Advances in understanding the physiology and pharmacology of the endogenous cannabinoid system have potentiated the interest of cannabinoid receptors as potential therapeutic targets. The endogenous cannabinoid system is involved in mechanisms that regulate energy metabolism, and interacts with many other signalling systems that play an important role in metabolic regulation(Reference York1). The cannabinoid type 1 (CB1) receptor has been strongly implicated in the regulation of food intake(Reference Di Marzo and Matías2). CB1 receptors are present in the brain and in the periphery. In the brain, CB1 receptors have been identified in pathways responsible for reward and energy balance(Reference Cota, Marsicano and Tschop3–Reference Pickel, Chan and Kash5), whereas in the periphery, CB1 receptors have been identified in the gut(Reference Croci, Manara and Aureggi6–Reference Coutts, Irving and Mackie8), as well as in hepatocytes(Reference Osei-Hyiaman, DePetrillo and Pacher9) and adipocytes(Reference Cota, Marsicano and Tschop3, Reference Bensaid, Gary-Bobo and Esclangon10). CB1 receptor agonists such as Δ9-tetrahydrocannabinol, together with the endocannabinoids, anandamide and 2-arachidonoylglycerol, increase food intake in both man and animals(Reference Harrod and Williams11). The enhancement of food intake can also be induced by other cannabinoid CB1 receptor agonists such as WIN 55,212-2(Reference Gómez, Navarro and Ferrer12). This action is blocked or reversed by the selective CB1 receptor antagonist SR 141716A(Reference Kirkham and Williams13) and by the structurally and pharmacologically similar compound AM 251(Reference Hildebrandt, Kelly-Sullivan and Black14, Reference Chambers, Sharkey and Koopmans15).
Since the nutritive utilization of any foodstuff can be significantly affected by the amount of food consumed, we undertook the study of food intake regulation at the central and peripheral level using the CB1 receptor agonist WIN 55,212-2 and the CB1 inverse agonist AM 251 administered at different doses by means of intracerebroventricular (icv) or intraperitoneal (ipt) injection. We chose to examine the effects of WIN 55,212-2 and AM 251 on food intake in presatiated rats to further delineate the role of endogenous cannabinoids in energy balance. Our data represent the first attempt to directly examine the effect of cannabinoids on serotonin (5-HT) release; there are few reports in the literature examining the interactions of catecholamine and cannabinoids and the resulting effect on food intake(Reference Avraham, Ben-Shushan and Breuer16).
Materials and methods
Drugs
CB1 receptor agonist R(+)-[2,3-dihydro-5-methyl-3-[(4-morpholinyl)methyl] pyrrolo[1,2,3-de]-1,4-benzoxazin-yl]-(1-naphthalenyl) methanone mesylate (WIN-55,212-2) and CB1 receptor inverse agonist N-(piperidin-1-yl)-5 (4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM-251) (Tocris, Bristol, UK) were dissolved in dimethyl sulfoxide–saline solution mixture (60:40).
Animals
Male Wistar rats weighing 210 (sd 20) g were housed individually for at least 10 d before the surgery under controlled environmental conditions (22 ± 1°C, 55 ± 15 % relative humidity, 12 h light/dark cycle, light on at 11.00 hours) and fed ad libitum a standard rat chow and water. All experiments were undertaken according to Directional Guides Related to Animal Housing and Care (17).
Chemical composition of the standard diet
According to the manufacturer, the macronutrient composition of the standard diet used in the present study was sufficient to meet the nutrient requirement of the growing rat; a fact corroborated under our experimental conditions for crude energy, total nitrogen and ash content. Diet composition was as follows: energy, 13·0 MJ/kg; nitrogen, 26·1 g/kg; fat, 41 g/kg; carbohydrates, 690 g/kg; fibre, 45 g/kg; ash, 60·8 g/kg. Energy was measured with an adiabatic bomb calorimeter (Gallenkamp, Loughborough, UK). Moisture content was determined by drying to constant weight in an oven at 105 ± 1°C. Ash was measured by calcination at 500°C to a constant weight, and total N was determined according to Kjeldahl's method. Crude protein was calculated as N × 6·25.
Intracerebroventricular injection
The animals were anaesthetized with chloral hydrate (400 mg/kg) and placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA, USA). A stainless-steel cannula guide (26 gauge) was implanted lowered into the brain and placed 2 mm above the ventromedian hypothalamic nucleus at the following coordinates: AP − 2·0 mm, L − 0·7 mm, DV 6·0 mm from brain surface. The cannulation assembly was fixed to the skull with miniature stainless-steel screws and acrylic dental resin. Wire stylet was used to seal the indwelling cannula guide and to ensure the guide remained patent throughout the experiments. A 10 d post-surgical recovery period was allowed to stabilize body weight prior to the experimental period.
