinical efficacy. Regression lines and coefficients were obtained from the two separate ‘within arm’ models, see Statistical models for details. There was a significant association between pre-treatment expectancy and response to escitalopram as assessed using the HAM-D, BDI, MADRS and STAI-T scales (blue lines), in contrast, there was no association for any outcome in the psilocybin arm (red lines). Note that the significance level is different between the two arms on four scales (HAMD, BDI, MADRS, STAI-T), but the difference reached significance only on two of them (HAMD, MADRS), see between-arm models for details. Boxes show the regression coefficients (β) associated with the expectancy × timepoint term in the two separate arms and the associated Bonferroni adjusted p values. Negative values indicate improved symptoms except for WEMWBS where positive values indicate improved well-being, see online Supplementary Tables S2 and S3 for details.
Within-treatment-arm association among suggestibility, absorption, and therapeutic outcomes
In the escitalopram arm, we found no significant interaction between baseline suggestibility and timepoint when predicting outcomes on any of the scales (HAM-D est. ± s.e.: 0.9 ± 1.07, adj. p = 1; BDI est. ± s.e.: 1.03 ± 1.83, adj. p = 1; MADRS est. ± s.e.: 2.78 ± 1.53, adj. p = 0.490; QIDS-SR-16 est. ± s.e.: 1.08 ± 1.01, adj. p = 1; STAI-T est. ± s.e.: 1.1 ± 1.71, adj. p = 1; WEMWBS est. ± s.e.: −2.78 ± 1.55, adj. p = 0.516), suggesting a lack of association between baseline suggestibility and therapeutic response to escitalopram, see online Supplementary Table S2 for details. Equivalence testing the suggestibility × timepoint interaction term yielded non-significant results on all scales except BDI and STAIT, see Equivalence testing and online Supplementary Table S6 for details; however, even on these two scales, the significance did not survive the Bonferroni correction.
In the psilocybin arm, we found a significant interaction between suggestibility and therapeutic response on all scales (HAM-D est. ± s.e.: −3.46 ± 0.92, adj. p = 0.005**; BDI est. ± s.e.: −7.16 ± 1.94, adj. p = 0.006**; MADRS est. ± s.e.: −6.36 ± 1.37, adj. p = 0.001***; QIDS-SR-16 est. ± s.e.: −3.31 ± 1.04, adj. p = 0.022*; STAI-T est. ± s.e.: −9.64 ± 2.1, adj. p = 0.001*; WEMWBS est. ± s.e.: 6.44 ± 2.02, adj. p = 0.022*), implying a robust association between baseline suggestibility and therapeutic response to psilocybin, see online Supplementary Table S3 for details. The findings suggest that, on the HAM-D scale, each standard deviation increase (~10 points on the Short Suggestibility Scale) of suggestibility is associated with 3.46 reduction in depression scores, etc. Figure 3 shows the suggestibility v. outcomes regression lines for both treatment arms.
