Ca is an essential nutrient and the most abundant mineral in the human body. It has a structural role in skeleton, and the majority of Ca (approximately 99 %) resides in bones and teeth, which is essential for the maintenance of bone health. Less than 1 % is found in soft tissues and body fluids, which is required for many biological functions including vascular tone, nerve transmission and muscle contraction( Reference Wang, Manson and Sesso 1 – 3 ). Serum ionised Ca is tightly maintained within a physiological range in order to avoid toxicity( Reference Peacock 4 ). Ca homoeostasis is controlled by calciotropic hormones including the parathyroid hormone (PTH) and calcitriol (1,25 dihydroxyvitamin D)( Reference Wang, Manson and Sesso 1 , Reference Theobald 2 ).
The Institute of Medicine (US)’s current dietary Ca recommendations for individuals aged 19 years or older range from 1000 to 1200 mg/d( 5 ). Milk and other dairy products are the main sources of dietary Ca. A significant proportion of people throughout the world fails to achieve this recommendation( Reference Shin and Kim 6 ), and Ca supplements are an alternative to meet daily Ca intake( 3 , Reference Peacock 4 ). Supplements of Ca are widely used in many countries( Reference Reid and Bolland 7 ). A study conducted in the USA observed that 43 % of the individuals used supplemental Ca( Reference Bailey, Dodd and Goldman 8 ).
Despite some epidemiological studies reporting an inverse association between dietary Ca and/or dairy intake and cardiovascular events( Reference Larsson, Orsini and Wolk 9 – Reference Soedamah-Muthu, Ding and Al-Delaimy 11 ), recent evidence suggests that supplemental Ca may be associated with an increased risk of cardiovascular events, especially acute myocardial infarction( Reference Bolland, Barber and Doughty 12 – Reference Mao, Zhang and Tang 16 ).
The proposed mechanism for the possible increase in cardiovascular events with Ca supplements includes the acute rise in serum Ca that follows its intake, which is not observed after dietary Ca consumption (dairy intake has minimal effects on calcaemia). This transient increase in serum Ca may favour vascular calcification and atherosclerotic plaque thickness( Reference Reid and Bolland 7 ). However, in some studies, Ca supplements were not associated with increased vascular calcification( Reference Samelson, Booth and Fox 17 ) nor with carotid artery intima-media thickness( Reference Lewis, Zhu and Thompson 18 ). Thus, acute rise in serum Ca may contribute to cardiovascular events through other mechanisms such as changes in blood pressure (BP)( Reference Bristow, Gamble and Stewart 19 , Reference Reid, Gamble and Bolland 20 ), but at present there is no consensus because of a lack of studies evaluating this issue.
As Ca supplements are most often taken with meals, their potential deleterious effects could probably occur during the postprandial period. High-fat meals are frequently consumed, and there is evidence that this kind of meal can impair endothelial function( Reference Ramírez-Vélez 21 – Reference Marinos, Celedonio and Ramirez 23 ) and may also raise BP( Reference Lithander, Herlihy and Walsh 24 , Reference Esser, Oosterink and op’t Roodt 25 ). Thus, if dietary and/or supplemental Ca have beneficial and/or deleterious acute effects on vascular function and BP, these could mitigate or aggravate the deleterious effects of a high-fat meal.
To the best of our knowledge, no previous study has evaluated the acute effects of supplemental v. dietary Ca on BP and vascular function during the postprandial period. Therefore, the purpose of this study was to evaluate the effects of supplemental or dietary Ca on BP and microvascular function during the postprandial period in obese women challenged with a high-fat meal.
Methods
This cross-over, randomised-controlled trial was performed at the Laboratory of Clinical and Experimental Pathophysiology (CLINEX), located at Pedro Ernesto University Hospital, Rio de Janeiro State University. It was conducted in accordance with the Declaration of Helsinki. All procedures involving human subjects/patients were approved by the committee on ethics and research of the Pedro Ernesto University Hospital (339.112-CEP/HUPE – CAAE: 19393213.7.0000.5259), and all participants provided their written informed consent. The present trial was registered at www.clinicaltrials.gov (ID no. NCT02137434).
