Fatty acids (FA) are strong modulators of a number of cell functions and regulate the potential risk and/or prevention of chronic diseases. The benefits of increasing the consumption of n-3 long-chain PUFA (LC-PUFA) and decreasing the intake of SFA are well known. However, the role of the proportions of dietary unsaturated fatty acids (UFA) on lipid metabolism is an attractive, although not very well-known, issue. There are many possible mechanisms involved in lipid regulation by UFA. For example, FA regulate the functionality of cell membranes, modify the plasma and tissue FA composition( Reference Abbott, Else and Atkins 1 ) and bind to and modulate nuclear receptors that regulate genes related to lipid metabolism( Reference Kersten 2 – Reference Calder 4 ). Therefore, FA can modulate enzymes involved in de novo lipogenesis and MUFA synthesis, as well as plasma and tissue lipid content. Moreover, the balance of the dietary FA, and more specifically the n-6:n-3 PUFA ratio, can be crucial to prevent inflammatory processes, which could mediate some non-communicable chronic diseases( Reference Calder 4 – Reference Jump 6 ).
On the other hand, a high intake of industrial trans-fatty acids (TFA), mainly rich in trans-elaidic acid, has a negative impact on human health( Reference Misra, Singhal and Khurana 7 ). The chronic consumption of industrial TFA has been associated with alterations in blood lipids, endothelial dysfunction, risk of CVD( Reference Roach, Feller and Ward 8 , Reference Mozaffarian, Aro and Willett 9 ) and increased incidence of different types of cancer( Reference Voorrips, Brants and Kardinaal 10 – Reference Vinikoor, Millikan and Satia 12 ). TFA have shown to be incorporated into many tissues in both humans( Reference Clifton, Keogh and Noakes 13 ) and experimental animal models( Reference Moore, Alfin-Slater and Aftergood 14 , Reference Kinsella, Bruckner and Mai 15 ). We have recently published that, even at low intake levels of TFA, they are incorporated into the tissues and their retention is related to the UFA proportion of the diet( Reference Saín, González and Lavandera 16 ). In addition, we have demonstrated that the different biological effects of TFA are associated with the incorporation and retention of TFA into the tissues, the metabolic fate, the TFA bioconversion and the biosynthesis of LC-PUFA derivates, as well as with the alteration of the FA metabolic pathways. However, little is known about the effects of TFA on TAG levels in liver or skeletal muscle( Reference Teegala, Willett and Mozaffarian 17 , Reference Wallace and Mozaffarian 18 ) in animals fed diets containing different UFA proportions. Thus, the aim of the present study was to investigate the effects of TFA from partially hydrogenated vegetable oil on liver and serum TAG regulation in mice fed experimental diets rich in edible oils containing different UFA proportions. Therefore, the effects of both dietary variables on the activity and expression of hepatic enzymes involved in lipogenesis and FA oxidation, as well as their transcription factor expressions, were also assessed. In order to clarify some of the mechanisms involved in the serum TAG levels, we also assessed the VLDL rich in TAG (VLDL-TAG) secretion rate from the liver, and epididymal white adipose tissue (EWAT) and gastrocnemius muscle (GM) lipoprotein lipase (LPL) activity.
Methods
Animals, diet preparation and experimental design
The experiment was conducted with two sets of thirty-six male CF1 mice (six mice per group) at 2 weeks after weaning (22 g), provided from the facilities at our University according to the regulations of the School of Biochemistry, Guide to the Care and Use of Experimental Animals of Laboratory( Reference Bayne 19 ). The animals were kept under controlled conditions (23±2°C and 12 h light–12 h dark cycle), and they had free access to food and water. Each set of mice was randomly divided into six groups of six animals each fed on experimental diets for 30 d, differing in the source of fat: group O was fed a diet containing olive oil, group C was fed a diet containing maize oil, group R was fed a diet containing rapeseed oil. Groups Ot, Ct and Rt were fed an O, C or R diet, respectively, partially substituted with partially hydrogenated vegetable oil. The FA composition of dietary fats was determined by GC, as shown in Table 1. The partially hydrogenated vegetable oil contained a mixture with three main TFA isomers: trans-9, trans-10 and trans-11-18 : 1. O, C and R oils provided oleic acid (OA), linoleic acid (LA) and α-linoleic acid (ALA) in the proportions of (OA/LA/ALA) 55·2/17·2/0·7; 32·0/51·3/0·9 and 61·1/18·4/8·6, respectively. The experimental diets were freshly prepared every 3 d, gassed with N2 and stored at 0–4°C. The diet composition is shown in Table 2, and it provided 64·4 % of dietary energy as carbohydrates and 19·9 % of dietary energy as protein. O, C and R diets contained 15·7 % of dietary energy as total fat, whereas Ot, Ct and Rt contained 15·7 % of dietary energy as total fat, of which 1·5 % of energy as TFA.
