As populations in developed countries continue to get older, age-associated decrements in mobility and cognition, even in the absence of neurodegenerative disorders, will become increasingly prevalent. Strategies to retard or reverse these deficits are needed to increase healthy ageing and reduce healthcare costs. Research in animals and humans has shown that age-related deficits in cognitive, motor and neuronal functions may arise as a result of an increasing inability of the ageing organism to protect itself against inflammation and oxidative stress(Reference Perry1–Reference Miller and Shukitt-Hale3), but that nutritional factors can preserve some behavioural function from age-related decline(Reference Spencer, Korosi and Layé4,Reference Lamport, Saunders and Butler5) . Berry fruits contain a variety of bioactive phytochemicals and polyphenolic compounds that have shown anti-inflammatory and antioxidant properties that may provide protection as well as health benefits against chronic disease(Reference Miller and Shukitt-Hale3,Reference Miller, Feucht and Schmid6) .
Previous animal studies have shown that strawberry (SB) improved several parameters of brain ageing (e.g. deficits in cell communication) as well as age-related motor and cognitive deficits when fed to aged rats for 2 months (from 19 to 21 months of age), compared with those fed a control diet(Reference Joseph, Shukitt-Hale and Denisova7,Reference Shukitt-Hale, Bielinski and Lau8) . Specifically, consuming SB-supplemented diets led to enhanced performance on a test of general balance and coordination, improved performance in a working memory version of the Morris water maze, increased neurogenesis (cell survival) in the dentate gyrus and increased insulin-like growth factor-1 (IGF-1) in the hippocampus, a neuronal signal that has been shown to be associated with learning and memory(Reference Shukitt-Hale, Bielinski and Lau8). Another study showed that pre-feeding rats for 8 weeks with 2 % SB and blueberry (BB) prior to exposure with whole-body irradiation (1·5 Gy of 1 GeV/n high-energy 56Fe particles, which produces deficits similar to those seen in ageing) were better protected than control diet-fed irradiated rats in Morris water maze performance(Reference Shukitt-Hale, Carey and Jenkins9).
Prospective studies using SB have shown that a higher intake of flavonoids, particularly from berries, appears to reduce the rates of cognitive decline in older adults. The first found that greater long-term intakes of berries, specifically BB and SB, and total flavonoids were associated with slower rates of cognitive decline, by up to 2·5 years, in older women(Reference Devore, Kang and Breteler10). Another study that was part of the Rush Memory and Aging Project showed that the consumption of SB and related bioactives (i.e. vitamin C, pelargonidin, anthocyanidins and total flavonoids) may reduce the risk of Alzheimer’s dementia(Reference Agarwal, Holland and Wang11). Clinical studies with berries have also demonstrated anti-inflammatory effects and improved cognition(Reference Miller, Feucht and Schmid6,Reference Ellis, Edirisinghe and Kappagoda12–Reference Krikorian, Shidler and Nash14) . For example, chronic consumption of SB for 6 weeks reduced fasting levels of serum C-reactive protein, IL-1β and TNF-α in overweight men and women, relative to placebo controls(Reference Ellis, Edirisinghe and Kappagoda12). Consumption of freeze-dried whole BB powder for 90 d improved cognitive function in healthy older adults compared with placebo-supplemented controls(Reference Miller, Hamilton and Joseph13). Specifically, we found that the addition of BB to older adults’ diet improved measures of executive function, such as decreased repetitive errors on a List Learning Test and reduced switch stimuli errors on a Task-Switching Test, relative to controls. However, measures of gait and balance were not improved by BB supplementation, perhaps due to the healthiness of the sample population and the lack of a challenge when walking/balancing(Reference Miller, Hamilton and Joseph13). Other studies using BB as an intervention have found improvements in mobility and cognition in older adults following chronic consumption (reviewed in(Reference Hein, Whyte and Wood15)), although to date no studies have looked at these endpoints following long-term SB intervention. Specifically, one study showed improved performance following 6 weeks of dietary BB compared with carrot juice in older adults performing a complex, adaptive gait task involving dual task performance, as indicated by fewer step errors, even after adjusting for individual differences in gait speed(Reference Schrager, Hilton and Gould16). Another study showed that BB improved brain perfusion and activation in brain areas associated with cognitive function, specifically working memory, in healthy older adults(Reference Bowtell, Aboo-Bakkar and Conway17). Two studies using wild BB showed both acute cognitive benefits in middle-aged adults(Reference Whyte, Rahman and Bell18) and improved episodic memory performance after 3 months compared with placebo in an elderly population(Reference Whyte, Cheng and Fromentin19).
These findings suggest that dietary supplementation with berry fruit has the potential for a multiplicity of effects, in addition to their antioxidant and anti-inflammatory properties, including the activation of a variety of signalling pathways that result in neuroprotection, neurogenesis and, ultimately, spared cognitive and motor behaviour. The present study was conducted to determine whether long-term dietary SB could improve age-related motor and cognitive decline among healthy older adults, similar to our study with BB(Reference Miller, Hamilton and Joseph13). Because different berry fruits might not be acting in the same brain region or via a singular mechanism, we used a similar protocol in the present SB study to see which measures were improved, and whether they were the same as those enhanced in the BB study.
Methods
Study design
This randomised, double-blind, placebo-controlled, parallel-group trial investigated the effects of a 90-d dietary SB (Fragaria) intervention on mobility and cognition in older men and women (60–75 years; Fig. 1). Following screening, participants visited the USDA/ARS Jean Mayer Human Nutrition Research Center on Aging (HNRCA) at Tufts University in Boston, MA, four times for pre-baseline (visit 1), baseline (visit 2, day 0), midpoint (visit 3, day 45) and endpoint (visit 4, day 90) study visits. The primary and secondary outcome measures were cognition and mobility, respectively, and tasks were selected to parallel behavioural tasks in which behavioural enhancement is observed when aged rodents’ diet is supplemented with berry fruit. The present study follows the same procedures as in our previous study on the effects of dietary BB(Reference Miller, Hamilton and Joseph13).