Subjects
Inclusion criteria were as follows: female sex, BMI≥30 and <40 kg/m2, aged between 20 and 50 years, low habitual intake of dairy products (≤1 portion/d) and premenopausal status. Pregnant or lactating women were not allowed to participate. Exclusion criteria were as follows: smoking; current use of dietary supplements (including supplements of Ca and vitamin D) or of medication known to interfere with Ca metabolism, body weight, metabolic profile or BP; clinical history of thyroid dysfunction; and diagnoses of diabetes mellitus, hypertension, dyslipidaemia (with drug treatment) and/or chronic diseases severely affecting the cardiovascular, gastrointestinal and renal systems. Those who reported recent (within previous 3 months) changes in dietary intake, body weight (≥3 kg) and intensity or frequency of physical exercise were also excluded.
Participants who engaged in physical activities, including light ones, such as walking, for at least 40 min three times a week were considered physically active.
Study design
Potential participants were recruited from the waiting rooms of the Department of Plastic Surgery among candidates for lipoplasty and of the Department of Gynaecology among participants in the Family Planning Program of Pedro Ernesto University Hospital. Those who met initial eligibility criteria (preliminary evaluation) and agreed to take part in the study were scheduled for a screening visit in order to evaluate other eligibility criteria. At this visit, they arrived at the Laboratory after a 12-h fasting period and abstinence from alcohol for 3 d. While fasting, they were subjected to clinical, nutritional and laboratory evaluations. The following week, participants returned to the laboratory, and those eligible for this study were randomly assigned to one of three possible sequences of the three test meals with a washout period of 1 week between meals.
During the 3 d before each test meal, participants were instructed to (1) eat no more than 1 portion of dairy products/d and 2 portions of fruits; (2) to avoid food items that are rich in fat, alcohol, green tea and dark chocolate; and (3) to avoid hard physical activities. On the test day, participants were scheduled to arrive at the Laboratory between 08.00 and 10.00 hours after a 12-h fasting period. While fasting, they were subjected to baseline evaluation of anthropometric parameters, cutaneous microvascular reactivity, BP (continuously during 15 min) and Ca metabolism variables. In sequence, participants were instructed to eat the test meal within 15 min. After test meal intake, BP was evaluated continuously for 120 min. At minute 120, blood samples were collected and skin microvascular reactivity was assessed again.
Test meals
The three test meals provided similar energy, macronutrient and Na contents (Table 1). However, the amount and source of Ca differed among the test meals: high dietary Ca meal (HDCM; 547 mg of dietary Ca), high supplemental Ca meal (HSCM; 500 mg of supplemental Ca and 42 mg of dietary Ca) and low-Ca meal (LCM; 42 mg of dietary Ca) or control meal. All three meals consisted of cracker (26 g) with butter (10 g) and a beverage. In the HDCM, this beverage consisted of a blend of skimmed powdered milk (39 g), milk cream (50 g), sugar (16 g), butter (15 g), maltodextrin (20 g), vanilla essence (5 ml) and water (40 ml). In HSCM, the beverage was a mix of powdered calcium carbonate (1250 mg), vanilla flavoured powdered albumin (15 g), milk cream (50 g), sugar (36 g), butter (15 g), maltodextrin (20 g) and water (40 ml). Finally, the beverage in the LCM was the same as in the HSCM but without calcium carbonate.
Table 1 Nutrient composition of the test meals
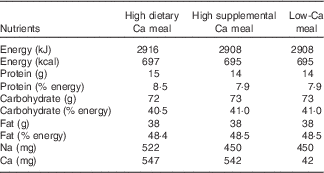
Meal composition was analysed for proximate composition (moisture, protein, fat and ash) by the standard procedures of the Association of Analysis Chemists( 26 ). Total carbohydrates were calculated by difference. Energy content was estimated using the usual conversion factors: 17 kJ/g (4 kcal/g) for protein and carbohydrates and 38 kJ/g (9 kcal/g) for lipids. The amount of Ca was analysed by complexometric titration with EDTA, and Na content was analysed by atomic emission flame spectrophotometry. These analyses utilised standards and were conducted at Nutritional Biochemistry Laboratory and at Bromatology Laboratory of Federal University of the State of Rio de Janeiro.