Table 1 Fatty acid composition of the experimental diets (% of total fatty acids methyl esters)

O, olive oil diet; C, maize oil diet; R, rapeseed oil diet; Ot, olive oil diet supplemented with trans-fatty acids; Ct, maize oil diet supplemented with trans-fatty acids; Rt, rapeseed oil diet supplemented with trans-fatty acids; ND, not detected.
Table 2 Composition of the experimental diets (g/kg dry diet)
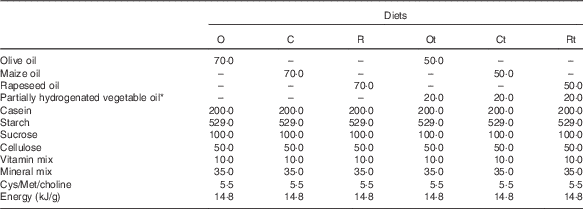
O, olive oil diet; C, maize oil diet; R, rapeseed oil diet; Ot, olive oil diet supplemented with trans-fatty acids; Ct, maize oil diet supplemented with trans-fatty acids; Rt, rapeseed oil diet supplemented with trans-fatty acids.
* Partially hydrogenated vegetable oil contains about 42 % of trans-fatty acids.
The partially hydrogenated vegetable oil was kindly provided by Compañía Argentina de Levaduras S.A. Olive oil, maize oil, rapeseed oil, sucrose, cellulose, casein and maize starch were obtained from local sources. Vitamin and mineral mixes were formulated according to the AIN-93G guidelines( Reference Reeves, Nielsen and Fahey 20 ) using compounds of food grade or better. Cysteine, methionine and choline were purchased from Sigma.
Extraction of tissues and serum samples
After 30 d of dietary treatment, one set of animals (n 36) was fasted overnight, anaesthetised and killed (09.00–11.00 hours) under anaesthesia (1 mg azepromazine+100 mg ketamine/kg body weight) by cardiac exsanguination. Blood was collected and serum was obtained after centrifugation (1000 g for 10 min at 4°C). Liver, GM and EWAT were dissected, weighed and immediately frozen. All samples were stored at −80°C until analysis.
Determination of TAG content in serum and tissues
Serum TAG levels were determined by spectrophotometry using a commercial kit (Wiener Lab).
To assess the liver and GM TAG content, portions of frozen tissue (0·5 g) were powdered and homogenised in saline (10 %, w/v) for TAG content quantification. Tissue TAG levels were determined by the method of Laurell( Reference Laurell 21 ). In brief, approximately 200 mg of frozen tissue were homogenised in saline solution 1:10 with a homogeniser and sonicated for 30 s. Complete cell destruction was achieved by one freeze–thaw cycle and two 15 s cycles of sonication (amplitude: 10 μm). TAG content was analysed using the same protocol cited by Laurell.
Liver enzyme activities
Liver samples (1·0 g) were homogenised in 2·5 ml of buffer (pH 7·6) containing 150 mm-KCl, 1 mm-MgCl2, 10 mm-N-acetyl-cysteine and 0·5 mm-dithiothreitol. After centrifugation at 100 000 g for 40 min at 4°C, the supernatant fraction was used for quantification of enzyme activities. Acetyl-CoA carboxylase (ACC, EC 6.4.1.2), fatty acid synthase (FAS, EC 2.3.1.85), glucose-6 phosphate dehydrogenase (G6PDH, EC 1.1.1.49) and malic enzyme (ME, EC 1.1.1.40) activities were measured following a previously used protocol( Reference Sain, Gonzalez and Lasa 22 ). Enzyme activities were expressed either as nmol NADH consumed (ACC), as nmol NADPH consumed (FAS) or as nmol NADPH produced (G6PDH and ME), per min and per mg of protein. Protein content was determined using bovine serum albumin as standard( Reference Lowry, Rosebrough and Farr 23 ).