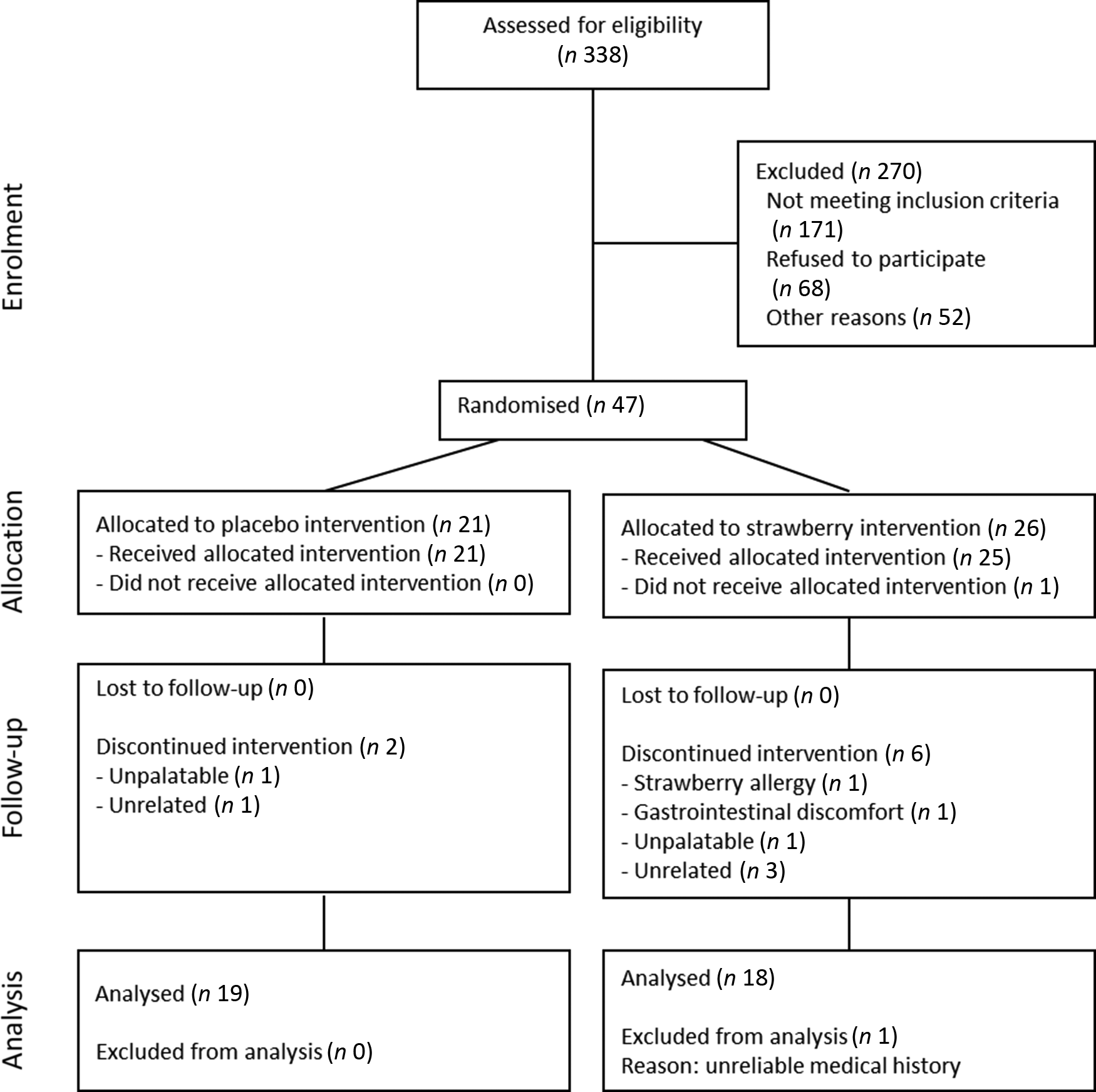
Fig. 1. Participant flowchart showing the progress through the phases of a 90-d randomised, double-blind, placebo-controlled study evaluating the effects of 24 g/d dietary strawberry in healthy older adults.
Participants
The age range for participants (60–75 years) was selected because older adults showed measurable age-related motor and cognitive decline at these ages relative to young adults(Reference Miller, Hamilton and Joseph20). Inclusion criteria included BMI between 18·5 and 29·9 kg/m2, ability to walk 20 min unassisted, English fluency, self-reported adequate visual acuity and >12 months postmenopausal. Exclusion criteria included self-reported psychological or psychiatric disorders, chronic disease including diabetes mellitus, history of significant brain injury and neurological insults/diseases, use of medications or dietary supplements that may impact study outcomes, Mini Mental Status Examination score <24, recent surgery or injury to head or lower extremities, SB allergy, vegetarian/vegan, fall within the past year, ethanol >2 drinks per d, smoking or illicit drug use. Participants were recruited from the community through direct mailings based on potential participant databases at the HNRCA’s Metabolic Research Unit and enrolled through telephone and on-site screening at the HNRCA. Participants received up to $390 for completing all study visits. All procedures were approved by the Tufts Health Sciences Institutional Review Board, and this study is registered on Clinicaltrials.gov (ClinicalTrials.gov identifier NCT02051140; 31 January 2014).
Participants’ usual diet was assessed using the National Cancer Institute’s Diet History Questionnaire II (DHQ-II). The DHQ-II is a comprehensive, 124-item online inventory that collects data on commonly consumed foods over the past year (National Cancer Institute, 2010). Participants also completed a supplemental questionnaire on berry consumption, which assessed the usual intake of berry fruits. Physical activity was assessed using a modified version of the Paffenbarger Physical Activity Questionnaire (PAQ). Hours spent at various activity levels were weighted based on the oxygen consumption associated with each type of activity (sleeping 1, sitting 1·1, light activity 1·5, moderate activity 2·4, heavy activity 5)(Reference Laugero, Falcon and Tucker21,Reference Paffenbarger, Hyde and Wing22) . Familiarity with testing apparatus was assessed using a Computer/Treadmill Proficiency (PROF) questionnaire. Participants also completed the Falls Efficacy Scale–International (FES-I)(Reference Yardley, Beyer and Hauer23) to measure fear of falling, and a Fall History (FH) questionnaire to verify their lack of recent falls. Participants completed the DHQ-II, PAQ, PROF and FH questionnaires during visit 1. Participants completed the FES-I during study visits 2, 3 and 4. Anthropometric measurements, including height, weight, waist circumference, blood pressure, body temperature, pulse and respiration rate, were measured at all study visits.