Test meals were formulated to have low levels of antioxidants that are known to improve vascular function and BP such as polyphenols( Reference Lekakis, Rallidis and Andreadou 27 – Reference Alexopoulos, Vlachopoulos and Aznaouridis 29 ).
Nutritional assessment
To assess usual dietary Ca intake over the last 6 months, a semi-quantitative FFQ containing eighty items and usual portions was used. It was developed for the Brazilian population on the basis of commonly consumed foods and was validated against more accurate methods of dietary intake assessment( Reference Sichieri and Everhart 30 ). Software SAS® and Brazilian Table of Food Consumption( 31 ) were used to process data from the FFQ. Alcohol intake was considered when the reported frequency was one or more times per week.
Height was measured using a stadiometer accurate to ±0·5 cm, and weight was obtained using a calibrated scale accurate to ±0·1 kg (Filizola S.A.), with participants wearing light clothing and no shoes, after they attempted to empty their bladder. BMI was calculated using the standard equation (kg/m2)( 32 ). Waist circumference (WC) was measured in the standing position, midway between the lower margin of the last rib and the iliac crest, at mid exhalation. Hip circumference was measured at the widest point of the hip/buttocks area with the measuring tape parallel to the floor. Waist:hip ratio was determined by dividing WC (cm) by hip circumference (cm). Waist:height ratio was obtained by dividing WC (cm) by height (cm). Neck circumference (NC) was measured in the standing position with the participants’ head positioned in the Frankfurt horizontal level following the description of Zhou et al.( Reference Zhou, Ge and Zhu 33 ). Anthropometric measurements were taken twice and mean values were used.
At the screening visit, percentage body fat was estimated by electrical bioimpedance using a Biodynamics BIA-450 body fat analyser (Biodynamics Corp.).
Laboratory parameters
At the screening visit, blood samples were collected to evaluate serum levels of glucose, insulin, total cholesterol and fractions, TAG and 25 hydroxyvitamin D (25(OH)D). On test days, serum samples were stored to evaluate Ca, total protein, albumin and PTH.
Glucose was determined by the glucose hexokinase method using the equipment Cobas Integra 400 Plus (Roche Diagnostics) with reagents provided by the manufacturer. Insulin levels were determined by the RIA method using the commercially available human insulin-specific kit (EMD Millipore Corporation). Insulin resistance status was assessed using the homoeostasis model assessment for insulin resistance index( Reference Matthews, Hosker and Rudenski 34 ). Total cholesterol, HDL-cholesterol and TAG concentrations were assessed by enzymatic colorimetry with the equipment Cobas Integra 400 Plus (Roche Diagnostics) with reagents provided by the manufacturer. When TAG values were <4.5 mmol/l, LDL-cholesterol was calculated using Friedewald’s formula( Reference Friedewald, Lévy and Fredckson 35 ). Levels of 25(OH)D were determined by electro-chemiluminescence immunoassay (ECLIA) using a commercial kit (Roche Diagnostics). Vitamin D deficiency was defined as serum 25(OH)D<20 ng/ml( Reference Holick, Binkley and Bischoff-Ferrari 36 ).
Serum Ca was determined by complexometry with the equipment Cobas Integra 400 Plus (Roche Diagnostics) with reagents provided by the manufacturer. Total protein and albumin were assessed by colorimetric techniques with the same equipment used for determining serum Ca. Ionised Ca was determined using the following formula: (6×serum Ca (mg/dl)−(albumin (g/dl)+(0·19×total protein (g/dl))/3)/(albumin (g/dl)+(0·19×total protein (g/dl))+6). Plasma PTH (intact molecule) was determined by ECLIA using commercially available kits (Roche Diagnostics).