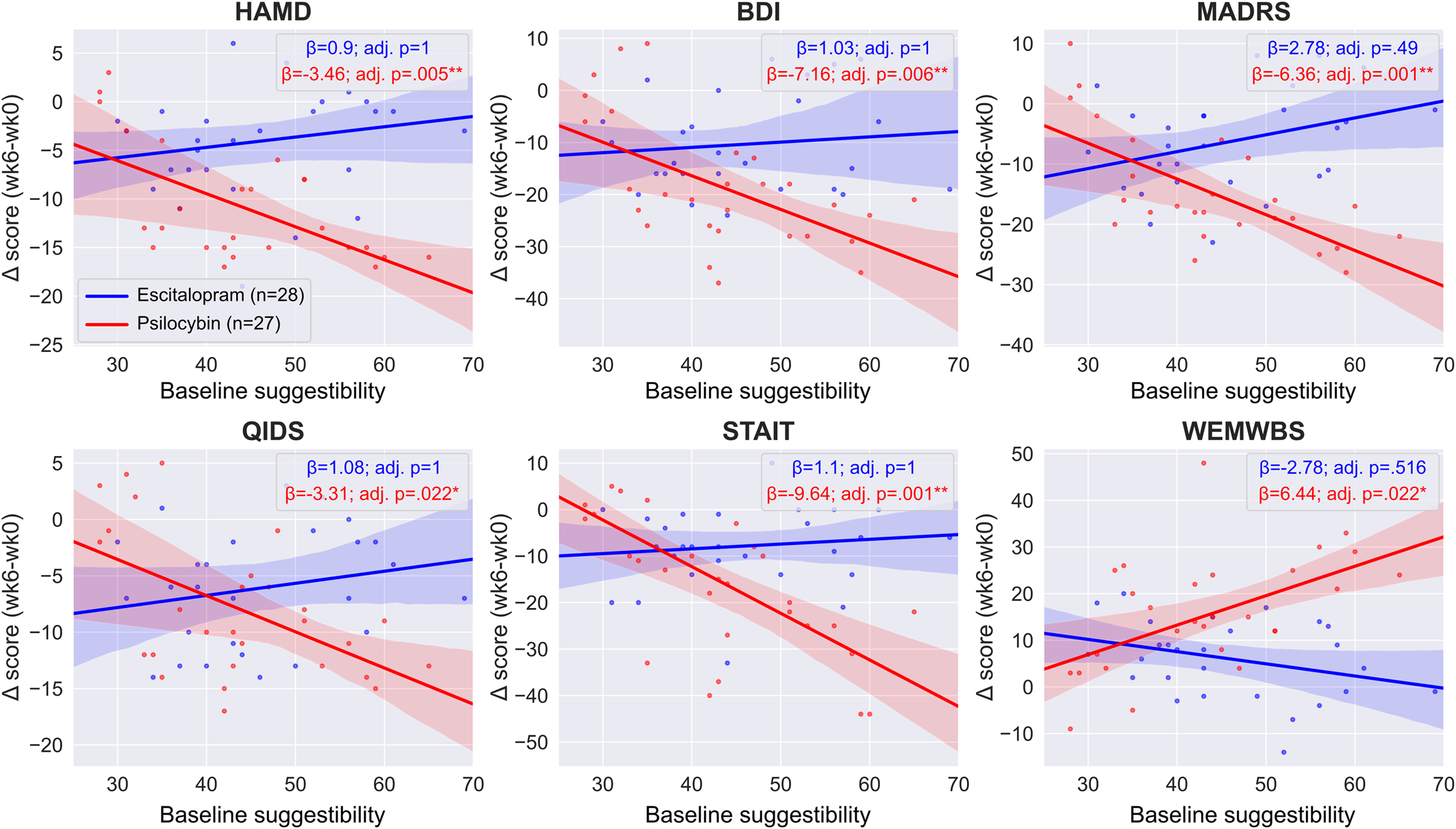
Figure 3. Regression lines of trait suggestibility v. clinical efficacy. Regression lines and coefficients were obtained from the two separate ‘within arm’ models, see Statistical models for details. There was a significant association between baseline suggestibility and outcomes on all scales in the psilocybin arm (red lines) that was maintained after adjusting for multiple comparisons. In contrast, there was no such significant relationship in the escitalopram arm (blue lines). Between-arm models indicate that not only the significance level was different between the treatment arms on all scales, but that the difference was also significant (Nieuwenhuis et al., Reference Nieuwenhuis, Forstmann and Wagenmakers2011). Boxes show the regression coefficients (β) associated with the suggestibility × timepoint term in the two separate arms and the associated Bonferroni adjusted p values. Negative values indicate improved symptoms except for WEMWBS where positive values indicate improved well-being, see online Supplementary Tables S2 and S3 for details.
We found no significant interaction between absorption and timepoint in either the escitalopram or the psilocybin arm on any of the scales, suggesting a lack of association between baseline absorption and response, see online Supplementary Tables S2 and S3 for details.
Between-treatment difference in models adjusted for expectancy and suggestibility
When adjusting the trial results for pre-trial expectancy, there was no significant interaction term between timepoint and treatment on any of the scales after adjusting for multiple comparisons (HAMD est. ± s.e.: −3.06 ± 1.67, adj. p = 0.438; BDI est. ± s.e.: −3.32 ± 3.49, adj. p = 1; MARDS est. ± s.e.: −4.52 ± 2.85, adj. p = 0.711; QIDS est. ± s.e.: 0.82 ± 1.93, adj. p = 1; STAI-T est. ± s.e.: −3.03 ± 3.92, adj. p = 1; WEMWBS est. ± s.e.: 7.82 ± 3.61, adj. p = 0.214), implying that there is no difference between the treatments after adjusting for expectancy. Equivalence testing yielded non-significant results on all scales, suggesting that we cannot rule out a true effect as large as the minimum important difference, see Equivalence testing and online Supplementary Table S6 for details.