Treatments and randomisation
An online randomiser (randomization.com) was used to divide participants into one of two intervention groups (SB or placebo) using block randomisation (eleven blocks of four) and a 1:1 allocation ratio. Participants in the SB group consumed 12 g of a lyophilised, standardised blend of SB sourced from equal parts of Albion, San Andreas, Camino Real and Well-Pict 269 varieties, twice daily (24 g/d, equivalent to two cups per serving of fresh SB). The phenolic composition of the SB powder has been previously reported(Reference Rutledge, Fisher and Miller24). Participants in the placebo group consumed 12 g of a colour-matched, isoenergetic (361 kcal (1510 kJ)/100 g), SB-flavoured placebo powder twice daily. The placebo comprised of maltodextrin, fructose, dextrose, sucrose, cellulose and xanthan gum, food starch, citric and malic acids, sugar beet fibre, dipotassium phosphate, potassium citrate, silicon dioxide, natural SB flavour and food colouring. SB and placebo powders were provided by the California Strawberry Commission and individually packaged according to Good Manufacturing Practices (GMP; We Pack It All Inc.). SB and placebo powders were packaged in serving-sized, opaque, coded packets, which were distributed to participants by a blinded study dietician according to the experimenters’ randomisation scheme. Both the research team and participants were blind to the treatment. Study powders were stored in participants’ home refrigerator and reconstituted in water prior to consumption with breakfast and dinner respectively. Starting at their pre-baseline visit, participants abstained from consuming berry fruit (from a list of forty-six commonly consumed, bite-sized fruit, e.g. grapes, cherries, BB, blackberries, cranberries, acai, etc.) and products made from or containing these berries (e.g. berry-flavoured yogurt, berry juice, raisins, wine, etc.); otherwise, participants maintained their usual diet. Adherence to protocol was assessed through intake calendars, weekly telephone calls and counts of empty packages returned at study visits 3 and 4.
Cognition
Executive function was assessed using a computerised Task-Switching Test (TST)(Reference Rogers and Monsell25–Reference Monsell, Sumner and Waters27) in which participants repeatedly and predictably switch between two number categorisation tasks for 30 min. An abbreviated practice session was conducted during visit 1, and the full TST was administered during visits 2, 3 and 4. Participants also completed the Trail-Making Test (TMT)(28), in which they alternated connecting a series of numbers (part A) or numbers and letters (part B) on a sheet of paper during visits 2 and 4. Long-term memory was assessed using the California Verbal Learning Test, Second Edition (CVLT-II; Pearson Inc.)(Reference Delis, Kramer and Kaplan29). In the CVLT-II, participants learn a sixteen-word list containing words from four semantic categories. The list is first presented for recall five times. A distractor list (list B) is then presented for recall before the participants’ recall of the initial list is tested with and without categorical clues. Delayed recall, cued recall and yes/no word recognition were tested again after a 20-min delay. During recognition testing, participants had to discriminate between semantically similar list and non-list words. After a further 10-min delay, participants are given a forced choice test wherein they are given word pairs containing a list word and a distractor word, and participants indicated which word was from the initial list. Correct responses, repetition errors, intrusion errors and false-positives were scored manually. This test was administered to participants only during study visits 2 and 4, and both the standard and alternate versions of the test were used in counterbalanced order. Short-term memory was assessed using the forward and backward versions of the digit span (DS) task during study visits 2, 3 and 4(Reference Wechsler30,Reference Wechsler31) . Spatial cognition was measured using a virtual version of the Morris water maze (vMWM)(Reference Hamilton, Johnson and Redhead32). In this task, participants use arrow keys to locate an invisible platform within a circular virtual arena that is surrounded by multiple cues as presented on a desktop computer(Reference Jacobs, Laurance and Thomas33). During this test, participants completed sixteen hidden platform acquisition trials (60 s), a probe test in which the platform is absent (40 s) and four visible platform trials (60 s). Different virtual environments were used during each administration to minimise task learning effects. Participants completed an abbreviated practice version of the task during study visit 1 and the full vMWM task during study visits 2, 3 and 4. Attention was measured using the Attention Network Task (ANT), which tests three different attentional networks (alerting, orienting and executive control) within a single computerised task(Reference Fan, McCandliss and Sommer34). A practice version of this task was administered during visit 1 and the full task was administered during study visits 2, 3 and 4. Mood was assessed using the Geriatric Depression Scale (GDS)(Reference Yesavage, Brink and Rose35) and a computerised version of the Profile of Mood States (POMS)(Reference McNair, Lorr and Droppleman36) during study visits 2, 3 and 4. For a more detailed description of these tasks, see Miller et al. (Reference Miller, Hamilton and Joseph13).
Mobility
Dynamic stance and gait analyses were performed using a high-density array of force sensors mounted beneath the tread belt of a commercial treadmill (Zebris™). During stance measurements, participants stood in their preferred stance with eyes opened and closed, narrow stance with feet together, tandem stance with their dominant foot in front (heel to toe) and on one foot (dominant). During gait analysis, participants were habituated to walking on the treadmill and then completed a procedure that determined their preferred walking speed (±0·1 mph) as well as the upper and lower bounds of their preferred walking range. Participants then continued to walk for 2 min at their preferred walking speed while their gait was measured. Stance and gait were measured during visits 2, 3 and 4.