Carnitine palmitoyltransferase-Ia (CPT-Ia, EC 1.3.99.3) activity was assessed in the mitochondrial fraction. Liver samples (0·5 g) were homogenised in 3 vol (w/v) of buffer pH 7·4 containing 0·25 mol/l sucrose, 1 mmol/l EDTA and 10 mmol/l Tris-HCl. Homogenates were centrifuged (700 g for 10 min at 4°C) and supernatant fluid was again centrifuged (12 000 g for 15 min at 4°C). Pellets were resuspended in 70 mmol/l sucrose, 220 mmol/l mannitol, 1 mmol/l EDTA and 2 mmol/l HEPES buffer at pH 7·4. CPT-Ia activity was assayed by means of the Bieber method( Reference Bieber, Abraham and Helmrath 24 ). The pellet protein content was determined as described above. CPT-Ia activity was expressed as nmol CoA formed/min per mg protein.
The removal capability of TAG-rich lipoproteins was evaluated by LPL activity in the main tissues responsible for the uptake of TAG: adipose tissue and muscle. The LPL enzyme activity was measured using a fluorimetric method based on Del Prado et al.( Reference Del Prado, Hernandez-Montes and Villalpando 25 ). Basically, the dibutyryl fluorescein was used as substrate for the enzyme, and the fluorescein liberated by enzymatic hydrolysis of the substrate was measured. Dibutyryl fluorescein was synthesised as described by Kramer & Guilbault( Reference Kramer and Guilbault 26 ).
The enzymatic activity of adipose tissue LPL was quantified in EWAT acetone powder. In brief, EWAT samples were delipidated by a double extraction with cold acetone followed by a double extraction with diethyl ether. The powders obtained were resuspended and incubated in buffer (25 mm-NH4Cl, pH 8·1 containing 1 IU/ml of heparin). The enzymatic reaction was carried out in a medium containing dibutyryl fluorescein as enzyme substrate. The quantification of LPL activity was performed by measuring the increase in fluorescence (λexcitation=490 nm; λemission=530 nm). In parallel, an identical assay was carried out in the same samples but in the presence of NaCl during incubation to inhibit specific enzyme activity. LPL activity was estimated as the difference between the total lipolitic and the non-specific lipolitic activity. Values were expressed as μmol fluorescein/min per g of tissue and as μmol fluorescein/min per total tissue.
To assess muscle LPL activity, GM samples were homogenised in NH4Cl/NH4OH – heparin buffer. Then, the quantification of LPL activity in GM was performed as previously described for EWAT without the step of delipidation. The measured activity was expressed as μmol fluorescein/min per total muscle.
Extraction and analysis of mRNA and quantification by real-time PCR
Total RNA was isolated from 100 mg of liver using Trizol (Invitrogen), according to the manufacturer’s instructions. RNA samples were then treated with a DNA-free kit (Applied Biosystems) to remove any contamination with genomic DNA. The yield and quality of the RNA were assessed by measuring absorbance at 260, 270, 280 and 310 nm and by electrophoresis on 1·3 % agarose gels. A quantity of 1·5 μg of total RNA of each sample was reverse-transcribed to first-strand complementary DNA (cDNA) using an iScriptTM cDNA Synthesis Kit (Bio-Rad).
Relative ACC, FAS, sterol regulatory element-binding protein (SREBP)-1a, SREBP-1c, CPT-Ia and PPAR-α mRNA levels were quantified using real-time PCR with an iCycler™ – MyiQ™ Real-Time PCR Detection System (Bio-Rad). β-Actin mRNA levels were similarly measured and served as the reference gene. A quantity of 0·1 μl of each cDNA was added to a PCR reagent mixture, SYBR® Green Master Mix (Applied Biosystems), with the upstream and downstream primers (300 nm for SREBP-1a, 600 nm for FAS and ACC and 900 nm for CPT-Ia, SREBP-1c and PPAR-α). Specific primers were designed (Genbank: NM_133360 ACC; NM–007988 FAS; NM_011480 SREBP-1a and SREBP-1c; NM–031559 CPT-Ia; AJ312092 β-actin; NM_011144.6 PPAR-α) and commercially synthesised (TIB Molbiol), and the sequences were as follows:
ACC: 5'-GGA CCA CTG CAT GGA ATG TTA A-3' (forward); 5'-TGA GTG ACT GCC GAA ACA TCT C-3' (reverse).