Experimental design
Food intake studies
The effects of CB1 receptor agonist or inverse agonist on food intake were analysed in presatiated rats(Reference Williams, Rogers and Kirkham18). To this end, animals were deprived of food but not of water for 24 h prior to the experiments and then allowed to eat for 1 h before drug testing. Drug administration was done intracerebroventricularly or intraperitoneally. For the icv microinjections, stylets were removed from the guide cannula and a 33 gauge injector with a 2 mm protrusion was lowered into the ventromedial hypothalamus nucleus. The injector was connected with polyethylene tubing to a precision micropump. Infusions of WIN 55,212-2 or AM 251 (1 μg) solutions were made at a rate of 1 μl/min and the volume injected was 5 μl. The injector remained in place for 1 min to allow diffusion of the drugs into the brain and to reduce backflow through the cannula track. The stylet was then immediately replaced. Vehicle injections were carried out in another series of animals. For the ipt injection, different WIN 55,212-2 or AM 251 doses (0·5, 1, 2 and 5 mg/kg body weight) were tested. Thirty minutes after drug or vehicle administration, rats were allowed free access to food pellets and the amount consumed, corrected for food spillage, was recorded at 1, 2, 4, 6 and 24 h after the injection.
Microdialysis studies
The effects of icv administration of WIN 55,212-2 (1 μg/5 μl), AM 251 (1 μg/5 μl) and vehicle on hypothalamic 5-HT and acetic acid 5-hydroxy-indol (5HIAA) release were analysed in partially satiated animals as for food intake studies. After surgery (1 week), the rats were deprived of food 24 h before the experiment. On the day of the experiment, each rat was placed, at 08.00 hours, into a plastic bowl (CMA/120 System for freely moving rats) and the probe was inserted through the guide cannula. After a 2 h stabilization period, four 15 min baseline dialysis samples were collected. Then the rats were allowed to eat for 1 h prior to drug or vehicle injection; 30 min after drug or vehicle administration, rats were re-allowed free access to food pellets. Dialysis samples were collected every 15 min before and after drug or vehicle administration. A CMA/11 microdialysis probe (CMA Mycrodialysis, Sweden) with polycarbonate membrane (2 mm long, 0·5 mm outer diameter molecular weight cut-off = 6000) showed a 15–17 % relative recovery for 5-HT in the in vitro calibration tests. Perfusion was made with a Ringer solution containing: NaCl 140 mm, KCl 3·0 mm, CaCl2 1·2 mm, MgCl2 1·0 mm (pH 7·4), at flow rate of 2 μl/min using a CMA/100 microdialysis pump (CMA Microdialysis). This methodology allowed us to assess simultaneously different parameters involved in the regulation of food intake that would not provide a complete view of such regulation if studied separately.
Open field test
The animals selected for motor activity studies were randomly divided in two groups (five animals per group). WIN 55,212-2 (5 mg/kg) was administered intraperitoneally to one of the experimental group, whereas the vehicle was administered to the second experimental group. Motor behaviour in the open field was studied in an opaque open field box (45 × 30 × 23 cm). Rats were habituated to the field for 10 min the day before testing. On the experimental day, the animals were treated and placed in the centre of the field, and locomotor activity (number of lines crossed) and rearing (number of rearings) was scored every 10 min for a total of 60 min after drug or vehicle injection. Behaviour was scored by trained observers who were unaware of the experimental conditions.
c-fos expression
The effects of icv administration of WIN 55,212-2 (1 μg/5 μl), AM 251 (1 μg/5 μl) and vehicle on hypothalamic c-fos expression were analysed in partially satiated animals as for food intake studies. After surgery (48 h), the rats were deprived of food, but not water, for 24 h prior to the experiment. On the day of the experiment, the rats were allowed to eat for 1 h before drug testing. Drug administration was done intracerebroventricularly as previously described. After drug administration (1 h) animals were anaesthetized with 4 % chloral hydrate and transcardially perfused with 60 ml PBS followed by 120 ml 4 % paraformaldehyde in PBS. The animals' brains were removed and placed in 20 % sucrose in PBS. Using a microtome, brains were sliced into 40 μm sections (twelve sections: three for each rat, four rats per experiment) that were preserved in protective solution (30 % ethylene glycol–30 % glycerol in 0·1 m-NaKPO4). Brain samples from each of the three experiments were processed for c-fos in three separate groups, one corresponding to each experiment. For immunocytochemistry, samples were washed in 0·5 m-2-amino-2-hydroxymethyl-1,3-propanediol (Tris)-buffered saline (TBS) between different treatments and stained using the avidin-biotin method. Sections were treated with 3 % H2O2 to block endogenous peroxidases and then, as a blocking step, incubated for 1 h in 5 % normal goat serum. The samples were then incubated for 24 h in the primary antibody solution comprised of polyclonal rabbit anti c-fos diluted 1:5000, as well as 0·5 % Triton X-100, 1 % normal goat serum and TBS. Next, tissue samples were incubated for 1 h in the secondary antibody mixture consisting of anti-rabbit IgG, TBS and normal goat serum, and then for 30 min in the avidin-biotinylated horseradish peroxidase complex. Staining was visualized using a solution of 0·5 % of the chromogen diaminobenzidine, 0·01 % H2O2 and TBS. After diaminobenzidine staining, the sections were washed again with TBS before being mounted and dehydrated. c-fos immunoreactive cells were counted using a photonic microscope with the aid of a computerized image analysis system (Leica QWin).