Procedure
Participants consented during study visit 1. Participants completed abbreviated versions of the ANT, TST, vMWM and gait assessment to minimise practice effects, as well as the DHQ-II, PROF, FH and PAQ. Participants then returned after a week for visit 2 (day 0). A study nurse collected anthropometric measurements, vital signs and fasting (about 12–15 h) blood. Following a low-polyphenol, standardised breakfast (corn muffin, banana, apple juice and optional coffee to prevent caffeine withdrawal among usual drinkers; about 50 % energy from carbohydrates, 31 % fat and 19 % protein), unconsumed food was weighed back and participants completed the POMS, FES, ANT, CVLT-II, TST, vMWM, DS, TMT and stance and gait measurements. Before departing, participants consumed their first dose of the supplement (either SB or placebo) and were provided with SB or placebo powder packets to consume twice daily until their next visit.
Study visit 3 (day 45) was identical to visit 2 (day 0) except that participants consumed either an SB or placebo packet with the study breakfast according to their randomly assigned group. The breakfast was adjusted to match each participant’s actual consumption during visit 2. The CVLT-II and TMT were not administered at this time point. All empty and unused powder packets and compliance calendar were collected from the first 45 d, and another set of packets and compliance calendar were provided.
During visit 4, participants consumed their final SB or placebo packet with the study breakfast. The study breakfast during visit 4 (day 90) was again adjusted to match actual consumption of visits 2 and 3. They returned all their used and unused powder packets and their compliance calendar. Otherwise, visit 4 was identical to study visit 2.
Statistical analyses
A sample size of twenty participants per treatment group was estimated to detect a significant effect of treatment with at least 80 % power at the 5 % α level based on vMWM and gait speed data from a previous study(Reference Miller, Hamilton and Joseph20). Analyses were conducted using a mixed-model ANOVA to compare between the two diet groups (placebo, SB) and across days of supplementation (0, 45, 90), with additional factors included for tasks involving a repeated measure within test sessions. Effects that were significant at the 5 % α level were then subjected to Bonferroni-corrected post hoc testing. Effect size was measured using the standardised mean difference approach. All statistical analyses were conducted using Systat 10.2 (Systat Software Inc.).
Results
Participants
A total of 338 people responded to the recruitment mailings and were pre-screened telephonically (Fig. 1). From these, ninety-seven people were invited to participate in on-site screening, and fifty-four volunteers consented and enrolled into the study. Sixteen participants did not complete the study and were not included in the analyses; as a result, more people were allocated to the SB group to achieve equal numbers in both groups. The final per protocol analysis consisted of thirty-seven participants, of which nineteen consumed a placebo powder and eighteen consumed the SB powder (for participant demographics, see Table 1). Compliance to supplementation was >97 %, and no significant differences in adherence were observed between the treatment groups (Table 1).
Table 1. Participants randomised into the study groups*
(Mean values and standard deviations; numbers and percentages)
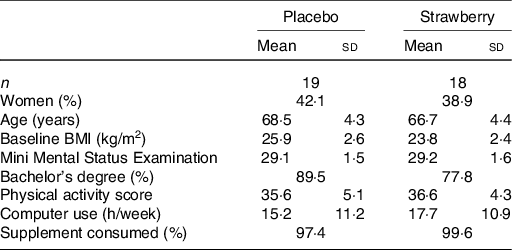
* No significant differences were observed between the two groups (P > 0·05).
At baseline, the analysis of food intake data from the DHQ-II revealed only one dietary measure on which the control and SB groups significantly differed. Participants in the SB group consumed significantly less folic acid than did participants in the placebo group (F(1,35) = 11·263, P = 0·002) (104·9 v. 188·7 μg/d, respectively). No significant differences between SB and placebo groups were observed among the other dietary intake measures (Table 2) nor among participant demographics, including physical activity levels (P = 0·509; Table 1). No significant differences between groups in body weight, waist circumference or vital signs were observed.
Table 2. Diet History Questionnaire II*
(Mean values and standard deviations)
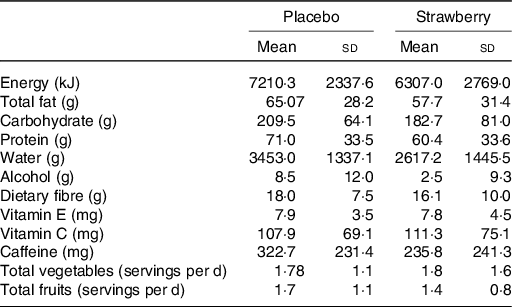
* Estimated daily intake of key nutrients and food groups over the 12 months prior to the study indicated no significant group differences in these key dietary measures (P > 0·05).
Cognition
On the California Verbal Learning Test (Table 3), all participants increased the number of words correctly recalled across the five immediate free recall presentations of the primary word list (F(4,140) = 146·913, P < 0·001), indicating that participants did learn the lists with repeated presentations during both visits. Furthermore, participants had steeper learning curves at visit 4, relative to visit 2 (F(4,140) = 2·606, P = 0·038). Similarly, word recall after a 20-min delay improved at visit 4, relative to visit 2 (F(1,35) = 4·568, P = 0·040). These performance improvements indicate a practice effect. However, during a 20-min delayed recognition paradigm, when participants were presented with list and non-list words, a significant group by study visit interaction was observed (F(1,35) = 6·653, P = 0·014, η p 2 = 0·159), indicating that, while participants in the SB group identified fewer list words at study visit 2, they increased the number of correctly identified list words at study visit 4, while those in the placebo group maintained baseline performance levels (Fig. 2). No effects of SB on cued recall, forced choice word recognition, false-positive responses during recognition or word recall after a short delay were observed (Table 3).