FAS: 5'-AGC CCC TCA AGT GCA CAG TG-3' (forward); 5'-TGC CAA TGT GTT TTC CCT GA-3' (reverse).
SREBP-1a: 5'-GGC TGT GGA ACA GGC ACT G-3' (forward); 5'-AGC TGG AGC ATG TCT TCG ATG-3' (reverse).
SREBP-1c: 5'-GCG GAC GCA GTC TGG G-3' (forward); 5'-ATG AGC TGG AGC ATG TCT TCA AA-3' (reverse).
CPT-Ia: 5'-GCA GAG GAC GGG CAT TGT A-3' (forward); 5'TGT AGC CTG GTG GGT TTG G-3' (reverse).
PPAR-α: 5'-CTC TTT CGT TTT GAC TTT CGT CTC T-3' (forward); 5'-GAA GGG CGG GTT ATT GCT G-3' (reverse).
The PCR parameters were as follows: initial 2 min at 50°C, denaturation at 95°C for 10 min followed by forty cycles of denaturation at 95°C for 15 s and combined annealing and extension at 60°C for 1 min. All sample mRNA levels were normalised to the values of β-actin, and the results were expressed as fold changes of threshold cycle (C
t
) value relative to controls using the
![]() $${\rm 2}^{{{\minus}\Delta \Delta C_{t} }} $$
method(
Reference Livak and Schmittgen
27
).
$${\rm 2}^{{{\minus}\Delta \Delta C_{t} }} $$
method(
Reference Livak and Schmittgen
27
).
Estimation of ‘in vivo’ VLDL-TAG secretion rate
Another set of animals (n 36) submitted to the same dietary treatment was fasted overnight, and anaesthetised as indicated above. Then, 600 mg/kg of body weight of Triton WR 1339 (Sigma-Aldrich) in saline solution, an agent known to inhibit the peripheral removal of TAG-rich lipoproteins, was injected intravenously( Reference Otway and Robinson 28 ). Blood samples were taken immediately before and 120 min after the injection of Triton solutions for estimation of TAG accumulation. The VLDL-TAG secretion rate was estimated based on plasma TAG concentrations at 0 and 120 min, plasma volume and body weight. Further details have been previously reported( Reference Bernal, Basilico and Gutman 29 ).
Statistical analysis
The statistical analysis was performed using SPSS 17.0 (SPSS Inc.). Values were expressed as means with their standard errors, and were statistically analysed by 2×3 ANOVA. All post hoc multiple comparisons were made using Tukey’s critical range test. For the mRNA statistical analysis, Student’s t test was performed comparing ∆∆C t between pairs of groups. Significant differences were considered at P<0·05 for both analyses.
Results
Tissues weights, liver, muscle and serum TAG levels, VLDL-TAG secretion rate and lipoprotein lipase activities
Results concerning tissue weights, liver and GM TAG content and serum TAG levels, as well as TAG secretion rate and LPL activities, are shown in Table 3. No significant differences were observed in liver, GM and EWAT weights either in animals fed O, C, R diets or in animals fed diets containing TFA v. their respective controls. The partial substitution with TFA to diets increased the TAG content in liver of Ot and Rt compared with O and R groups, respectively. In GM, no significant differences were observed in the TAG content in the groups fed O, C and R diets. However, dietary TFA decreased this parameter in the Ot group with respect to O. On the other hand, serum TAG levels were dependent on the source of fat (P<0·001). Specifically, animals fed diets containing rapeseed oil showed the highest TAG levels, independently of the presence of dietary TFA.
