Metabolic syndrome (MS) comprises of a cluster of risk factors for CVD that includes central obesity, dyslipidaemia, hyperglycaemia and hypertension(Reference Raven1, Reference Alberti, Zimmet and Shaw2). The prevalence of MS is increasing and it now affects 27 % of the US population(Reference Ford, Giles and Dietz3). This epidemic correlates with pronounced changes in the environment, behaviours and lifestyles, and is considered one of the main threats to human health worldwide(Reference Eckel, Scott and Zimmet4). Underdeveloped countries are also facing high levels of MS; 26 % of the adult Mexican population suffers from this syndrome(Reference Aguilar-Salinas, Rojas and Gómez-Pérez5). Mexico is also facing epidemic levels of CVD and type 2 diabetes mellitus (T2DM), illnesses associated with MS. Ford and colleagues have estimated that the population-attributable fraction associated with MS is ∼6–7 % for all-cause mortality, 12–17 % for CVD and 30–52 % for T2DM(Reference Ford6).
Several public health studies have linked sweetened beverage intake and negative health outcomes(Reference Vartanian, Schartz and Brownell7), including serious metabolic disorders such as obesity(Reference Schulze, Manson and Ludwig8–Reference Denova-Gutiérrez, Jiménez-Aguilar and Halley-Castillo11), T2DM(Reference Schulze, Manson and Ludwig8, Reference Johnson and Segal12), CVD(Reference Johnson and Segal12) and hypertension(Reference Yoo, Nicklas and Baranowski13). Since Mexico has the third highest rate of sweetened beverage consumption in the world, and 20 % of total energy intake comes from sweetened beverages(Reference Rivera, Muñoz-Hernández and Rosas-Peralta14), this sweetened beverage consumption seems likely to be related to the elevated national prevalence of MS. However, MS and its risk factors are understudied in Mexico. We thus used data from the Health Workers Cohort Study(Reference Denova-Gutiérrez, Jiménez-Aguilar and Halley-Castillo11) to examine the relationship between sweetened beverage consumption and the prevalence of MS components in Mexican adults.
Experimental methods
Study population
We performed a cross-sectional analysis of data from adults participating in the baseline assessment of the Health Workers Cohort Study in the Mexican states of Morelos and Mexico. The present analysis was performed on data from healthy employees and their relatives from three different health and academic institutions: (i) Instituto Mexicano del Seguro Social (IMSS) and (ii) Instituto Nacional de Salud Pública (INSP), both located in Cuernavaca, Morelos State; and workers at (iii) Universidad Autónoma del Estado de México (UAEM) in Toluca, Mexico State. Subjects recruited for the study were participating in the first stage of an ongoing, long-term cohort study focusing on lifestyle and health. The specifics of the study design, methodology and participants’ baseline characteristics have been detailed elsewhere(Reference Salmeron-Castro and Arillo-Santillán15–Reference Tamayo, Lazcano-Ponce and Muñoz17). The ethics committees of all participating institutions approved the study protocol and consent forms. Out of a total population of 13 275 study candidates identified between March and April 2006, 9467 employees were invited to participate in the cohort study and a total of 8307 adults were formally enrolled.
For the present analysis we excluded participants with diagnoses of diabetes, hypertension, dyslipidaemia, gout, rheumatoid arthritis and degenerative arthritis (n 2320), and those who had values of plasma glucose ≥7·08 mmol/l (n 628) because this is a criterion for the diagnosis of diabetes. We also excluded subjects who did not satisfy the a priori criterion of a daily energy intake between 2510·4 and 29 288 kJ, and those providing incomplete information on their sweetened beverage consumption (n 119). The remaining 5240 participants were included in our analysis.
Data collection
Demographic characteristics were evaluated by means of self-administered questionnaires. Participants were asked about their physical activity during leisure time, at work and during housework. Each activity was given a value in metabolic equivalent tasks (METS) and total daily METS were computed(Reference Ainsworth, Haskell and Whitt18).
Participants were also asked about the weight changes they had experienced within the past year and this information was categorized as no weight change, weight loss or weight gain in the past year (less/more than 5 kg).