Table 3. California Verbal Learning Test
(Mean values and standard deviations)
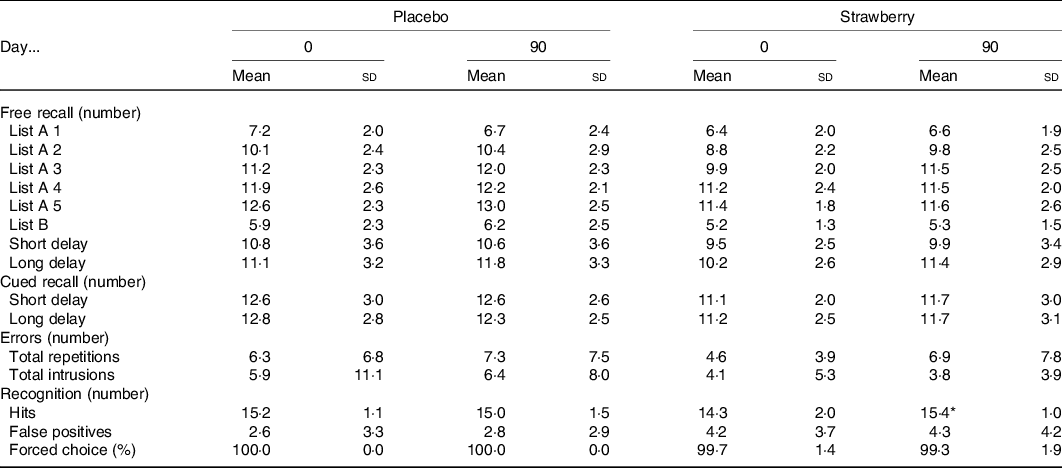
* Significant diet group × visit interaction (F(1,35) = 6·653, P = 0·014, η p2 = 0·159).
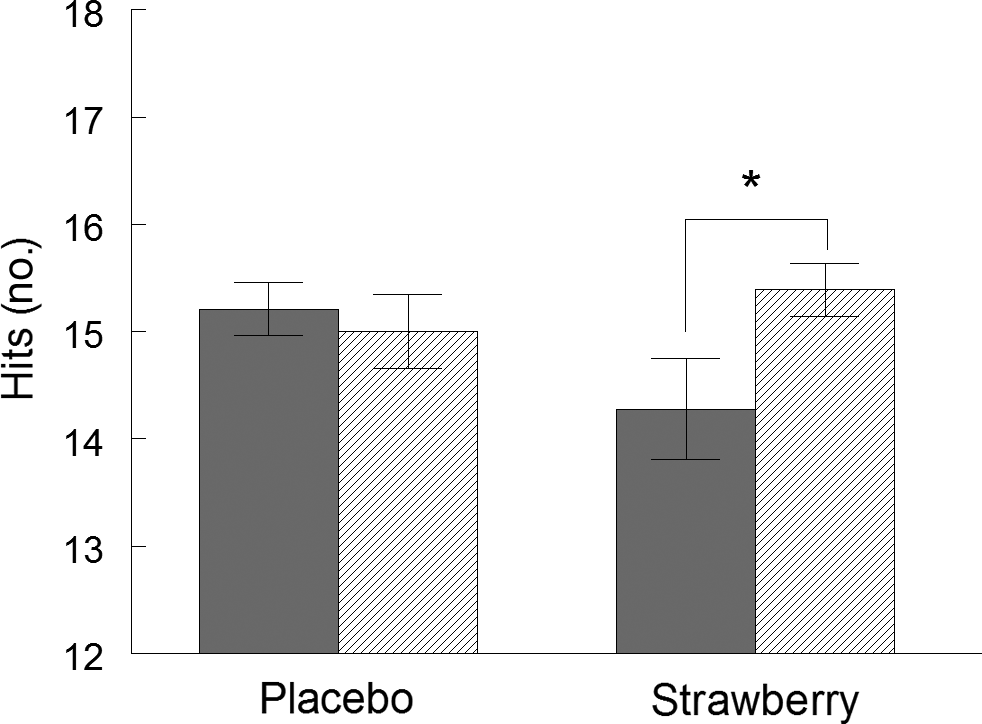
Fig. 2. California Verbal Learning Test II. Correctly identified list words (hits) during word recognition in the California Verbal Learning Test. Values are means, with standard errors represented by vertical bars. *P < 0·05. ![]() , Visit 2;
, Visit 2; ![]() , visit 4.
, visit 4.
In the vMWM task, participants were able to locate a hidden platform more quickly throughout acquisition trials during each testing day (F(3,105) = 8·933, P < 0·001). Overall acquisition latencies also decreased across intervention days (F(2,70) = 8·145, P = 0·001). These findings show that participants were able to learn the platform’s location over time; however, there were no group differences (Fig. 3(a)). An analysis of probe tests revealed a significant group × study visit interaction (F(2,70) = 4·140, P = 0·020, η p 2 = 0·106), in which participants who consumed SB showed improved spatial learning at study visit 3, that is, they spent more time searching for the platform in the correct quadrant on the trial when the platform was removed (Fig. 3(b)). However, performance was similar to baseline at visit 4, suggesting a potential short-term improvement (Fig. 3(b)). No differences in performance were observed when participants searched for a visible platform (P = 0·340).