Table 3 Tissue weights, TAG levels in serum and tissues, liver TAG secretion rate and lipoprotein lipase activity in peripheral tissues (Mean values with their standard errors for six animals per group)

O, olive oil diet; TFA, trans-fatty acids; Ot, olive oil diet supplemented with TFA; C, maize oil diet; Ct, maize oil diet supplemented with TFA; R, rapeseed oil diet; Rt, rapeseed oil diet supplemented with TFA; EWAT, epididymal white adipose tissue; GM, gastrocnemius muscle; LPL, lipoprotein lipase.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* F, t and F×t correspond to P values of 2×3 ANOVA to the effect of fat, TFA and interaction of fat×TFA.
The in vivo VLDL-TAG secretion rate was increased in the Ot and Rt groups compared with their respective controls and also the VLDL-TAG secretion rate was Ot>Rt, showing different effect of TFA depending the source of fat. No significant differences were observed in LPL activity in EWAT when it was expressed per g of tissue between experimental groups. However, the contribution of this tissue to the total TAG removal rate was significantly lower in the O and R groups compared with C, and the intake of diets containing TFA increased this parameter in the Ot and Rt groups compared with their respective controls. In GM, the LPL activity was lower in the R group, reaching significant differences v. O. The presence of TFA reduced this parameter when maize oil was the source of fat.
Lipogenic and oxidative enzyme activities
No significant differences were observed in the G6PDH, FAS and ACC activities between the animals fed O, C and R diets (Table 4). The partial substitution with TFA in the olive oil diet increased the activities of lipogenic enzymes, and the ACC activity was lower in Rt v. their respective control. In liver, the activity of CPT-Ia, an indicator of mitochondrial FA oxidation, was increased in R compared with the O and C groups. The presence of dietary TFA decreased this parameter only in the Rt group v. its respective control.
Table 4 Liver enzyme activities (nmol/min per mg protein) (Mean values with their standard errors for six animals per group)
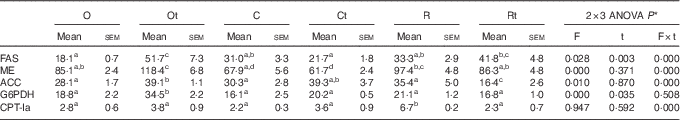
O, olive oil diet; TFA, trans-fatty acids; Ot, olive oil diet supplemented with TFA; C, maize oil diet; Ct, maize oil diet supplemented with TFA; R, rapeseed oil diet; Rt, rapeseed oil diet supplemented with TFA; FAS, fatty acid synthase; ME, malic enzyme; ACC, acetyl-CoA carboxylase; G6PDH, glucose-6 phosphate dehydrogenase; CPT-Ia, carnitine palmitoyltransferase-Ia.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* F, t and F×t correspond to P values of 2×3 ANOVA to the effect of fat, TFA and interaction of fat×TFA.
mRNA levels of enzymes and transcriptional factors in liver
The partial substitution with TFA increased the expression of Fas and Acc in animals fed the olive oil diet (Fig. 1(A) and (B)), as well as the Srebf1a (SREBP-1a gene) (Fig. 2(A)). No changes were observed in the expression of Srebf1c (SREBP-1c gene) (Fig. 2(B)). With regard to FA oxidation, the expression of Cpt1a in liver was higher in the R group and reduced by the presence of TFA (Fig. 1(C)). The expression of Ppara followed the same trend (Fig. 2(C)).

Fig. 1 Liver mRNA levels of key enzymes involved in lipogenesis and β-oxidation. O, olive oil diet; Ot, olive oil diet supplemented with trans-fatty acids; C, maize oil diet; Ct, maize oil diet supplemented with trans-fatty acids; R, rapeseed oil diet; Rt, rapeseed oil diet supplemented with trans-fatty acids; FAS, fatty acid synthase; ACC, acetyl-CoA carboxylase; CPT-Ia, carnitine palmitoyltransferase-Ia. Values are means (six animals per group), with their standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P<0·05). F, t and F×t correspond to P values of 2×3 ANOVA to the effect of fat, TFA and interaction of fat×TFA.

Fig. 2 Liver mRNA levels of transcription factors of key enzymes involved in lipogenesis and β-oxidation. O, olive oil diet; Ot, olive oil diet supplemented with trans-fatty acids (TFA); C, maize oil diet; Ct, maize oil diet supplemented with TFA; R, rapeseed oil diet; Rt, rapeseed oil diet supplemented with TFA; PPAR-α, peroxisome proliferator-activated receptor; SREBP, sterol regulatory element-binding protein. Values are means (six animals per group), with their standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P<0·05). F, t and F×t correspond to P values of 2×3 ANOVA to the effect of fat, TFA and interaction of fat×TFA.