HPLC analysis
The analytical HPLC system consisted of a pump (model LC6A; Shimadzu, Japan), analytical column C18-5 mm and electrochemical detector (L-ECD-6A; Shimadzu). A guard cell was placed between the column and injector to oxidize compounds which could eventually interfere with the analysis. The chromatograms were collected, stored and processed with a computerized integrator (model CR4A; Shimadzu). The optimal composition of the mobile phase was: 0·05 m-sodium acetate anhydrate, 0·05 m-citric acid, 0·15 mm-EDTA, 0·4 mm-octylsulphonate as the counter ion and 8 % methanol. The pH was adjusted to 3·5 with acetic acid. Octylsulphonate concentration and pH value were chosen in preliminary tests to allow the best compromise of peak separation. The freshly prepared mobile phase was filtered (0·22 μm; Millipore, USA) and degassed under vacuum (Branson 2200; UK) for 10 min. The flow rate was 1 ml/min. The system was run continuously with the mobile phase being recycled back into the reservoir and used for up to 1 week. The detector operated in the oxidative mode, with the guard cell potential at 700 mV. The sensitivity was 10 nA.
Analysis of hypothalamic serotonin and acetic acid 5-hydroxy-indol
Dialysis samples were analysed immediately by means of reverse-phase HPLC with electrochemical detection as descri bed earlier using an inverse C18 column. The identification of the various neurotransmitters and the calibration curve were achieved by injections in the HPLC system of standard solutions of 5-HT, and its metabolite 5HIAA, with concentrations ranging from 50 to 500 pmol/20 μl. In this range of concentrations, the response of the detector proved to be linear; the height and the surface of the peaks being proportional to the concentration of the compound injected.
Statistical analysis
The effect of CB1 receptor agonist (WIN 55,212-2) or inverse agonist (AM 251) administered intracerebroventricularly and time on relative cumulative food intake was analysed by 3 × 5 factorial ANOVA with time, treatment, and time × treatment interaction as the main components (Table 1). The effect of CB1 receptor agonist (WIN 55,212-2) or inverse agonist (AM 251) administered intraperitoneally at different doses on relative cumulative food intake was analysed by 5 × 5 factorial ANOVA with time, dose, and time × dose interaction as the main treatments (Tables 2 and 3). T pairwise comparisons were performed among the different experimental groups at each one of the different time-points selected (1, 2, 4, 6, 24 h) after drug administration. Statistical differences between the levels of neurotransmitters at each time-point after drug administration compared to the baseline values or the vehicle-injected control (Figs. 1 and 2) were analysed by time-repeated measurement ANOVA with time, treatment, and time × treatment interaction as main effects. Differences on motor behaviour measured in the open field between dimethyl sulfoxide and WIN 55,212-2 (5 mg/kg) administered intraperitoneally (Fig. 3) were evaluated by Student's t test. Statistical differences in ventromedial hypothalamus c-fos expression among control, CB1 receptor agonist (WIN 55,212-2) or inverse agonist (AM 251)-administered rats (Fig. 4) were evaluated by one-way ANOVA. Differences between means were compared with Duncan's test. Statistical analysis was applied with the use of Statgraphics 2.1 System software (Statistical Graphics Corp, Rockville, MD, USA). Results are given as mean values and standard errors of the mean. The level of significance was set at P < 0·05.
Table 1 Effect of intracerebroventricular administration of WIN 55,212-2 (1 μg/5 μl) and AM 251 (0·1 μg/5 μl) on the relative cumulative food intake (g/100 g body weight)*
(Mean values with their standard errors for four rats)
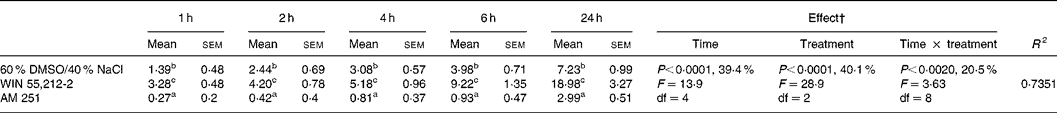
DMSO, dimethyl sulfoxide.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·05) after T pairwise comparison.
* For details of procedures, see Materials and methods. The effect of cannabinoid type 1 (CB1) receptor agonist (WIN 55,212-2) or inverse agonist (AM 251) administered intracerebroventricularly, and time on relative cumulative food intake was analysed by 3 × 5 factorial ANOVA with time, treatment, and time × treatment interaction as the main components.
† Percentages represent the contribution to total variance of the specific ANOVA component.