Anthropometric and clinical assessment
Weight was measured with a previously calibrated electronic scale (model BC-533; Tanita, Tokyo, Japan), with participants wearing minimal clothing. Height was measured using a conventional stadiometer. Waist circumference was measured at the high point of the iliac crest at the end of normal expiration, to the nearest 0·1 cm, with a steel measuring tape. BMI was calculated as the ratio of weight to the square of height (kg/m2) from standardized measurements of weight and height; and the proportion of body fat was estimated via the reference technique of dual-energy X-ray absorptiometry performed with a Lunar DPXL whole-body X-ray densitometer (Lunar Radiation Corp., Madison, WI, USA; software version 1·35, fast scan mode).
Blood pressure was measured with an automatic digital blood pressure monitor. Participants were seated with their right arm resting at heart level. Up to three blood pressure measurements were obtained from each participant. All measurement procedures were performed by nurses trained to use standardized procedures (reproducibility was evaluated, resulting in concordance coefficients between 0·83 and 0·90).
A fasting venous blood sample was collected from each participant; fasting time was ≥8 h to be consistent with previous analyses of data from adults participating in the National Health and Nutrition Examination Survey(Reference Ford and Liu19). Plasma glucose was measured with the oxidized glucose method, TAG with a colorimetric method following enzymatic hydrolysis performed with the lipase technique, and HDL cholesterol (HDL-C) by the clearance method. All biomedical assays were performed with a Selectra XL instrument (Randox Laboratories Ltd, Antrim, UK), in concordance with the procedures of the International Federation of Clinical Chemistry and Laboratory Medicine(Reference Tate, Rifai and Berg20, Reference Halley Castillo, Borges and Talavera21).
Dietary assessment
A semi-quantitative FFQ validated in a Mexican population(Reference Hernández-Avila, Romieu and Parra22) was used to assess diet. This questionnaire included data on frequency of consumption of 116 food items during the previous year. Sweetened beverage consumption was estimated by means of this FFQ. This questionnaire gathered information on the consumption of colas, flavoured sodas, flavoured water with sugar (such as lemon or orange water prepared with artificial flavourings) and diet colas, using a standard serving size of 355 ml. Sweetened beverage intake frequency was divided into four consumption categories: (i) 0 servings/d; (ii) <1 serving/d; (iii) 1–2 servings/d; (iv) >2 servings/d. The energy intake derived from this sweetened beverage intake (kJ/d) was estimated by means of a comprehensive database of food composition(Reference Hernández-Avila, Resoles and Parra23). Total energy, dietary fat and alcohol intake were also estimated with this questionnaire. Outlier values in energy intake were eliminated using the standard deviation method(Reference Rosner24), and all values below 2510·4 kJ/d and above 29 288 kJ/d were excluded from the analysis.
Metabolic syndrome definition
The National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) defines MS as the presence of three or more of the following components: (i) fasting plasma glucose ≥5·6 mmol/l; (ii) serum TAG ≥1·7 mmol/l; (iii) systolic and/or diastolic blood pressure ≥130 and/or ≥85 mmHg, respectively; (iv) waist circumference (central obesity) ≥102 cm (40 in) for men and ≥88 cm (35 in) for women; and (v) HDL-C ≤1·03 mmol/l for men and ≤1·29 mmol/l for women(25). As suggested by Norman, we used a cut-off for plasma glucose level of ≥5.6 mmol/l, lower than that in the guidelines, in order to optimize our ability to assess diabetes risk(Reference Norman26).
Statistical analysis
We performed a descriptive analysis of the main characteristics of interest by sex, testing differences between groups with Fisher’s exact tests, Student t tests or tests for trend as appropriate.
Prevalences and 95 % confidence intervals of MS and its components were computed across sweetened beverage intake categories, and differences between groups that consumed different amounts of sweetened beverages were assessed with tests for linear trend. These tests entail non-parametric tests for trend across ordered groups, which are an extension of the Wilcoxon rank-sum test. A correction for ties is incorporated into the test.
We evaluated the influence of sweetened beverage consumption on the studied components of MS using multivariate regression models, in which these variables were analysed as continuous. In this case the increments of the components of MS were considered for each additional serving (standard portion of 355 ml) of sweetened beverages.
To estimate the magnitude of the association between specific categories of sweetened beverage consumption and MS and its components (central obesity, dyslipidaemia, hyperglycaemia and elevated blood pressure), we computed adjusted odds ratios and 95 % confidence intervals with multiple logistic regression models.