Discussion
To the best of our knowledge, this is the first study to investigate the potential effect of dietary TFA at different UFA proportions on liver and serum TAG regulation. Interestingly, in the present study, the partial substitution with TFA on diets containing different edible oils led to dissimilar effects on TAG metabolism. In liver, TFA induced an increase in TAG content in the Ot and Rt groups, and this effect was associated with an imbalance between lipogenesis and β-oxidation. Several studies have associated the accretion of hepatic lipids with changes in these parameters( Reference Takeuchi, Nakamoto and Mori 30 – Reference Sealls, Gonzalez and Brosnan 32 ). As previously demonstrated( Reference Sain, Gonzalez and Lasa 22 ), in mice fed diets containing olive oil as a source of fat and partially substituted with TFA, the exacerbated lipogenesis may be one of the mechanisms responsible for the elevated liver TAG accretion induced by the presence of TFA. Specifically, the increased expression and activity of the lipogenic enzymes associated with the high mRNA levels of SREBP-1a led to an increase in liver TAG content in the Ot group with respect to O. In the present study, the raised hepatic TAG accretion observed in Rt has been related to a decreased β-oxidation, compared with the R group. Specifically, we observed an increased expression and activity of CPT-Ia in R compared with the O and C groups, associated with a greater expression of Ppara. The partial substitution with TFA reduced these parameters. In agreement with these findings, Pawar et al.( Reference Pawar and Jump 33 ) reported that n-3 PUFA might activate the PPAR-α in rat primary hepatocytes, leading to a greater β-oxidation. Furthermore, Giudetti et al.( Reference Giudetti, Beynen and Lemmens 34 ) demonstrated that dietary TFA might affect the β-oxidation of FA reducing the CPT-1a activity in rat liver compared with diets without TFA. As regards the C and Ct groups, no alterations were observed in the expression and activities of enzymes involved in both lipogenesis and β-oxidation. Consequently, the lack of effects in the hepatic TAG content might be associated with the absence of changes in these parameters. As we have recently observed( Reference Saín, González and Lavandera 16 ) that the total TFA retained in the Ot, Ct and Rt groups was similar, the differences in liver TAG content, hepatic lipogenesis and β-oxidation between these groups cannot be explained by the differences in the hepatic incorporation of TFA isomers. In a recent research article published by our group( Reference Saín, González and Lavandera 16 ), it was shown that the liver of mice from the Ct group contained a significantly higher percentage of LA and a lower percentage of OA than those from the Ot and Rt groups. It has been demonstrated that LA reduces liver lipogenesis in comparison with OA( Reference Sabine, McGrath and Abraham 35 , Reference Musch, Ojakian and Williams 36 ). Thus, we could claim that the effect of TFA was mitigated by LA in the Ct group but not in the Ot and Rt groups. In contrast, several authors have observed that TFA increase the content of TAG in liver in diets containing maize oil( Reference Giudetti, Beynen and Lemmens 34 , Reference Colandre, Diez and Bernal 37 – Reference Dorfman, Laurent and Gounarides 39 ). In addition, compared with our results this discrepancy might be explained by differences in the experimental design: levels of dietary fat (7, 10, 12, 20 %), high content of TFA and the type of FA isomers, among others. Particularly, the fat used in the present study contains equimolecular levels of vaccenic and elaidic acid, and dissimilar and/or opposite biological effects have been reported between both isomeric TFA( Reference Wang, Jacome-Sosa and Ruth 40 – Reference Meijer, van Tol and van Berkel 42 ). Therefore, it cannot be excluded that there might exist a counteracting effect of vaccenic acid on elaidic acid attenuating the alterations induced by the TFA.