The treatment × timepoint × expectancy interaction term was significant on the HAMD and MADRS scales (HAMD est. ± s.e.: 6.02 ± 1.63, adj. p = 0.003**; MARDS est. ± s.e.: 7.79 ± 2.78, adj. p = 0.043*), suggesting that, on these two scales, the difference between the treatment arms reached significance; however, the difference was not significant on the other 4 scales (BDI est. ± s.e.: 7.88 ± 3.39, adj. p = 0.146; QIDS est. ± s.e.: 3.6 ± 1.87, adj. p = 0.361; STAI-T est. ± s.e.: 6.96 ± 3.81, adj. p = 0.441; WEMWBS est. ± s.e.: −6.82 ± 3.54, adj. p = 0.361). When adjusting the trial results for suggestibility, the results qualitatively remained the same as for the unadjusted models (Carhart-Harris et al., Reference Carhart-Harris, Giribaldi, Watts, Baker-Jones, Murphy-Beiner, Murphy and Nutt2021); specifically, there was a significant interaction term between timepoint and treatment on all scales except QIDS (HAMD est. ± s.e.: −5.88 ± 1.44, adj. p < 0.001***; BDI est. ± s.e.: −7.48 ± 2.7, adj. p = 0.047*; MARDS est. ± s.e.: −7.36 ± 2.09, adj. p = 0.006**; QIDS est. ± s.e.: −1.37 ± 1.46, adj. p = 1; STAI-T est. ± s.e.: −8.17 ± 2.75, adj. p = 0.027*; WEMWBS est. ± s.e.: 9.34 ± 2.59, adj. p = 0.004**). This finding suggests a between-treatment difference at the primary endpoint after adjusting for suggestibility, favoring the psilocybin condition on all scales, see online Supplementary Table S5 for details. The treatment × timepoint × suggestibility interaction term was significant on all scales (HAMD est. ± s.e.: −4.34 ± 1.42, adj. p = 0.021*; BDI est. ± s.e.: −8.12 ± 2.68, adj. p = 0.023*; MARDS est. ± s.e.: −9.12 ± 2.06, adj. p < 0.001***; QIDS est. ± s.e.: −4.37 ± 1.45, adj. p = 0.024*; STAI-T est. ± s.e.: −10.66 ± 2.73, adj. p = 0.002**; WEMWBS est. ± s.e.: 9.2 ± 2.57, adj. p = 0.005**), suggesting that not only was the significance level different between the treatment arms but that the difference was also significant (Nieuwenhuis, Forstmann, & Wagenmakers, Reference Nieuwenhuis, Forstmann and Wagenmakers2011); see within-arm suggestibility models.
Discussion
Analyzing pre-treatment efficacy-related expectations in a trial of escitalopram v. psilocybin for the treatment of depression (Carhart-Harris et al., Reference Carhart-Harris, Giribaldi, Watts, Baker-Jones, Murphy-Beiner, Murphy and Nutt2021), we found that patients had substantially higher expectancy for psilocybin therapy compared with escitalopram; however, when we assessed whether an association exists between pre-trial expectancy and therapeutic response, we found a significant association in the escitalopram arm, but not in the psilocybin arm.
The escitalopram results are consistent with previous findings pertaining to SSRIs (Bingel et al., Reference Bingel, Wanigasekera, Wiech, Ni Mhuircheartaigh, Lee, Ploner and Tracey2011). However, the lack of association for the psilocybin arm is surprising given that expectancy effects are associated with improved outcomes across a wide range of medical diagnoses and therapeutic approaches (Tambling, Reference Tambling2012), including one naturalistic study of psychedelic use that assessed expectancy with a self-constructed binary (yes/no) questionnaire (Weiss, Miller, Carter, & Keith Campbell, Reference Weiss, Miller, Carter and Keith Campbell2021), rather than using a continuous scale. Suspicion has been expressed that in psychedelic trials the combination of the lack of effective blinding, strong demand characteristics, and related confirmation biases may positively bias trial outcomes (Burke & Blumberger, Reference Burke and Blumberger2021; Muthukumaraswamy et al., Reference Muthukumaraswamy, Forsyth and Lumley2021; Szigeti et al., Reference Szigeti, Nutt, Carhart-Harris and Erritzoe2023). Our results partially alleviate these suspicions, as we did not find a significant association between psilocybin-specific efficacy-related expectations and efficacy-related outcomes.
What explanations can be given for the lack of an expectancy effect in the psilocybin arm? Given that most of our trial patients were self-referred and it is reasonable to assume that many were seeking psilocybin treatment in particular, a ceiling effect on pre-trial expectancy for psilocybin was considered and examined; however, the average psilocybin expectancy score was 51% from a possible 100%, i.e. far from the ceiling. A second possibility is that the relationship is not linear in nature. For example, one could speculate that patients with unrealistically high expectations may be disappointed, leading to worse outcomes with higher expectations; indeed, the slopes of the expectancy v. outcome regressions are positive, see Fig. 2, although all of them are highly non-significant. Our sample was too small to investigate complex, non-linear models; however, this would be worth exploring via larger samples – achievable e.g. via pragmatic trials or real-world data collection (Carhart-Harris et al., Reference Carhart-Harris, Wagner, Agrawal, Kettner, Rosenbaum, Gazzaley and Erritzoe2022).