Table 2 Effect of intraperitoneal administration of WIN 55,212-2 at different doses on the relative cumulative food intake (g/100 g body weight)*
(Mean values with their standard errors for ten rats)
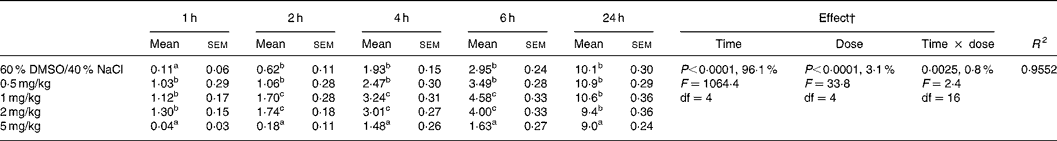
DMSO, dimethyl sulfoxide.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P(0·05) after T pairwise comparison.
* For details of procedures, see Materials and methods. The effect of cannabinoid type 1 (CB1) receptor agonist (WIN 55,212-2) administered intraperitoneally at different doses on relative cumulative food intake was analysed by 5 × 5 factorial ANOVA with time, dose, and time × dose interaction as the main treatments.
† Percentages represent the contribution to total variance of the specific ANOVA component.
Table 3 Effect of intraperitoneal administration of AM 251 at different doses on the relative cumulative food intake (g/100 g body weight)*
(Mean values with their standard errors for ten rats)
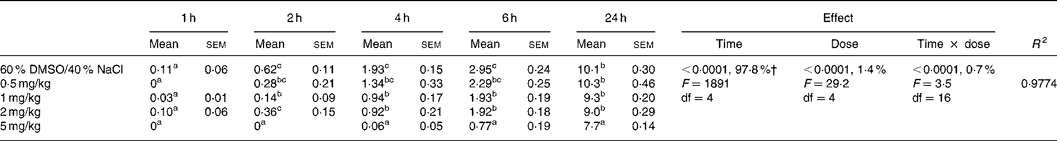
DMSO, dimethyl sulfoxide.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·05) after T pairwise comparison.
* For details of procedures, see Materials and methods. The effect of cannabinoid type 1 (CB1) receptor inverse agonist (AM 251) administered intraperitoneally at different doses on relative cumulative food intake was analysed by 5 × 5 factorial ANOVA with time, dose, and time × dose interaction as the main treatments.
† Percentages represent the contribution to total variance of the specific ANOVA component.

Fig. 1 Effect of intracerebroventricular administration of WIN 55,212-2 (1 μg/5 μl) and AM 251 (1 μg/5 μl) on extracellular levels of serotonin (5-HT) of freely moving rats. ↓ , Time of the presentation of food (1), suppression of food (2) and the injection (3) of WIN 55,212-2 (![]() ), AM 251 (
), AM 251 (![]() ) or vehicle dimethyl sulfoxide (60 %)–NaCl (40 %) solution (
) or vehicle dimethyl sulfoxide (60 %)–NaCl (40 %) solution (![]() ). Values are means with their standard errors depicted by vertical bars (n 10). Statistical differences between the levels of neurotransmitters at each time-point after drug administration compared to the baseline values or the vehicle-injected control were analysed by time-repeated measurement ANOVA. Treatment effect: P = 0·0053, F = 14·2, df = 2. Time effect: P < 0·0001, F = 259·2, df = 21. Time × treatment interaction: P < 0·0001, F = 146·7, df = 42.
). Values are means with their standard errors depicted by vertical bars (n 10). Statistical differences between the levels of neurotransmitters at each time-point after drug administration compared to the baseline values or the vehicle-injected control were analysed by time-repeated measurement ANOVA. Treatment effect: P = 0·0053, F = 14·2, df = 2. Time effect: P < 0·0001, F = 259·2, df = 21. Time × treatment interaction: P < 0·0001, F = 146·7, df = 42.

Fig. 2 Effect of intracerebroventricular administration of WIN 55,212-2 (1 μg/5 μl) and AM 251 (1 μg/5 μl) on extracellular levels of acetic acid 5-hydroxy-indol (5HIAA) of freely moving rats. ↓ , Time of the presentation of food (1), suppression of food (2) and the injection (3) of WIN 55,212-2 (![]() ), AM 251 (
), AM 251 (![]() ) or vehicle dimethyl sulfoxide (60 %)–NaCl (40 %) solution (
) or vehicle dimethyl sulfoxide (60 %)–NaCl (40 %) solution (![]() ). Values are means with their standard errors depicted by vertical bars (n 10). Statistical differences between the levels of neurotransmitters at each time-point after drug administration compared to the baseline values or the vehicle-injected control were analysed by time-repeated measurement ANOVA. Treatment effect: P = 0·04, F = 5·8, df = 2. Time effect: P < 0·0001, F = 481·8, df = 21. Time × treatment interaction: P < 0·0001, F = 146·2, df = 42.