All laboratory analyses were performed at the University Hospital Central Laboratory and at the Lipids Laboratory of Rio de Janeiro State University. These laboratories have control procedures and quality assurance certification according to national and international standards.
Cutaneous microvascular reactivity
Cutaneous microvascular reactivity was assessed after a 20-min resting period, with subjects lying down in the supine position in a room with temperature control (23±1°C) using the laser speckle contrast imaging technique. This technique provides a non-invasive evaluation of a wide area of tissue in real time with very good spatial resolution and excellent reproducibility( Reference Roustit, Millet and Blaise 37 , Reference Hellmann, Roustit and Cracowski 38 ). The equipment used was PeriCam PSI-NR analyser with a 780-nm wavelength (Perimed AB). Variations in the microvascular flow (endothelium-dependent and independent) were analysed using postocclusive reactive hyperaemia (PORH). A cuff on the non-dominant arm of the participants was inflated to 50 mmHg above systolic BP for 3 min to occlude arterial flow and then induce PORH after it was deflated. Cutaneous blood flow on the forearm was evaluated before arterial occlusion (baseline period) and after fast cuff deflation. Images were analysed by PIMSoft software (Perimed AB), and the measurements of cutaneous microvascular flow were expressed in arbitrary perfusion units (APU). Values of cutaneous vascular conductance (CVC) were obtained by dividing APU by medium BP, expressed in AUP/mmHg. To quantify skin microvascular reactivity, the following parameters were used: (1) hyperaemia peak, which is the maximum value of CVC in the postocclusion period; (2) amplitude of the PORH response, obtained by the difference between peak CVC and baseline CVC; and (3) AUC in the postocclusion period.
Blood pressure
BP and heart rate were continuously measured (beat-by-beat), in the lying state after a 20-min rest period, with a photoplethysmography finger cuff placed on the left middle finger using the equipment Finometer Pro (Finapres Medical System)( Reference Langewouters, Settels and Roelandt 39 ). A return-to-flow calibration was performed to provide adjustment of the finger arterial pressure with the brachial artery pressure( Reference Bos, van Goudoever and van Montfrans 40 ). During the evaluation, a digital cuff was automatically calibrated every seventy pulses to guarantee the physiological conditions of the digital artery.
At baseline, before meals intake, records of BP were obtained for 15 min. Data from the first 10 min were discarded, and the mean of the values recorded in the final 5 min was considered as the baseline BP. In order to eat test meals, participants were seated in a semi-recumbent position. After completion of the meals, they returned to a lying position and values of BP were obtained for 120 min. In the statistical analyses, during the postprandial period, we included the mean values of each interval of 10 min.
Statistical methods
Categorical variables are expressed as percentages and compared by the χ 2 test. Means with their standard errors are used to summarise continuous variables. Normality was tested by the Shapiro–Wilk normality test, and skewed data were log transformed to improve normality.
Statistical analyses included the following: (i) baseline comparisons between the 3 test days using ANOVA, (ii) baseline comparisons with the response at 120 min (Ca metabolism and microvascular reactivity) or over 120 min (BP) after each test meal (time effects) using repeated-measures ANOVA and (iii) comparison of treatments (test meals) for modifications from baseline to 120 min (treatment effects or meal×time interaction) using repeated-measures ANOVA.
To test for carry- over effect, the order of treatment was included as a covariate in the model, and no carry-over effect was observed for any of the variables. All statistical analyses were performed using STATA version 12 (STATA Corp.) software. A P value<0·05 was considered statistically significant.
It was not possible to determine the desired sample size to achieve a significant change in BP and/or skin microvascular reactivity, because of the non-existence of a previous clinical trial with a similar intervention.
Results
A total of ninety-five women were interviewed, of which forty-two agreed to participate in the study. Among them, twenty met the eligibility criteria and were scheduled for the screening visit. The reasons for exclusion were BMI<30 or ≥40 kg/m2 (n 9), hypertension (n 4), smoking (n 3), use of medications (n 3), menopause (n 2) and hypothyroidism (n 1). After the screening visit, three more women were not included in the study because of lack of interest. In all, seventeen participants were randomised and sixteen completed the study (Fig. 1). The non-completer left the study because of changes in work schedule.