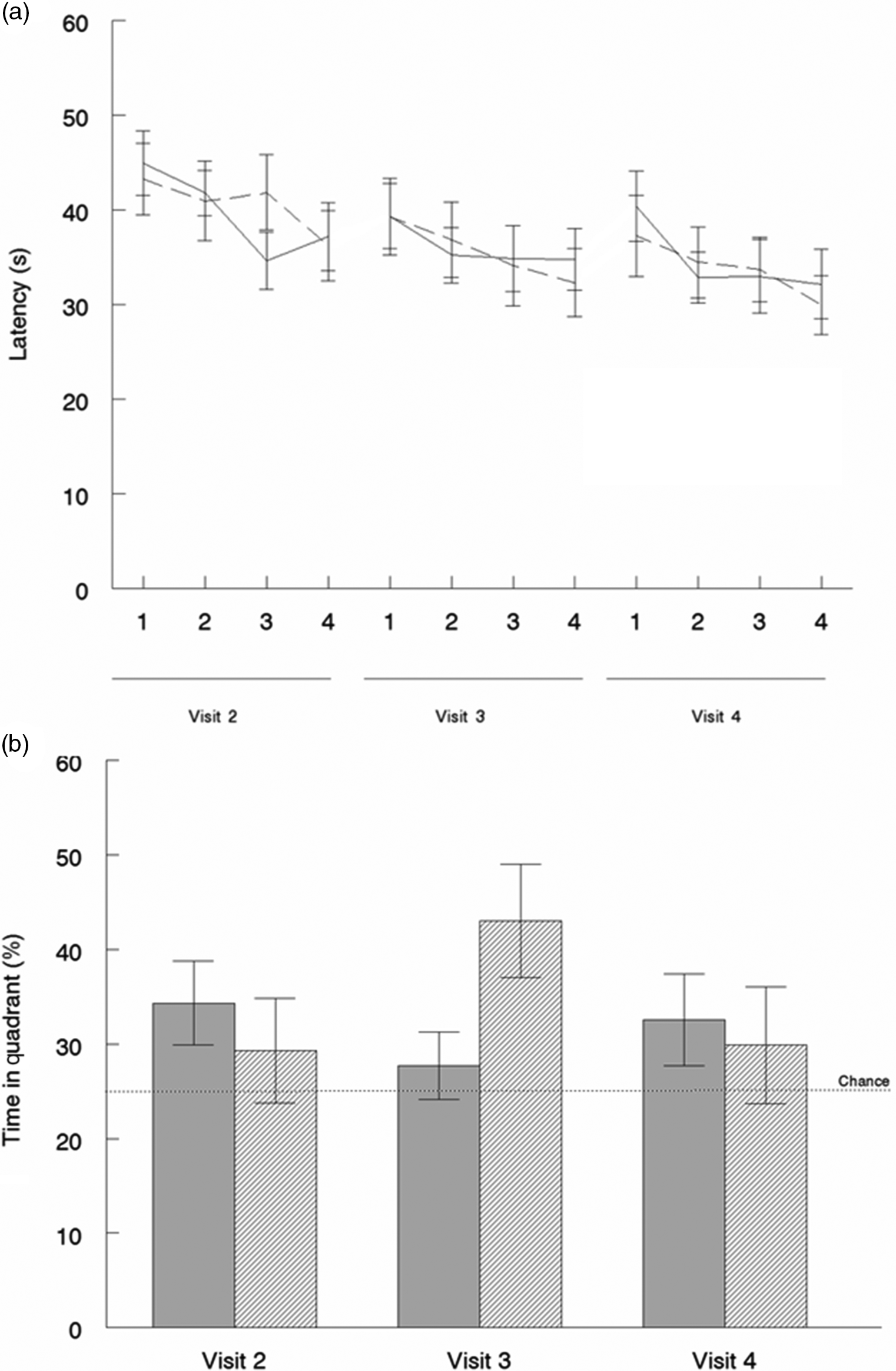
Fig. 3. Virtual Morris water maze. (a) Mean latency to complete blocks of four acquisition trials with counterbalanced start locations. No significant difference between groups was observed. ![]() , Placebo;
, Placebo; ![]() , strawberry. (b) Quadrant preference during probe trials. A significant group × visit interaction was observed (P = 0·02). Values are means, with standard errors represented by vertical bars.
, strawberry. (b) Quadrant preference during probe trials. A significant group × visit interaction was observed (P = 0·02). Values are means, with standard errors represented by vertical bars. ![]() , Placebo;
, Placebo; ![]() , strawberry.
, strawberry.
No significant effects of SB were detected on the Attention Network Test, Task-Switching Test, Trail-Making Test, digit span or on either the Geriatric Depression Scale or the Profile of Mood States.
Mobility
An analysis of the FES-I revealed no baseline differences or effect of SB on fear of falling during the study (P = 0·288 and 0·518, respectively). An analysis of postural sway throughout the study showed no evidence of a positive effect of SB on balance in any of the stance conditions (Table 4). Habituation to walking on the treadmill was observed among participants in both the SB and placebo groups, as evidenced by a general decline in cadence across study visits (F(2,64) = 4·393, P = 0·016). Additionally, a dynamic analysis of gait detected no effect of SB on gait speed or variability (Table 5).
Table 4. Postural sway*
(Mean values and standard deviations)
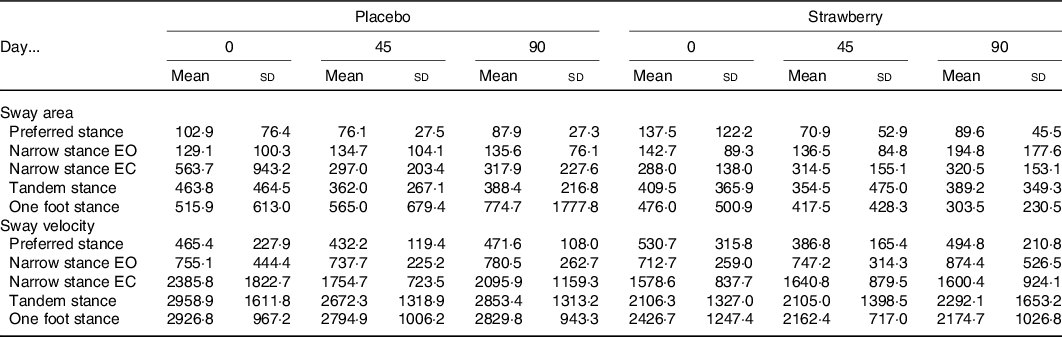
EO, eyes open; EC, eyes closed.
* Centre of pressure area (95 % confidence ellipse) and path length (mm/min) during 1-min stance measurements revealed no significant differences between intervention groups. Narrow stance is reported for both EO and EC conditions.