). Values are means with their standard errors depicted by vertical bars (n 10). Statistical differences between the levels of neurotransmitters at each time-point after drug administration compared to the baseline values or the vehicle-injected control were analysed by time-repeated measurement ANOVA. Treatment effect: P = 0·04, F = 5·8, df = 2. Time effect: P < 0·0001, F = 481·8, df = 21. Time × treatment interaction: P < 0·0001, F = 146·2, df = 42.

Fig. 3 Effect of peripheral (intraperitoneal) administration of vehicle (control) and cannabinoid type 1 (CB1) receptor agonist WIN 55,212-2 (5 mg/kg) on motor behaviour measured in the open field (■, locomotor activity; □, exploratory activity). Values are means with their standard errors depicted by vertical bars (n 5). a,b Mean values with unlike letters were significantly different (P < 0·05).

Fig. 4 Effect of intracerebroventricular injection of WIN 55,212-2 (1 μg/5 μl) and AM 251 (1 μg/5 μl) on ventromedial hypothalamus c-fos expression. Values are means with their standard errors depicted by vertical bars (n 4; three sections counted per rat). a,b,c Mean values with unlike letters were significantly different (P < 0·05).
Results
Changes in relative cumulated DM intake in response to intracerebroventricular injection of WIN 55,212-2 and AM 251 at different time-points after injection
The two-way factorial analysis applied showed a significant time effect (P < 0·0001), treatment effect (P < 0·0001), and time × treatment interaction (P = 0·0020) (Table 1). Nevertheless, partitioning the total variation of the ANOVA treatment into its various components (time, treatment, and time × treatment interaction) gave the highest magnitude to the time effect and treatment effect that exhibited similar values, followed by time × treatment interaction.
The central administration of cannabinoid receptor agonist WIN 55,212-2 (1 μg/5 μl) stimulated daily food intake in presatiated rats, leading to considerable hyperphagia in comparison to the control group of rats injected with the vehicle (60 % dimethyl sulfoxide, 40 % NaCl). Hyperphagia was noticeable from the first hour after injection and lasted for 24 h, a time when the food intake of rats administered WIN 55,212-2 was 2·5-fold higher than that of the vehicle-injected control group. The icv administration of the CB1 inverse agonist AM 251 (0·1 μg/5 μl) to presatiated rats caused a significant decrease in daily food intake in comparison to that of the vehicle-injected control group, following a similar although inverse trend to that observed after the administration of WIN 55,212-2 (Table 1).
Effect of intracerebroventricular administration of WIN 55,212-2 and AM 251 on extracellular levels of serotonin and its metabolite acetic acid 5-hydroxy-indol
The time-repeated measurement ANOVA applied to the data showed significant time effects (P < 0·05), treatment effects (P < 0·0001), and time × treatment interactions (P < 0·0001) for extracellular levels of 5-HT and its metabolite 5HIAA (Figs. 1 and 2). Compared to fasting baseline values in the three experimental groups studied, 5-HT levels increased from 30 min after food was presented to the animals (90 min from the beginning of the experimental period) up to the time when drugs were injected (150 min; Fig. 1). In the vehicle-injected experimental group, 5-HT levels remained high up to 270 min, when they returned to basal values. The icv administration of WIN 55,512-2 caused a considerable reduction (average 38 %) in 5-HT levels up to 270 min in comparison to the vehicle-injected control group or to the baseline values of the three experimental groups. Nevertheless, basal 5-HT levels were recovered after the 270 min time-point.
Administration of AM 251 caused a 36 % increase in the levels of 5-HT (Fig. 1), and it took a longer time period to recover the baseline values of this neurotransmitter in comparison to the WIN 55,212-2 administration. In fact, 330 min after administration of the CB1 inverse agonist, 5-HT levels were still higher than the baseline values.
The levels of 5HIAA increased 45 min after the animals were offered free access to food (105 min from the beginning of the experimental period) in the three experimental groups studied (Fig. 2), and remained higher than the baseline values until the drugs were injected (150 min). Almost immediately after injection (165 min) of WIN 55,212-2, there was a strong decrease in the extracellular levels of 5HIAA, to values similar to the fasting baseline for the rest of the experimental period. An average 47 % reduction in 5HIAA values was found from the injection time up to 270 min, leading to 5HIAA levels that were lower in the WIN 55,212-2-administered group than those found in the control vehicle-administered group until the 240 min time-point.
After the icv administration of AM 251, a drastic increase in 5HIAA levels (average 57·6 %) was apparent, lasting for about 75 min after the injection, exhibiting a strong decrease thereafter until it reached values similar to those of the vehicle administered-group and the baseline values of the three experimental groups.
Changes in the cumulated food intake in response to intraperitoneal administration of WIN 55,212-2 and AM 251 at different doses
The two-way factorial analysis applied showed significant time effects (P < 0·0001), dose effects (P < 0·0001), and time × treatment interactions (P < 0·05) (Tables 2 and 3). Nevertheless, partitioning the total variation of the ANOVA treatment into its various components (time, dose, and time × dose interaction) gave the highest magnitude to the time effect, followed by treatment effect, and, finally, time × treatment interaction.