We failed to observe a significant expectancy effect in the psilocybin arm, but such a non-significant result should not be mistaken as evidence from which the absence of an effect can be inferred (Lakens et al., Reference Lakens, McLatchie, Isager, Scheel and Dienes2020). We performed equivalence testing to confirm the null hypothesis; however, this was non-significant as well. Therefore, from a strict inferential perspective, we cannot either rule out or confirm expectancy effects in the psilocybin arm, more data is needed to test and infer on this matter. We note that our data suggests that ‘negative expectancy’, i.e. higher expectancy associated with worse response, may be more likely than the generally assumed positive expectancy (Muthukumaraswamy et al., Reference Muthukumaraswamy, Forsyth and Lumley2021), as indicated by the positive, although non-significant, slopes in Fig. 2. Thus, if there is a ‘true’ expectancy effect that we were underpowered to detect, it may be that higher expectancy for psilocybin could actually be associated with worse response to psilocybin.
If future research enabled us to accept the null hypothesis, i.e. that there is no association between expectancy and therapeutic response in psilocybin therapy, then this would imply that psilocybin therapy has a direct treatment effect that is independent of positive expectancy. More work is needed to determine what psilocybin's precise therapeutic action is, but some empirical clues and models are emerging (Carhart-Harris & Friston, Reference Carhart-Harris and Friston2019; Daws et al., Reference Daws, Timmermann, Giribaldi, Sexton, Wall, Erritzoe and Carhart-Harris2022; Murphy et al., Reference Murphy, Kettner, Zeifman, Giribaldi, Kartner, Martell and Carhart-Harris2022; Zeifman, Wagner, Monson, & Carhart-Harris, Reference Zeifman, Wagner, Monson and Carhart-Harris2023).
In this trial, response to psilocybin was not predicted by baseline expectancy, but the response to escitalopram was, therefore, the between-arm difference is also affected by expectancy. When we adjusted the models for baseline expectancy, there was no between-treatment difference in efficacy on any of the scales. In contrast, models unadjusted for expectancy produced a significant between-arm difference for all depression-related outcomes except on the QIDS-SR-16 scale, as originally reported (Carhart-Harris et al., Reference Carhart-Harris, Giribaldi, Watts, Baker-Jones, Murphy-Beiner, Murphy and Nutt2021). This result implies that the observed expectancy imbalance biased the results in favor of psilocybin's superiority, see online Supplementary Table S7 for a direct comparison of the unadjusted and expectancy-adjusted between-arm models. Notably, this expectancy bias is not a result of the patients in the psilocybin arm benefitting from high expectations, as we found no expectancy effect in the psilocybin arm, but rather due to patients having low expectancy in the escitalopram arm, which can be interpreted as a nocebo effect.
Trait suggestibility was predictive of psilocybin efficacy here. Previous research indicates a link between verbal suggestibility and placebo responsiveness (Oakley, Walsh, Mehta, Halligan, & Deeley, Reference Oakley, Walsh, Mehta, Halligan and Deeley2021; Parsons, Bergmann, Wiech, & Terhune, Reference Parsons, Bergmann, Wiech and Terhune2021). Together, these findings could be interpreted as evidence for extra-pharmacological factors driving the response in the psilocybin arm, demand characteristics, and/or the Hawthorne effect, playing a role in psilocybin's efficacy, future trials may further examine this possibility. In a recent prospective naturalistic study on ayahuasca, suggestibility was associated with a greater reduction in trait neuroticism after ayahuasca (Weiss et al., Reference Weiss, Miller, Carter and Keith Campbell2021). One other naturalistic study failed to see a relationship between baseline trait suggestibility and either acute subjective experience or changes in well-being (Haijen et al., Reference Haijen, Kaelen, Roseman, Timmermann, Kettner, Russ and Carhart-Harris2018); however, this latter null findings may have been a product of a multivariate regression approach and potential collinearity between model components. Baseline absorption has previously been found to be predictive of the acute subjective intensity of psychedelic effects (Aday et al., Reference Aday, Davis, Mitzkovitz, Bloesch and Davoli2021; Haijen et al., Reference Haijen, Kaelen, Roseman, Timmermann, Kettner, Russ and Carhart-Harris2018), which in turn may predict therapeutic outcomes (Murphy et al., Reference Murphy, Kettner, Zeifman, Giribaldi, Kartner, Martell and Carhart-Harris2022; Roseman, Nutt, & Carhart-Harris, Reference Roseman, Nutt and Carhart-Harris2017); however, here we did not find a direct link between absorption and response in either treatment arms. More work is needed to test the reliability with which trait suggestibility can predict response to psilocybin therapy, as well as what the mechanisms are for this apparent effect – e.g. is it more biologically grounded (Ott et al., Reference Ott, Reuter, Hennig and Vaitl2005), or more psychologically based (De Pascalis, Chiaradia, & Carotenuto, Reference De Pascalis, Chiaradia and Carotenuto2002), or are the two inter-related and do they interact? High trait absorption could imply elevated sensitivity to direct drug effects (Ott et al., Reference Ott, Reuter, Hennig and Vaitl2005), while high suggestibility could imply elevated attunement to acute insights, and influence from therapy personnel such as the therapist or clinical staff (Cherniak et al., Reference Cherniak, Brulin, Mikulincer, Ostlind, Carhart-Harris and Granqvist2021; Murphy et al., Reference Murphy, Kettner, Zeifman, Giribaldi, Kartner, Martell and Carhart-Harris2022).