Table 5. Dynamic gait analysis*
(Mean values and standard deviations)
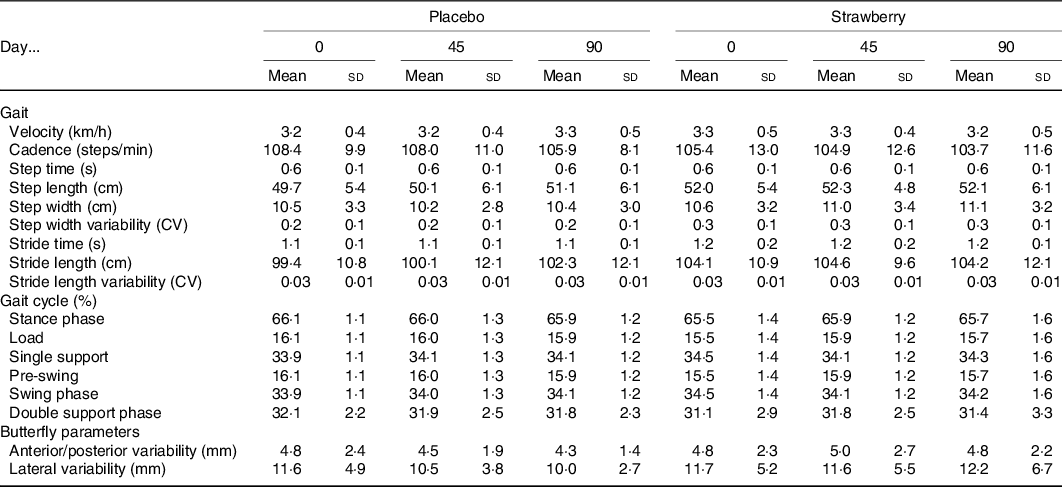
* No significant group differences were observed during 2 min of treadmill walking at preferred walking speed. Step times and lengths represented as average between left and right feet. Variability represented by coefficient of variation (CV).
Discussion
This study found that 90 d of dietary intervention with SB resulted in (1) improved word recognition and (2) improved spatial learning and memory in a virtual navigation task among healthy older adults. Interestingly, as seen in our previous rat study, these improvements in memory function were observed in hippocampally mediated tasks(Reference Shukitt-Hale, Carey and Jenkins9), for example, retained place information, measured by spending more time in the platform quadrant on the probe trial in the MWM. However, these results were in contrast to those seen in our recent clinical trial where BB was seen to improve measures of executive function, not memory(Reference Miller, Hamilton and Joseph13). Interestingly, in our rat study(Reference Shukitt-Hale, Carey and Jenkins9), BB improved measures of reversal learning and mental flexibility, similar to executive functions in humans. As hypothesised then, and somewhat confirmed in our clinical trials, it is possible that the polyphenolic compounds in BB are exerting their effects mainly in the striatum/frontal cortex, while those in SB are primarily affecting the hippocampus. Moreover, one clinical study using a mixed berry intervention, which included SB, found improvement on measures of executive function(Reference Whyte, Cheng and Butler37), while another found improvements in working memory(Reference Nilsson, Salo and Plaza38), suggesting that a mixture of berries could improve a variety of cognitive domains. More testing needs to be done to confirm that different berries are having their effects in different parts of the brain, including measuring the polyphenolic compounds in different brain regions following supplementation with different berry fruits, because it may be that different polyphenolics localise or concentrate in different brain regions.
Improvements in motor control or other cognitive domains were not observed in the present study. It is likely that the relatively short duration of the intervention and the exceedingly healthy and physically fit older adults recruited into the study precluded the detection of any improvements in mobility, particularly on the simple measures of gait and balance. Likewise, in another study, 6 weeks of dietary BB (two cups per d) in older adults (61–81 years) only resulted in improved performance on a complex dual performance task of walking within a narrow path while performing a concurrent cognitive task, and not simple measures of grip strength and gait(Reference Schrager, Hilton and Gould16). Similarly, the beneficial effects of dietary SB were only discernible on the most challenging cognitive tests. These results confirm those seen in our BB clinical trial(Reference Miller, Hamilton and Joseph13) and in other trials studying the effects of wild BB intervention in children(Reference Whyte, Schafer and Williams39) and middle-aged adults(Reference Whyte, Rahman and Bell18) where the benefits of BB treatment were most evident on more cognitively demanding high-load or incongruent trials. Similarly, in a recent review of the effects of BB on cognitive performance, the authors observed greater effects on tests that were more cognitively demanding, noting that the level of task demand should be carefully considered when designing intervention trials(Reference Hein, Whyte and Wood15).
In the present study, participants completed the California Verbal Learning Test at two timepoints: at baseline and again after 90 d of dietary intervention with SB or placebo. At baseline, participants randomised to the SB group correctly recognised fewer words than did those who were randomised to the placebo group. However, following the intervention, the participants who consumed SB showed improved word recognition, while those who consumed the placebo showed no change in performance. Importantly, this improvement in word recognition was not due to a change in the rate of false-positive responses, that is, the improvement in detection is not because they are simply saying that they recognise list and non-list words indiscriminately. Recognition memory, knowing that a stimulus has previously been experienced, is known to decline with age(Reference Wang, Kruggel and Rugg40), albeit usually not to the same extent that free and cued recall are impacted by age. Improvements in recognition memory have previously been observed in animal models of ageing following feeding with berry fruit(Reference Goyarzu, Malin and Lau41,Reference Malin, Lee and Goyarzu42) , and SB has been shown to improve recognition memory in a rodent model of accelerated ageing(Reference Rabin, Carrihill-Knoll and Hinchman43). Discrimination between new and previously experienced stimuli is thought to be dependent on medial temporal lobe function, specifically the perirhinal cortex(Reference Eichenbaum, Yonelinas and Ranganath44). Similar to the results seen in the present study, a polyphenol-rich extract from grape and BB improved verbal recognition and memory-free recall in healthy adults aged 60–70 years(Reference Bensalem, Dudonne and Etchamendy45). Supplementation with the polyphenol-rich extract from grape and BB also improved visuospatial episodic memory and delayed recall word recognition, but only in a subset of participants, that is, healthy older subjects presenting the highest memory decline at baseline(Reference Bensalem, Dudonne and Etchamendy45). This finding confirmed results from a recent animal study in our laboratory that showed that consuming BB-supplemented diets led to enhanced spatial working memory performance in animals that were poor performers at baseline, as well as preserved cognition in good performers(Reference Shukitt-Hale, Thangthaeng and Miller46).