The ipt administration of WIN 55,212-2 to presatiated rats at doses of 0·5, 1 and 2 mg/kg body weight caused a significant increase in food intake from 1 h after injection, which was not directly related to the amount of drug administered in the range of doses mentioned earlier (Table 2). The observed hyperphagia tended to disappear 2 h after injection in the 0·5 mg/kg-injected animals, and persisted for up to 6 h in the 1 and 2 mg/kg-injected animals, with no significant differences being found between the two latter doses. In comparison to the vehicle-injected control group, no significant differences in food intake were found for any of the three doses studied 24 h after injection.
When a higher WIN 55,212-2 dose (5 mg/kg body weight) was administered to the animals, contrasting effects to those elicited by the 0·5, 1 and 2 mg/kg doses were obtained. A significant decrease in food intake was observed from 2 to 24 h after injection, although a trend towards recuperation of food intake up to values similar to those observed in the vehicle-injected control was observed at the end of the experimental period. In fact, upon studying the non-cumulated food intake between 6 and 24 h after injection, it was observed that the animals injected with 5 mg/kg WIN 55,512-2 ingested similar amounts of food, expressed in absolute values, in comparison to the vehicle-injected control animals (dimethyl sulfoxide: NaCl, 7·17 mg/100 g; WIN 55,512-2, 7·39 mg/100 g). On the other hand, the inhibitory effects on food intake caused by the high dose of WIN 55,212-2 were accompanied by a significant reduction of motor behaviour measured in the open field (Fig. 3).
Under the present experimental conditions, presatiated rats injected with the vehicle or any dose of AM 251 consumed hardly any feed for 1 h after injection, with no significant differences being found among any of the experimental doses administered (Table 3). Administration of any of the AM 251 doses studied (0·5, 1, 2, 5 mg/kg) led to a significant decrease in the amount of food ingested from 2 h after the injection, in comparison to the vehicle-injected control group, and the most striking effect was observed when the 5 mg/kg dose was injected. Nevertheless, such inhibitory effect of AM 251 administration on food intake by presatiated rats was not directly related to the amount of drug administered in the 0·5–2 mg/kg dose interval.
Hypophagia was maintained for 4 h after injection in the animals treated with 0·5, 1 and 2 mg/kg AM 251, but this effect on food intake was even more severe for the 5 mg/kg-injected animals. After 6 h, hypophagia started to recede in the animals injected with 0·5, 1 or 2 mg/kg AM 251, but continued to be acute in the animals injected with 5 mg/kg AM 251. Between 6 and 24 h, the cumulated food intake (g/100 g body weight) was similar in the experimental groups injected with 0·5, 1 and 2 mg/kg AM 251 when compared to the vehicle-injected control animals. With regard to the experimental group injected with 5 mg/kg, the animals ingested a similar amount of food (non-cumulated) during the 6–24 h post-injection period in comparison to the other experimental groups, but did not reach their levels of cumulated food intake due to the significantly lower levels of food intake by the former group, injected with 5 mg/kg, during the first 6 h after injection.
Effect of intracerebroventricular administration of WIN 55,212-2 and AM 251 on c-fos expression
Administration of WIN 55,212-2 and AM 251 significantly increased c-fos expression when compared to the vehicle-administered control (1·3- and 2·2-fold, respectively), and c-fos expression was highest in the AM 251-administered experimental group (P < 0·05; Fig. 4).
Discussion
Under the present experimental conditions, and in a similar way to what has been reported by other authors(Reference Currie and Coscina19, Reference Orosco, Rouch and Dauge20), the mechanism involving central hypothalamic control of hunger and satiety is responsive to food intake and takes place in parallel to an increment in the levels of 5-HT(Reference Fetissov, Meguid and Chen21), which probably contributes to the satiation signal. CB1 receptors have been detected not only in the hypothalamus(Reference Gonzalez, Manzanares and Berrendero22), but also in other brain areas involved in the control of food intake, including the nucleus accumbens shell(Reference Robbe, Kopf and Remaury23). Recent findings reported by Sano et al. (Reference Sano, Mishima and Koushi24) demonstrated that Δ9-tetrahydrocannabinol, which usually stimulates the feeding behaviour, reduces 5-HT in the nucleus accumbens. Taken together, these results also support the hypothesis regarding quantifiable changes in palatable food self-administration caused by the interaction between cannabinoids and 5-HT receptor system as described previously by Jane et al. (Reference Jane, Lefever and Jackson25), although they do not specifically identify the mechanism of action underlying these effects. Nevertheless, the orexigenic action induced by WIN 55,512-2 (1 μg/5 μl) 1 h after the icv administration, which lasted for up to 24 h, is indicative per se of the active participation of hypothalamic nuclei in the regulation of food intake carried out via stimulation of central CB1 receptors. Furthermore, the in vivo microdialysis study may contribute to a better understanding of this feeding behaviour, given that a marked reduction in the extracellular levels of 5-HT and 5HIAA was observed after the CB1 receptor agonist administration. The reduction in 5-HT and 5HIAA release was sharp and persisted for the entire experimental period, although no exact relationship was observed between the changes found in the levels of 5-HT and 5HIAA and the degree of hyperphagia, a finding that suggests the possible participation of other neurotransmitters in this feeding behaviour, or the potential desensitization of CB1 receptors during the experimental period. Recently, Lau & Schloss(Reference Lau and Schloss26) have shown that cannabinoids can regulate the serotonergic neurotransmission not only by inhibition of 5-HT release but also by inhibition of 5-HT re-uptake. Under the present experimental conditions, icv administration of WIN 55,212-2 caused a rapid return of 5-HT levels to their basal values, and, therefore, may have prompted the induction of a hunger sensation sooner compared to the vehicle-injected control, thus increasing the amount of food ingested. A greater reduction in 5-HT levels caused by WIN 55,512-2 could have been prevented by the extensive metabolization of the CB1 receptor agonist during the course of its action.