Changes in the hepatic TAG content are regulated by a number of mechanisms including TAG synthesis and VLDL-TAG assembly and secretion to plasma, among others. Several authors( Reference Dashti, McConathy and Ontko 43 – Reference Mitmesser and Carr 46 ) have studied, both in vivo and in vitro, the secretion of VLDL-TAG-rich particles from the liver, showing a link between the liver TAG pool and/or the apo synthesis. In the present study, the high levels of the hepatic TAG in the Ot and Rt groups were correlated to a greater VLDL-TAG secretion. This suggests that, under our experimental conditions, the TFA regulating the hepatic TAG accretion promote the TAG secretion in VLDL particles. In addition, we cannot preclude that the increased VLDL-TAG secretion rate could be associated with an enhanced apo-B synthesis. In this matter, Arrol et al.( Reference Arrol, Mackness and Durrington 44 ) demonstrated that a high TAG content in isolated hepatocytes increased the apo-B secretion to the medium. On the other hand, Mitmesser & Carr( Reference Mitmesser and Carr 46 ) demonstrated that, compared with OA, elaidic acid increased the apo-B secretion in Hep-G2 cells. In the same way, rumenic acid (c9,t11-18 : 2) also showed the same effect in comparison with LA, and this indicates that trans-isomers could enhance the apo-B secretion more than their cis counterpart. In contrast, Dashti et al.( Reference Dashti, McConathy and Ontko 43 , Reference Dashti, Feng and Freeman 45 ) demonstrated that elaidic acid did not increase the apo-B secretion compared with OA. Nevertheless, the authors reported that the different FA had more effects on VLDL composition than in the amount of lipoprotein secreted by hepatocytes.
Despite the increase in liver TAG in the Ot and Rt groups, and the consequent enhanced VLDL-TAG secretion, the serum TAG levels did not show greater levels in these groups. Instead, the effects found in serum TAG levels were associated with the source of UFA rather than with the presence of TFA. Specifically, serum TAG levels were increased in animals fed rapeseed oil, compared with the animals fed maize and olive oil diets, independently of TFA intake. These results do not seem to agree with the beneficial effects associated with the n-3 FA intake on serum lipids. Nevertheless, these effects have been attributed to the intake of n-3 LC-PUFA: EPA and DHA( Reference Takeuchi, Nakamoto and Mori 30 , Reference Sealls, Gonzalez and Brosnan 32 , Reference Connor and Connor 47 , Reference Ruiz-Gutierrez, Perez-Espinosa and Vazquez 48 ). In this respect, in mice fed rapeseed oil it was observed that serum TAG levels were higher than in mice fed fish oil( Reference Sealls, Gonzalez and Brosnan 32 ). The authors claimed that dietary LC-PUFA might have different metabolic fates from those synthesised in vivo from ALA and thus could govern different regulatory pathways.
Previously, in our laboratory, it was shown that rats fed high levels of a trans-fat rich in elaidic acid showed increased levels of serum TAG( Reference Colandre, Diez and Bernal 37 , Reference Bernal, Rovira and Colandre 38 ). However, in the present study, no hyperlipidaemic effects were found in animals fed diets containing TFA. This discrepancy might be explained by both the type of hydrogenated fat used and the experimental animal model used. The hydrogenated fat used had equivalent levels of three t-18 : 1 isomers: vaccenic acid, elaidic acid and trans-10 18 : 1. According to our results, Tyburczy et al.( Reference Tyburczy, Major and Lock 49 ) demonstrated that hamsters fed diets rich in either vaccenic acid or elaidic acid and a diet containing a hydrogenated fat similar to the one used in this study did not show alterations in serum TAG, suggesting that other variables, different from the type of isomer, could be present.