The participants also completed vMWM at baseline and again after 45 and 90 d of dietary intervention. Latency to locate a hidden platform during vMWM acquisition trials decreased both within and between study visits, indicating that the participants were able to learn the platform’s location and show improvement with additional trials. Overall, acquisition latencies in the present study were shorter than in our previous study (i.e. participants found the platform sooner during acquisition trials(Reference Miller, Hamilton and Joseph20)), perhaps due to the extended task instruction during study visit 1, relative to the instruction given in the previous study. An analysis of swim paths during probe trials showed that, after 45 d, participants who consumed SB spent more time searching in the correct platform quadrant, showing improved place learning, although the effect was not observed at the 90-d timepoint. Importantly, a similar pattern was not observed during visible platform trials, suggesting that the observed improvement was not due to confounding factors such as a change in vision or motivation. Previous studies have shown that spatial learning and memory in virtual and real-world environments are impaired during ageing(Reference Miller, Hamilton and Joseph20,Reference Goyarzu, Malin and Lau41) . This type of spatial learning and memory is associated with medial temporal lobe function, specifically the hippocampus and parahippocampal cortex(Reference Ploner, Gaymard and Rivaud-Pechoux47), as well as the striatum(Reference Devan, Goad and Petri48).
Ageing is associated with decreases in postural stability and gait speed and increases in gait variability, and these changes are related to the risk of falls, dementia and death. Because dietary SB was able to improve balance and mobility in aged rats(Reference Joseph, Shukitt-Hale and Denisova7,Reference Shukitt-Hale, Bielinski and Lau8) , we hypothesised that dietary intervention with SB might also improve gait and balance parameters among older adults. However, the present study did not detect an effect of SB on postural or gait stability or on gait speed. Because the potential participants who had sustained a fall within the past year and those unwilling/unable to walk unassisted (i.e. not holding on to the sides) on a treadmill were excluded from the study to reduce the risk of an injurious fall during testing, the participants who were recruited into the study may not have had sufficient age-related postural instability to detect a positive effect of the SB intervention. Similarly, the participants in this study preferred gait speeds that were faster than gait speed norms for their age group(Reference Öberg, Karsznia and Öberg49). Therefore, the failure to detect an effect of SB on motor control is unsurprising given the high level of overall health among study participants.
Possible mechanisms behind the protective effects of SB supplementation may be posited from other studies using blood from participants in this study. It is possible that SB’s beneficial effects on cognitive performance may be due to a decrease in neuroinflammation, as serum collected from these SB-supplemented older adults reduced LPS-induced inflammatory signals in stressed HAPI (highly aggressively proliferating immortalised) microglia in vitro (Reference Rutledge, Fisher and Miller24). Additionally, a second study found that several polyphenolic compounds, including anthocyanins, and their conjugated metabolites were detected in the plasma samples of these older adults consuming SB powder(Reference Sandhu, Miller and Thangthaeng50). These polyphenols from SB were absorbed, extensively metabolised and remained persistent in circulation, but whether this retention is part of delivering the beneficial effects of SB requires further investigation(Reference Sandhu, Miller and Thangthaeng50).
Given the promising neuroprotective effects of SB in cell and animal models of ageing, it is surprising that more robust effects were not detected. Limitations in this study include the healthiness of the sample, extended practice with the cognitive tests, the small sample size and the duration of the intervention. We did not reach our target of 80 % power, which was based on twenty subjects per intervention. Two months were sufficient to see improved cognitive performance and reductions in oxidative and inflammatory biomarkers in the brains of aged rats(Reference Joseph, Shukitt-Hale and Denisova7,Reference Shukitt-Hale, Bielinski and Lau8) . When translating these findings to humans, it is possible that a similar amount of time may be sufficient to achieve similar results at the cellular level. However, 2 months represents a considerably larger percentage of total lifespan for rats than it does for humans. Therefore, it is possible that a longer intervention, perhaps ≥6 months, may be required for evaluating efficacy. As an example, a recent randomised, placebo-controlled study of the memory effects of 8 oz pomegranate juice daily in middle-aged and older adults found that visual memory performance, specifically the ability to learn visual information over repeated learning trials, was only improved following 12 months, and not 6 months, of supplementation(Reference Siddarth, Li and Miller51).
In conclusion, these findings suggest that the inclusion of SB in the diet may aid in preserving some aspects of hippocampal cognitive function during normal ageing. Given the current lack of therapeutics for age-related cognitive decline, a cost–benefit analysis suggests that adding foods such as SB that are safe to consume has an overall positive net value. Additionally, a recent article showed that diet could also be an important contributor to reduce the risk of Alzheimer’s disease and related dementias(Reference Shishtar, Rogers and Blumberg52). Specifically, diets higher in flavonols, anthocyanins and flavonoid polymers were associated with a lower risk of developing Alzheimer’s disease and related dementias(Reference Shishtar, Rogers and Blumberg52), showing that a higher long-term flavonoid intake from foods such as berry fruits could reduce the risk of Alzheimer’s disease and related dementias(Reference Devore, Kang and Breteler10,Reference Agarwal, Holland and Wang11,Reference Shishtar, Rogers and Blumberg52) . Because berry fruits contain polyphenolic compounds that have anti-inflammatory and antioxidant properties, dietary interventions with SB might present a possible strategy for combatting some of the deleterious effects of chronic oxidative stress and inflammation, which have been linked with both ‘normal’ and pathological brain ageing.
Acknowledgements
The authors would like to acknowledge the contributions of the late James A. Joseph, PhD, who initiated work on this project, and Derek Hamilton, PhD, who provided the vMWM.
The study was funded by the U.S. Department of Agriculture and California Strawberry Commission.
All authors read and approved the final manuscript.
The authors declare that they have no conflicts of interest.
Ethics approval
This study was approved by the Tufts Health Sciences Institutional Review Board and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All study participants gave informed consent prior to participation in the study.
Consent for publication
All authors agreed with the content and gave explicit consent to submit the manuscript for publication. Consent to publish was obtained from responsible authorities of ARS-USDA and Tufts University.
Availability of data and material
Available upon request.