All P values presented are two-tailed; P < 0·05 was considered statistically significant. All statistical analyses were performed using the STATA statistical software package version 9·2 (StataCorp LP, College Station, TX, USA).
Results
MS as defined by the NCEP ATP III criteria was evident in 26·6 % of our population. On average, participants were middle-aged (mean 39·4 (sd 11·5) years), 71·6 % were women and 56·6 % had BMI ≥ 25 kg/m2 (mean 26·1 (sd 4·2) kg/m2). Men consumed more sweetened beverages (1·9 servings/d) than women (1·6 servings/d; P < 0·01). Men also consumed more energy derived from sweetened beverages (1100·4 kJ/d) than women (907·9 kJ/d; P < 0·001). However, after adjusting for body weight no differences between men and women were observed. The prevalence of each component of MS was: raised fasting plasma glucose, 14·4 %; raised TAG, 37·5 %; raised blood pressure, 18·3 %; central obesity (higher-than-recommended waist circumference), 38·0 %; and low HDL-C, 76·6 % (Table 1).
Table 1 Distribution of participants according to demographic variables: selected adults (aged 20 to 70 years; n 5240) from the baseline assessment of the Health Workers Cohort Study, Mexican states of Morelos and Mexico
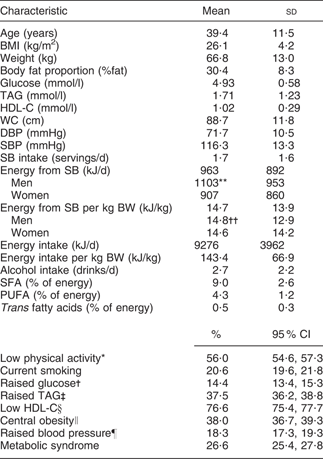
HDL-C, HDL cholesterol; WC, waist circumference; DBP, diastolic blood pressure; SBP, systolic blood pressure; SB, sweetened beverage(s); BW, body weight.
*Subjects with <30 min physical activity/d.
†Fasting plasma glucose ≥ 5·6 mmol/l.
‡Plasma TAG ≥ 1·7 mmol/l.
§Plasma HDL-C ≤ 1·29 mmol/l for women, ≤1·03 mmol/l for men.
∥WC ≥ 88 cm for women, ≥102 cm for men.
¶SBP ≥ 130 mmHg, DBP ≥ 85 mmHg.
Significance of the difference between sexes (Student t test): **P < 0·001, ††P = 0·3.
Subjects with higher sweetened beverage intake tended to be less physically active, to smoke more and to have higher energy intakes. Intakes of total carbohydrates, sucrose and fructose were higher in subjects with greater sweetened beverage consumption (data not shown). In general, the prevalence of MS and its components increased with higher sweetened beverage intake. Participants in the high intake category had higher plasma glucose than subjects who did not consume sweetened beverages (P for trend <0·001). The prevalence of MS was higher among subjects who consumed >2 sweetened beverage servings daily than among non-consumers (P for trend <0·001; Table 2).
Table 2 Characteristics and components of the metabolic syndrome according to sweetened beverage intake in a Mexican population: selected adults (aged 20 to 70 years; n 5240) from the baseline assessment of the Health Workers Cohort Study, Mexican states of Morelos and Mexico
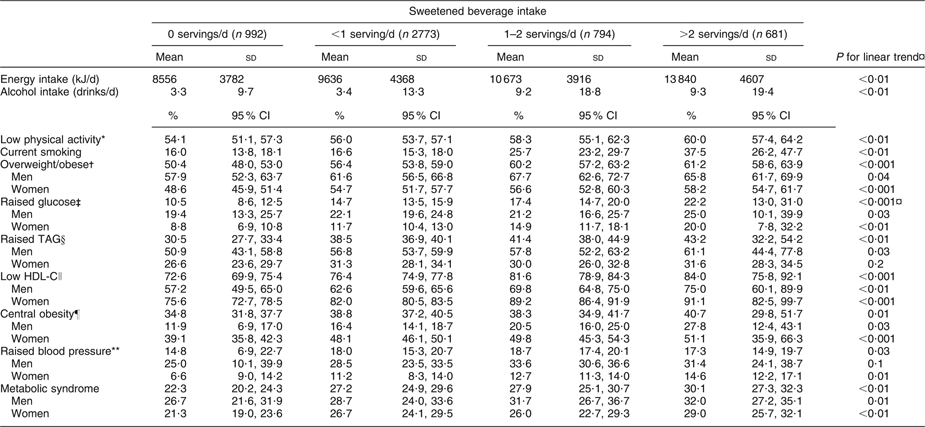
HDL-C, HDL cholesterol.