Fig. 1 Flow diagram of the study. HDCM, high dietary calcium meal; HSCM, high supplemental calcium meal; LCM, low-calcium meal.
The characteristics of the participants, their habitual intake of Ca and serum levels of 25(OH)D are presented in Table 2. None of the participants presented with vitamin D deficiency. Anthropometric parameters were similar on each day of test meal. Similarly, values at baseline of BP, serum Ca, PTH and variables related to cutaneous microvascular reactivity were not significantly different on the 3 test days (data not shown).
Table 2 Characteristics of the participants (Mean values with their standard errors; number of subjects and percentages)
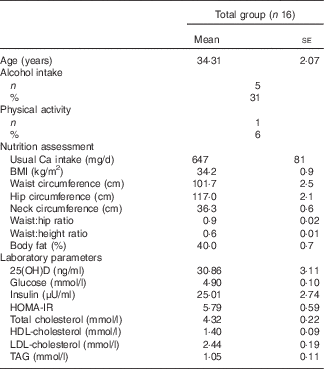
25(OH)D, 25 hydroxyvitamin D; HOMA-IR, homoeostasis model assessment for insulin resistance.
Postprandial responses in serum Ca (total and ionised) and PTH are shown in Fig. 2. Repeated-measures ANOVA revealed that postprandial changes in Ca and PTH had no significant treatment effects. However, total and ionised Ca increased significantly only after HSCM, whereas PTH presented a significant decrease after the three test meals.

Fig. 2 Mean values of (a) total calcium, (b) ionised calcium and (c) parathyroid hormone at baseline and 120 min after the consumption of low-calcium meal (![]() , LCM), high dietary calcium meal (
, LCM), high dietary calcium meal (![]() , HDCM) and high supplemental calcium meal (
, HDCM) and high supplemental calcium meal (![]() , HSCM). * P=0·01 for modification during the postprandial period in each test meal (repeated-measures ANOVA). ** P<0·0001 for modification during the postprandial period in each test meal (repeated-measures ANOVA). † P values refer to treatment effects (repeated-measures ANOVA).
, HSCM). * P=0·01 for modification during the postprandial period in each test meal (repeated-measures ANOVA). ** P<0·0001 for modification during the postprandial period in each test meal (repeated-measures ANOVA). † P values refer to treatment effects (repeated-measures ANOVA).
Comparisons of the modifications in BP from baseline to 120 min after each test meal intake are presented in Fig. 3. Comparative analysis of the three test meals on BP (systolic, diastolic and mean) revealed no significant treatment effects. After LCM and HDCM, there was a significant increase in systolic, diastolic and mean BP. However, after the intake of HSCM, only systolic and mean BP increased significantly.

Fig. 3 Mean values of (a) systolic blood pressure, (b) diastolic blood pressure, (c) mean blood pressure and (d) heart rate at baseline and at 120 min after the consumption of a low-calcium meal (![]() , LCM), high dietary calcium meal (
, LCM), high dietary calcium meal (![]() , HDCM) and high supplemental calcium meal (
, HDCM) and high supplemental calcium meal (![]() , HSCM). BP, blood pressure. * P<0·05 for modification during the postprandial period in each test meal. For systolic BP, mean BP and heart rate (HDCM, HSCM and LCM) and for diastolic BP (HDCM and LCM) (repeated-measures ANOVA). † P values refer to treatment effects (repeated-measures ANOVA).
, HSCM). BP, blood pressure. * P<0·05 for modification during the postprandial period in each test meal. For systolic BP, mean BP and heart rate (HDCM, HSCM and LCM) and for diastolic BP (HDCM and LCM) (repeated-measures ANOVA). † P values refer to treatment effects (repeated-measures ANOVA).