It is widely known that, in fasting, serum TAG levels are related to the balance between the hepatic secretion and the peripheral tissue clearance through LPL activity. Therefore, the potential discrepancy observed between the high liver TAG secretion and the normal serum TAG levels could be explained by a differential removal of TAG by LPL enzyme in extrahepatic tissues. The adipose tissue is a key organ involved in the incorporation of TAG from the VLDL particles. In the absence of TFA, the C group showed a higher LPL activity than O and R. These results are in agreement with those obtained by several authors( Reference Michaud and Renier 50 – Reference Murphy, Zampelas and Puddicombe 52 ) who demonstrated that high levels of dietary UFA led to an increase in the LPL activity. Accordingly, the C diet had the highest levels of total PUFA, and this could explain the enhanced LPL activity in EWAT. The lower activity in the R group than in the C group suggests that one of the mechanisms responsible for the high levels of TAG in serum in the R group might be related to the lower LPL activity in comparison with the C group. However, several authors have demonstrated that animals fed rapeseed oil show greater levels of LPL activity in adipose tissue in comparison with other oils enriched in n-3 and n-6 PUFA( Reference Baba, Antoniades and Habbal 53 – Reference Hulsmann, Geelhoed-Mieras and Jansen 55 ). Among other reasons, the differences found with our results might be related to disparities between species, differences between in vitro and in vivo experiments, and/or age of the animals. The presence of TFA in the diets increased the EWAT LPL activity in a fat source-dependent way. Thus, the animals fed Ot and Rt diets showed a higher LPL activity with respect to their controls. In contrast, other authors did not find( Reference Pereira-Assumpcao, Duarte dos Santos and de Mattos Machado Andrade 56 , Reference Silva, Guimaraes and Mizurini 57 ) or observe a low removal rate( Reference Saravanan, Haseeb and Ehtesham 58 ) in rats fed diets containing a hydrogenated fat. Nevertheless, the increased LPL activity in EWAT in the Ot and Rt groups v. O and R, respectively, is related to the absence of changes in serum TAG despite the enhanced VLDL-TAG secretion rate in those groups.
In addition to EWAT, GM is another tissue with an important contribution to the serum TAG removal( Reference Goldberg 59 , Reference Olivecrona, Liu and Hultin 60 ). In the present study, the R and Rt groups showed lower levels of LPL activity than O and Ot, respectively. Although it has been demonstrated that LPL expression and activity could lead to a muscle TAG accretion( Reference Voshol, Jong and Dahlmans 61 ), the mentioned alterations observed in R v. O did not induce changes in GM TAG levels. In animals fed rapeseed oil, the low removal rate induced by EWAT and/or GM might be, at least in part, responsible for the high serum TAG levels. On the other hand, the LPL activity in GM, as well as the muscle TAG levels, did not show any significant alterations in animals fed olive or maize oil. In addition, the low LPL activity induced by TFA in animals fed maize oil was not related to variations in GM TAG levels.
In brief, the effects induced by the partial substitution with TFA to diets containing different edible oils were dependent on the fat source. Hence, the animals fed both Ot and Rt diets showed a higher accretion of liver TAG than their respective controls, and this was associated with an imbalance between lipogenesis and β-oxidation. However, no differences were observed in liver TAG content between C and Ct groups. Specifically, in the Rt group, the increased liver TAG content might be related to a low β-oxidation, reflected by a decrease in the Ppara expression and Cpt1a expression and activity, whereas in the Ot group the liver TAG accretion was associated with an enhanced lipogenesis. In contrast, TFA did not induce changes in the lipogenesis and β-oxidation in animals fed maize oil. The high levels of liver TAG in Ot and Rt led to a high secretion rate of VLDL-TAG, suggesting that under our experimental conditions the TFA regulating the hepatic TAG accretion promote the VLDL-TAG secretion. Nevertheless, this effect was not reflected in serum TAG, probably by the compensation through the increased EWAT LPL activity in Ot and Rt. Moreover, the low TAG removal rate by the GM in the R group might be associated with the high serum TAG levels. Summing up, the effects of a diet containing low levels of TFA, with a high proportion of vaccenic acid on liver and serum TAG regulation, depend on the dietary proportions of n-3, n-6 and n-9 UFA. These results provide insights into some controversial findings associated with the intake of TFA and its effects on human metabolic alterations.
Acknowledgements
The authors thank Walter DaRú for his technical assistance.
This study was supported by Universidad Nacional del Litoral – Cursos de Acción para la Investigación y el Desarrollo (CAI+D 2011 no. 501 201101 00165 LI and no. 501201101 00166 LI) – Secretaría de Ciencia y Técnica – UNL.
C. A. B. designed and planned the study, as well as analysed results, interpreted findings and wrote the manuscript; J. S. conducted the study, performed the sample analysis, analysed the data, interpreted findings and prepared the manuscript; M. A. G. contributed to the design and planning of the study, interpreted findings and discussed the manuscript; M. V. S. and J. V. L. carried out collections and analytical determinations and assisted in the maintenance and killing of mice.
The authors have no conflicts of interest to disclose.