Limitations and future work
The analysis presented here was not pre-registered; thus, our results should be understood as exploratory rather than confirmatory (Jaeger & Halliday, Reference Jaeger and Halliday1998). Furthermore, in the absence of any experimental manipulation of expectancy, all relationships reported here should be interpreted as correlational, not causal. Further studies are needed to assess causation, e.g. by seeking to manipulate expectations in a controlled and measured way.
The non-significant equivalence tests for the expectancy-outcome association in the psilocybin arm suggest that we cannot rule out an expectancy effect as large as 0.5 standardized mean difference (s.m.d.), corresponding to the minimum important difference (Norman et al., Reference Norman, Sloan and Wyrwich2003). Our trial was not powered to reject effects as small as the minimum important difference, thus, the failed equivalence test may be a consequence of the small sample. Also, the expectancy measure used here was not a validated survey. It is possible that using validated expectancy measures would find different results from those presented in this paper.
No ‘treatment allocation guess’ data was collected either from patients or assessors in the current trial, meaning we could not evaluate blinding integrity or its interaction with expectancy. It is plausible that expectancy could interact with perceived treatment allocation – and the confidence level of this ‘guess’ – to influence response outcomes (e.g. disappointment at confidently realizing you have been allocated to the escitalopram arm). A new measure of blinding integrity that incorporates these features is introduced in another paper (Szigeti et al., Reference Szigeti, Nutt, Carhart-Harris and Erritzoe2023). We note that the expectancy measure used here was administered pre-trial for each arm when randomization had not yet determined treatment allocation. From the available data, we could derive a hypothetical treatment-agnostic expectancy measure, i.e., by averaging the expectancies for both treatments. However, this averaged or ‘treatment agnostic’ expectancy score did not qualitatively alter any of our conclusions, see online Supplementary materials for details.
We finally note that while the current paper has focused specifically on positive expectancy in relation to measures of therapeutic efficacy, i.e., mechanisms relevant to the so-called ‘placebo effect’, one could also examine expectancy regarding adverse effects - i.e., nocebo effects. The investigation of negative expectancy and negative outcomes in psychedelic trials is a worthwhile avenue for future investigations, as it could inform on risk type, prevalence, and mitigation.
Conclusions
We observed higher pre-trial positive expectancy for psilocybin v. escitalopram but found no evidence that efficacy-related expectations for psilocybin could predict therapeutic actual response to psilocybin. Conversely, pre-trial expectancy for escitalopram was reliably predictive of response to escitalopram across most of the efficacy-related outcome measures, in line with what is generally known about the influence of expectancy on response. Baseline trait suggestibility was predictive of response to psilocybin, but not to escitalopram.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291723003653
Funding statement
This work was funded by the Alexander Mosley Charitable Trust and Imperial College London's Centre for Psychedelic Research.
Competing interests
B.Sz., B.W., and F.E.R. declare no conflict. D.N. is an advisory to COMPASS Pathways, Neural Therapeutics, and Algernon Pharmaceuticals; received consulting fees from Algernon, H. Lundbeck, and Beckley Psytech; received lecture fees from Takeda and Otsuka and Janssen plus owns stock in Alcarelle, Awakn and Psyched Wellness. D.E. received consulting fees from Aya, Mindstate, Field Trip, and Clerkenwell Health. R.C.H. is an advisor to Mindstate, TRYP Therapeutics, Maya Health, Entheos Lab, and Journey Collab.