Table 3 contains the comparisons of mean values of cutaneous microvascular reactivity. There was no significant difference between the three test meals regarding microvascular reactivity (peak value of CVC, amplitude of CVC and AUC). However, after LCM intake, there was a significant decrease in these three variables, and after HDCM there was a significant reduction in the peak value of CVC and in the amplitude of CVC. Intake of HSCM resulted in the decrease of only one of these variables (peak CVC).
Table 3 Microvascular parameters at baseline and 120 min after consumption of a low-calcium meal (LCM), high dietary calcium meal (HDCM) and high supplemental calcium meal (HSCM) (Mean values with their standard errors)

Δ=minute 120−baseline; CVC, cutaneous vascular conductance; APU, arbitrary perfusion units; amplitude CVC=peak CVC−basal CVC.
* P values refer to time effect in each test meal (repeated-measures ANOVA).
† P values refer to treatment effects (repeated-measures ANOVA).
Discussion
In the present cross-over clinical trial, based on a sample of obese women, the main finding was that supplemental and dietary Ca do not influence the effects of a high-fat meal on BP and microvascular function.
We observed a significant increase in systolic BP after the three test meals and in diastolic BP after LCM and HDCM. There is evidence that a high-fat meal may be associated with an increment in BP, which may be due to the hypertriacylglycerolaemia and elevation in free fat acids with consequent increase in oxidative stress leading to endothelial dysfunction and to an increase in BP( Reference Lithander, Herlihy and Walsh 24 ).
To the best of our knowledge, only one randomised trial has evaluated the postprandial effects of Ca supplements on BP( Reference Bristow, Gamble and Stewart 19 ). In this study, 100 postmenopausal women were randomised to 1 g/d of Ca or a placebo. Ca was administered with a glass of water. A light breakfast was provided following the intervention. BP was measured at baseline and every 2 h up to 8 h. A light lunch was provided after 4 h, and an optional snack was provided after 6 h. Water and non-caffeinated tea without milk were allowed ad libitum. In both groups, over 8 h, BP declined, and the reduction in systolic BP was smaller in the Ca group compared with control group by >5 mmHg between 2 and 6 h (P≤0·02); the reduction in diastolic BP was smaller at 2 h (between-group difference 4·5 mmHg, P=0·004).
The results observed in the study conducted by Bristow et al.( Reference Bristow, Gamble and Stewart 19 ) are very different from ours. The first difference is the decrease and not the increase in BP in both study groups. Authors attributed this decline to the normal diurnal variation in BP, which is characterised by a surge in the morning. Another factor that may have also contributed to the reduction in BP is the inclusion of participants under antihypertensive treatment and the age of participants, which varied from 59 to 84 years. Postprandial hypotension in the elderly have already been reported( Reference Lipsitz and Fullerton 41 , Reference Trahair, Horowitz and Jones 42 ). Another factor that may have favoured the reduction in BP was the concomitant intake of fruits and tea, which contain antioxidant substances. The second difference is that they observed a lower reduction in BP in the Ca group and we observed a similar increase in BP in the HSCM, HDCM and LCM groups. This divergence may be attributed to the differences in (1) nutritional composition of the meals and (2) dose of supplemental Ca (500 mg v. 1 g). Thus, one could hypothesise that only in higher doses supplemental Ca may induce detrimental effects on BP probably due to greater increases in serum Ca.
The relation between serum Ca and BP has been evaluated in a few studies. There is evidence that acute hypercalcaemia induced by intravenous infusion of Ca can increase BP( Reference Nilsson, Rastad and Johansson 43 , Reference Kamycheva, Jorde and Haug 44 ) and that higher serum levels of Ca are related with higher values of BP and greater prevalence of hypertension( Reference Fraser, Williams and Lawlor 45 – Reference Walsh, Divitini and Knuiman 48 ). This is possibly mediated by changes in intracellular Ca affecting vascular smooth muscle contraction( Reference Reid, Gamble and Bolland 20 ). Burt et al.( Reference Burt, Mangelsdorf and Srivastava 49 ) observed an acute rise in serum Ca following the intake of Ca supplements (without a meal); however, BP remained unchanged. In the present study, serum levels of Ca remained in the normal range (2·2–2·6 mmol/l)( Reference Peacock 4 ) in all participants after the three test meals. The lack of a significant increase in serum Ca may be attributed to the high-fat content of test meals, which may have slowed Ca absorption. In a study conducted by Bristow et al.( Reference Bristow, Gamble and Stewart 50 ) when a Ca supplement was given with a high-fat meal the acute rise in serum Ca was only detected 4 h after its intake. After an extensive review of the literature, we did not find any study evaluating the acute effects of dietary Ca/dairy products on BP.