There is no agreement in the scientific literature with regard to the central effect of CB1 receptor agonists on feeding behaviour, given that some authors(Reference Gómez, Navarro and Ferrer12) did not find signs of hyperphagia after central administration of WIN 55,212-2 at doses of 0·4, 2 and 10 μg/5 μl, whereas others did find a significant improvement in food intake after central administration of anandamide(Reference Jamshidi and Taylor27). The experimental conditions of the present study have permitted a more precise study of the orexigenic effect of WIN 55,212-2 administration to presatiated rats, and are in agreement with a previous study(Reference Aaron, Iain and Gregorb28) that reported a stimulated feeding behaviour after intrahypothalamic administration of Δ9-tetrahydrocannabinol. On the other hand, it has been demonstrated that cannabinoids not only induce hyperphagia but also increase the desire to consume highly palatable food in man(Reference Abel29). Therefore, cannabinoid consumption stimulates appetite with a concomitant effect on food selection(Reference Cota, Marsicano and Tschop3). In addition to those effects, a possible role for the endogenous cannabinoid system in feeding motivation has also been suggested(Reference Williams, Rogers and Kirkham18).
The present results show that hyperphagia induced after the ipt administration of WIN 55,212-2 at doses of 0·5, 1 and 2 mg/kg was apparent shortly after injection of the CB1 receptor agonist, but did not maintain a dose–effect relationship for that range of drug concentrations administered. Furthermore, upon administration of the 0·5 mg/kg dose, hyperphagia receded 2 h after injection; after that time-point, food intake decreased temporarily to values below those measured in the vehicle-injected control animals. The present finding suggests that the drug was extensively metabolized during the 2 h post-injection period. Thus, the brief effect attained by the 0·5 mg/kg dose can be attributed to the low blood levels of this CB1 agonist, and the quick metabolization process of the drug.
When WIN 55,212-2 was administered at doses of 1 and 2 mg/kg, hyperphagia was apparent from 1 to 6 h after injection, with no significant differences being found in comparison to the vehicle-injected control group when food intake was expressed as non-cumulative values. The more extended effect of the doses mentioned earlier in comparison to the 0·5 mg/kg dose suggests that a longer time is needed to catabolize them, and so the blood levels remain high enough to prevent the expected decrease in food intake resulting from the metabolization of the CB1 receptor agonist.
The anorexigenic effect caused by administration of high doses of the CB1 receptor agonist WIN 55,212-2 (5 mg/kg) was maintained for up to 6 h and tended to recede 6–24 h after administration of the drug; this anorexigenic effect can be attributed to the motor impairment that may have masked the activation mechanisms involved in the stimulation of food intake. In fact, experiments that evaluated the effect of WIN 55,212-2 on motor behaviour in the open field showed that the administration of high doses of the CB1 agonist (5 mg/kg) led to a significant decrease in locomotor activity (Fig. 3). This effect of WIN 55,212-2 can also be ascribed to the exploratory conduct associated with locomotion. The inhibition of motor activity is not only reflected in locomotion, but also in the inability to start any movement, which leads to catalepsy(Reference Dewey30). Gómez et al. (Reference Gómez, Navarro and Ferrer12) have confirmed these effects of WIN 55,212-2 using a higher dose (10 mg/kg) than that administered in the present study. In this regard, it is worth pointing out the large number of CB1 receptors in the cerebellum and basal ganglia, a fact which suggests the existence of an endocannabinoid system that takes part in the regulation of motor activity(Reference Rodríguez de Fonseca, Del Arco and Martín-Calderón31) and may, at the same time, affect feeding behaviour.