*Subjects with <30 min physical activity/d.
†BMI ≥ 25·0 kg/m2.
‡Fasting plasma glucose ≥5·6 mmol/l.
§Plasma TAG ≥ 1·7 mmol/l.
∥Plasma HDL-C ≤ 1·29 mmol/l for women, ≤1·03 mmol/l for men.
¶Waist circumference ≥88 cm for women, ≥102 cm for men.
**Systolic blood pressure ≥130 mmHg, diastolic blood pressure ≥85 mmHg.
Table 3 shows the results of a multivariate regression analysis evaluating the effects of sweetened beverage intake on components of MS. After adjusting for demographic characteristics, energy intake and consumption of SFA, PUFA, trans fatty acids and alcohol, we found that for each additional serving of sweetened beverage, subjects’ glucose and TAG levels were increased. Furthermore, we observed that for each additional serving of sweetened beverages, subjects’ HDL-C concentrations decreased. The β coefficients did not change substantially after we controlled for several previously reported risk factors for MS. Age, sex, BMI and weight gain in the past year were positively associated with the five components of MS studied (data not shown).
Table 3 Multivariate regression model for evaluating the effect of sweetened beverages on components of the metabolic syndrome in a Mexican population: selected adults (aged 20 to 70 years; n 5240) from the baseline assessment of the Health Workers Cohort Study, Mexican states of Morelos and Mexico
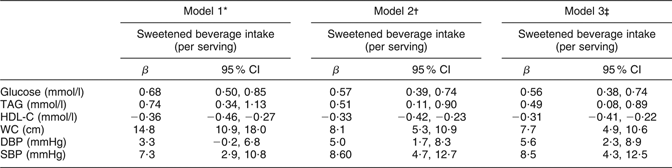
HDL-C, HDL cholesterol; WC, waist circumference; DBP, diastolic blood pressure; SBP, systolic blood pressure.
*Model 1 adjusted for age (continuous) and sex.
†Model 2 adjusted for variables in Model 1 plus BMI (two categories: <25·0 and ≥25·0 kg/m2).
‡Model 3 adjusted for variables in Models 1 and 2 plus weight change within past year (no weight change, weight gain, weight loss), physical activity, total energy intake (kJ/d), alcohol intake (drinks/d), SFA intake (% of energy), PUFA intake (% of energy), trans fatty acid intake (% of energy), cigarette smoking (never, past, current) and place of residence.
In the adjusted model, we found that subjects consuming >2 servings of sweetened beverages daily had a greater risk of low HDL-C compared with those who did not consume sweetened beverages. The subjects consuming >2 sweetened beverage servings daily had a greater risk of central obesity compared with non-consumers. The odds for MS were 2·0 for subjects consuming >2 sweetened beverage servings daily compared with those who did not consume sweetened beverages (Table 4).
Table 4 Odds ratio for components of the metabolic syndrome according to sweetened beverage intake: selected adults (aged 20 to 70 years; n 5240) from the baseline assessment of the Health Workers Cohort Study, Mexican states of Morelos and Mexico
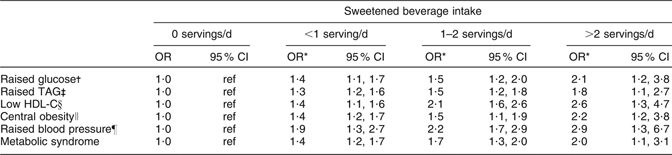
HDL-C, HDL cholesterol; ref, referent category.
*Adjusted for age (continuous), sex, BMI (two categories: <25·0 and ≥25·0 kg/m2), weight change within past year (no weight change, weight gain, weight loss), physical activity, total energy intake (kJ/d), alcohol intake (drinks/d), SFA intake (% of energy), PUFA intake (% of energy), trans fatty acid intake (% of energy), cigarette smoking (never, past, current) and place of residence.