We did not find studies evaluating the acute effects of supplemental and/or dietary Ca with a meal on vascular function. However, some studies have evaluated the effects of supplemental Ca or of milk, yogurt or cheese (without a meal). Burt et al.( Reference Burt, Mangelsdorf and Srivastava 49 ) observed no effects on endothelial function 3 h after a single oral dose of 1000 mg calcium citrate. In the cross-over study conducted by Yaron et al.( Reference Yaron, Roach and Izkhakov 51 ), 600 mg of Ca from calcium citrate or from dairy products was not associated with significant changes in arterial stiffness and endothelial function. Recently, Alba et al.( Reference Alba, Stanhewicz and Kennye 52 ) observed low-fat milk did not increase cutaneous nitric oxide-dependent vasodilatation assessed by laser-Doppler flowmetry when compared with a rice beverage and water. On the other hand, Ballard et al.( Reference Ballard, Mah and Guo 53 ) observed that low-fat milk acutely maintains vascular endothelial function by limiting postprandial hyperglycaemia, in comparison with an isoenergic volume of rice milk. The design of the present study is very different from those described above, but our findings are similar to a majority of them.
The strength of this study includes its contribution to elucidate the complex relationship between Ca intake and cardiovascular risk. The method used to evaluate BP is also a strength of the study: the digital photoplethysmography technique provides BP values non-invasively and continuously, beat to beat( Reference Langewouters, Settels and Roelandt 39 ), and has been validated in previous studies( Reference Schutte, Huisman and van Rooyen 54 ). A major advantage of this apparatus is the sensitivity to detect small changes in cardiovascular function, including BP, resulting from dietary interventions( Reference Schutte, Huisman and Van Rooyen 55 , Reference Cao and Pilowsky 56 ). There are several limitations to the present study, including a small sample size. The length of BP evaluation is probably another limitation, but other studies using the same method for BP measurement also evaluated it during 2 h( Reference Cao and Pilowsky 56 , Reference McMuller, Whitehouse and Shine 57 ). The need to keep volunteers at rest limited the evaluation of BP during a longer period of time. Finally, attention should be paid to the fact that this study was performed in young obese women following a high-fat meal. Therefore, our findings might not apply to other age groups, BMI or after the consumption of different meals, as age, BMI and meal composition can potentially influence BP and vascular function.
Conclusion
The findings of this study suggest that in obese women neither supplemental nor dietary Ca consumed with a high-fat meal has effects on BP or microvascular function.
Acknowledgements
The authors express their sincere gratitude to Maria de Lourdes Guimarães Rodrigues, Débora Cristina Torres Valença, Vagner Ismerin Lobão, José Firmino Nogueira Neto, Alex Itaborahy, Sergio Emanuel Kaiser, Maria Inês Barreto Silva, Orlando Marino Gadas de Moraes, Anderson Junger Teodoro, Diana Barbosa Cunha, Deborah Barbosa Vahia de Abreu, Bernardo Barreto da Silva Gaspar and Vittor Stern Pereira de Melo.
The present study was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro.
T. d. S. F., P. M. L., V. P. A., M. R. S. T. K.: contributed to the study conception and design, data collection, assembly, analysis and interpretation, manuscript drafting and the approval of the final version of the manuscript. A. F. S.: contributed to the study conception and design.
The authors declare that there are no conflicts of interest.