The acute inhibitory effect of AM 251 on food intake when administered by icv or ipt injection (Tables 1 and 3) confirms recent bibliographic data regarding the anorectic effect of this CB1 receptor inverse agonist. Chambers et al. (Reference Chambers, Sharkey and Koopmans15), Zhou & Shearman(Reference Zhou and Shearman32) and Shearman et al. (Reference Shearman, Rosko and Fleischer33) have reported a decrease in food intake after administration of AM 251, whereas McLaughlin et al. (Reference McLaughlin, Winston and Limebeer34) found this inhibitory effect on food intake to be dose-dependent. Nevertheless, under the present experimental conditions, a dose-dependent effect was only observed when the 5 mg/kg experimental group was compared to those injected with 0·5, 1 and 2 mg/kg, but not when the latter experimental groups were compared amongst each other. Other authors(Reference Rinaldi-Carmona, Barth and Heaulme35) have reported that ipt administration of the selective CB1 receptor antagonist SR141716 caused a reduction in food intake via interference with endogenously released endocannabinoids like anandamide or 2-arachidonoyl glycerol(Reference Arnone, Maruani and Chaperon36–Reference Cani, Montoya and Neyrinck40). However, McLaughlin et al. (Reference McLaughlin, Winston and Limebeer34) suggested that in addition to the CB1 receptor blocking effect, the suppression of food intake by the administration of AM 251 was related to the appearance of secondary collateral effects of the drug, which induced emesis.
The anorectic effects of AM 251 observed under the experimental conditions of the present study are also in agreement with what has been reported by Gómez et al. (Reference Gómez, Navarro and Ferrer12) using the CB1 receptor antagonist SR141716A. These authors found a dose-dependent inhibition of food intake after the drug administration, which they attributed exclusively to the blockade of peripheral receptors, given that no effect was found when the CB1 receptor antagonist was injected centrally using different doses (0·1, 0·4, 2 and 10 μg/5 μl) in contrast to what has been observed under the experimental conditions of the present study using AM 251 at 0·1 μg/5 μl. The anorectic effect of AM 251 on presatiated rats would be carried out via significant activation of 5-HT-releasing neurons; a finding that was reflected in significantly higher levels of this neurotransmitter being released.
WIN 55,212-2 and AM 251 induced changes in the fos-immunoreactive cells when compared to vehicle-administered controls. The present findings support the hypothesis that one of the neurochemical mechanisms underlying the feeding effects of WIN 55,212-2 and AM 251 could be related to their ability to differentially affect the functionality of serotonine-releasing neurons. Murillo-Rodríguez et al. (Reference Murillo-Rodríguez, Vázquez and Millán-Aldaco41) have reported cannabinoid-induced changes in the fos-immunoreactive cells in some brain areas, including hypothalamic nuclei. The present results showing an enhanced c-fos expression following synthetic cannabinoid administration suggest a CB1 receptor-mediated effect, and are consistent with the findings reported by Patel & Hillard(Reference Patel and Hillard42) who showed that increases in c-fos expression in A10 dopaminergic neurons following the synthetic cannabinoid CP55940 administration result from excitatory noradrenergic transmission.
Most papers published so far addressing the interaction between the endocannabinoid system and 5-HT in the brain have used systemic administration of drugs, making it difficult to interpret the data in terms of site of action(Reference Egashira, Mishima and Katsurabayashi43–Reference Tzavara, Davis and Perry45). The present results corroborate the role of hypothalamus in the orexigenic effect of cannabinoids, as well as the ability of cannabinoids to affect the activity of 5-HT-releasing neurons. However, it is not possible to conclude from the results of the present study whether the effect of WIN 55,212-2 on 5-HT release was caused via direct action of cannabinoids on the 5-HT-releasing neurons, or by an intermediate factor that alters c-fos expression and liberation of this neurotransmitter. The mechanisms of these functional interactions might be of multiple nature, although the present data seem to point out to a direct control of 5-HT release by CB1 receptors as a possible pathway. In the medium-term perspective, new approaches should be envisaged for the best characterization of 5-HT/CB1 interactions, as it is necessary to identify the mechanisms underlying synergistic cannabinoid–5-HT interactions. Therefore, further investigation is warranted to determine whether the observed synergy applies to other systems. In this regard, cannabinoids can represent a promising therapeutic strategy for the treatment of diseases related to alterations of food intake and body weight control, which involve both the endocannabinoid and the serotoninergic systems.
Acknowledgements
We want to thank Rosa Jiménez Llamas for skilful technical assistance. There is no conflict of interest that the authors should disclose. The sources of funding were Projects AM34/04 and AGR 02 704 from Junta de Andalucía, Spain, and PROTARS III D14/47 from CNRST, Morocco. I. M. did the biological experiments, chemical analysis and data analysis, M. E. designed the study and participated in the discussion of the results and drafting of the paper, H. H. did the biological experiments, chemical analysis and data analysis, G. U. designed the study and participated in the discussion of the results and drafting of the paper, J. M. P. did the biological experiments, chemical analysis, data analysis, discussion of the results and drafting of the paper, P. A. did the biological experiments, chemical analysis, data analysis, discussion of the results and drafting of the paper, J. L. designed the study and participated in the discussion of the results and drafting of the paper, M. L.-J. co-ordinated the scientific team of this paper, designed the study and participated in the discussion of the results and drafting of the paper.