†Fasting plasma glucose ≥5·6 mmol/l.
‡Plasma TAG ≥ 1·7 mmol/l.
§Plasma HDL-C ≤ 1·29 mmol/l for women, ≤1·03 mmol/l for men.
∥Waist circumference ≥88 cm for women, ≥102 cm for men.
¶Systolic blood pressure ≥130 mmHg, diastolic blood pressure ≥85 mmHg.
Discussion
After controlling for known risk factors, we found that sweetened beverage consumption is associated with risk of MS in a Mexican population.
The high prevalence of MS observed in our study population (26·6 %) is similar to that found by other studies using the NCEP ATP III criteria in Mexico (26 %)(Reference Aguilar-Salinas, Rojas and Gómez-Pérez5) and the USA (26·7 %)(Reference Ford, Giles and Dietz3). As expected, we found that MS occurrence was related to increased BMI and proportion of body fat.
The MS component most altered was low HDL-C. Low levels of HDL-C are common in the Mexican population, which has one of the highest rates of low HDL-C worldwide(Reference Aguilar-Salinas, Olaiz and Valles27). In our study the prevalence of low HDL-C was 76·6 %, and it was more prevalent in women (81·6 %) than in men (63·9 %).
We observed significant differences in the amount of sweetened beverage consumption by sex: men consumed about 15 % more sweetened beverages than women (1·9 v. 1·6 servings/d). Our study population, particularly the men, derived a considerable proportion of their energy intake from sweetened beverages (10·5 %). These results are consistent with previous national reports of daily energy derived from sweetened beverage intake in Mexican adult males, who derived ∼10 % of their daily energy intake from sweetened beverages(Reference Rivera, Muñoz-Hernández and Rosas-Peralta14).
Study participants with high sweetened beverage consumption also had relatively high levels of energy intake from other foods, indicating that sweetened beverages may induce hunger and food intake. Nevertheless, this might be the result of a possible report bias and cannot be interpreted as a causal relationship. In addition, experimental data on sweetened beverage consumption and food intake have not provided support for this hypothesis(Reference Almiron-Roig and Drewnowski28, Reference DiMeglio and Mattes29).
Our findings suggest that subjects who consume >2 servings of sweetened beverages daily are at 2·0 times greater risk for MS than those who do not consume sweetened beverages. This finding is in accordance with a previous study to evaluate the relationship between soft drink intake and risk of MS, which reported that individuals consuming ≥1 soft drink/d had a higher prevalence of MS (OR = 1·48; 95 % CI 1·30, 1·69) than those consuming <1 drink/d(Reference Dhingra, Sullivan and Jacques30).
Our analyses showed that each serving of sweetened beverages is associated with an increase in plasma glucose of 0·56 mmol/l (P < 0·001). This means that for subjects who do not consume sweetened beverages, increasing their consumption to a daily serving would elevate their plasma glucose from 5·09 to 5·66 mmol/l, a level that would be classified by our criteria as raised plasma glucose. We found that subjects consuming >2 servings of sweetened beverages daily are at 2·1 times greater risk of raised fasting plasma glucose compared with non-consumers. The ability of sweetened beverage consumption to raise fasting plasma glucose has been attributed the high amount of rapidly absorbable carbohydrates that these beverages provide(Reference Schulze, Manson and Ludwig8, Reference Akgun and Ertel31, Reference Drewnowski and Bellisle32). Sweetened beverages therefore increase consumers’ dietary glycaemic index, and diets with a high glycaemic index have been found to be a risk factor for diabetes in some cohort studies(Reference Schulze, Manson and Ludwig8, Reference Willett, Manson and Liu33–Reference Salmerón, Manson and Stampfer36).
Though a similar biological pathway, sweetened beverages might also increase the risk of raised plasma TAG and low plasma HDL-C. We observed that subjects consuming >2 servings of sweetened beverage daily are at 1·8 times greater risk of raised plasma TAG and 2·6 times greater risk of low plasma HDL-C compared with non-consumers. These results are consistent with those of a previous study, which reported that consumption of ≥1 soft drink/d was associated with hypertriacylglycerolaemia (OR = 1·25; 95 % CI 1·04, 1·51) and low HDL-C (OR = 1·32; 95 % CI 1·06, 1·64)(Reference Dhingra, Sullivan and Jacques30). Our analyses showed that each serving of sweetened beverage is associated with a 0·49 mmol/l increase in plasma TAG concentrations (P < 0·001). This means that for subjects who do not consume sweetened beverages, increasing their consumption by a serving daily would elevate their plasma TAG concentrations from 1·67 to 2·15 mmol/l, classifying them as having raised plasma TAG according to our criteria. Since an experimental study in adults has shown that consumption of fructose-sweetened beverages rapidly increases and peaks plasma TAG concentrations(Reference Teff, Elliott and Tschöp37), the large amounts of free fructose and fructose combined in sucrose derived from sweetened beverages may be a precursor to hepatic lipogenesis, since they provide a relatively unregulated source of carbon(Reference Petersen, Laurent and Yu38, Reference Basciano, Federico and Adeli39).
We also found that increased sweetened beverage consumption is associated with increases in systolic and diastolic blood pressure. Each serving of sweetened beverage was associated with an increase of 8·5 mmHg in systolic blood pressure (P < 0·001) and an increase of 5·6 mmHg in diastolic blood pressure (P < 0·01). Experiments have shown that high-fructose sweetened beverages induce hypertension in animals(Reference Elliot, Keim and Stern40, Reference Sánchez-Lozada, Tapia and Jímenez41) and that adults consuming sucrose-sweetened beverages exhibit an increase in both systolic and diastolic blood pressure(Reference Raben, Vasilaras and Moller42). The mechanism by which fructose-sweetened beverages induce hypertension is not well understood, but it may involve uric acid production, hyperinsulinaemia, aldehyde formation and/or altered vascular reactivity(Reference Johnson and Segal12, Reference Elliot, Keim and Stern40).
The present study’s cross-sectional design made it difficult to examine the potential causal relationship between sweetened beverage intake and MS occurrence, since the temporal relationship of these events could not be established. Further, this cohort contained adults from a specific segment of the Mexican population: working, seemingly healthy individuals. While these adults cannot be considered representative of the Mexican adult population as a whole, they may be considered representative of middle- to low-income adults residing in the urban areas of central Mexico. Even in light of these limitations, our results provide important information about the association between sweetened beverage intake and risk of MS in our population. However, MS is a multifactorial disorder, and diet plays an important role in its development. Dietary intake can be considered in terms of particular dietary patterns. This perspective accounts for the effect of dietary patterns as a whole and thus may provide insight beyond the effects described for single nutrients or foods(Reference Esmaillzadeh, Kimiagar and Mehrabi43, Reference Lutsey, Steffen and Stevens44).
Our findings support the need to develop public health strategies that will discourage sweetened beverage consumption in the Mexican population. All sectors of society, including private and governmental institutions, the health-care system and especially nutrition professionals, have important roles to play in reducing the population’s sweetened beverage consumption. Nutrition and health professionals should educate our population about the potential adverse effects of excessive sweetened beverage consumption. These professionals could also suggest strategies for limiting sweetened beverage intake, including limiting the availability of sweetened beverages in homes and workplaces and recommending smaller portion sizes. They could also promote healthier alternatives, particularly non-caloric beverages like water.
Acknowledgements
This project was partially financed through the grant of the Comisión Nacional de Ciencia y Tecnología (CONACYT grant no. M-7876), the Instituto Mexicano del Seguro Social (IMSS grant no. 2005-785-012) and the Universidad Autónoma del Estado de México (UAEM grant no. 1860/2004). None of the authors had a conflict of interest. E.D.-G. and J.S. designed the study; E.D.-G., G.H.-B., P.M.-H. and J.S. conducted the literature search and collected the data; E.D.-G., J.O.T. and J.S. conducted the statistical analyses; and E.D.-G. and J.S. prepared the first draft of the manuscript and wrote the final manuscript. All authors contributed to the editing and proofing of the final manuscript. We wish to express our gratitude to everyone who contributed to make this study possible: to all participants and their families, to the nursing staff for the extraordinary care given, and to the laboratory staff for their commitment to the study.






