Introduction
The diagnosis of a psychotic illness such as schizophrenia or bipolar disorder is associated with a reduced life expectancy of 15–25 years, mostly due to increased cardiovascular deaths (Saha et al. Reference Saha, Chant and McGrath2007; Kilbourne et al. Reference Kilbourne, Morden, Austin, Ilgen, McCarthy, Dalack and Blow2009; Brown et al. Reference Brown, Kim, Mitchell and Inskip2010; Chang et al. Reference Chang, Hayes, Perera, Broadbent, Fernandes, Lee, Hotopf and Stewart2011). A recent meta-analysis has demonstrated that the pooled relative risk of mortality among those with psychoses is 2.54 (2.35–2.75) times that of the general population, and higher rates of mortality for those with psychoses when compared with depressive and anxiety disorders (Walker et al. Reference Walker, McGee and Druss2015). Cardiometabolic risk factors are common in psychosis (McEvoy et al. Reference McEvoy, Meyer, Goff, Nasrallah, Davis, Sullivan, Meltzer, Hsiao, Scott Stroup and Lieberman2005; Leucht et al. Reference Leucht, Burkard, Henderson, Maj and Sartorius2007; Osborn et al. Reference Osborn, Levy, Nazareth, Petersen, Islam and King2007; De Hert et al. Reference De Hert, Dekker, Wood, Kahl, Holt and Moller2009; Kilbourne et al. Reference Kilbourne, Morden, Austin, Ilgen, McCarthy, Dalack and Blow2009). A recent meta-analysis of international studies reported prevalence rates of 44% for central obesity, 19.5% for hyperglycaemia, 10.9% for diabetes, 39% for hypertension and 39% for dyslipidaemias, with rates increasing with age (Mitchell et al. Reference Mitchell, Vancampfort, Sweers, van Winkel, Yu and De Hert2013).
Clustering of cardiometabolic risk factors is termed the metabolic syndrome (MetS) (Alberti et al. Reference Alberti, Zimmet and Shaw2005). Individuals with the MetS have a 3- to 6-fold increased risk of developing type 2 diabetes mellitus (Hanley et al. Reference Hanley, Karter, Williams, Festa, D'Agostino, Wagenknecht and Haffner2005; De Hert et al. Reference De Hert, Correll, Bobes, Cetkovich-Bakmas, Cohen, Asai, Detraux, Gautam, Moller, Ndetei, Newcomer, Uwakwe and Leucht2011) and a 2- to 6-fold risk of mortality due to cardiovascular disease (CVD) (Hanley et al. Reference Hanley, Karter, Williams, Festa, D'Agostino, Wagenknecht and Haffner2005). On meta-analysis, a third of patients with schizophrenia have the MetS, with this proportion increasing with duration of illness (Mitchell et al. Reference Mitchell, Vancampfort, Sweers, van Winkel, Yu and De Hert2013). Slightly higher MetS rates of 34% are seen in multi-episode patients (Vancampfort et al. Reference Vancampfort, Wampers, Mitchell, Correll, De Herdt, Probst and De Hert2013b ) while the deficit syndrome (i.e. negative symptoms of psychosis that are present as enduring traits) in schizophrenia is in itself associated with higher CVD risk (Arango et al. Reference Arango, Bobes, Kirkpatrick, Garcia-Garcia and Rejas2011). Similar rates of the MetS have been observed in bipolar disorder (37%) (Vancampfort et al. Reference Vancampfort, Vansteelandt, Correll, Mitchell, De Herdt, Sienaert, Probst and De Hert2013a ) and schizo-affective disorder (27–42%) (Basu et al. Reference Basu, Brar, Chengappa, John, Parepally, Gershon, Schlicht and Kupfer2004; Bobes et al. Reference Bobes, Arango, Aranda, Carmena, Garcia-Garcia and Rejas2012), although recently the Second Australian National Survey of Psychosis reported higher rates of 61% in those with psychotic disorders, suggesting an increasing prevalence with time (Morgan et al. Reference Morgan, McGrath, Jablensky, Badcock, Waterreus, Bush, Carr, Castle, Cohen, Galletly, Harvey, Hocking, McGorry, Neil, Saw, Shah, Stain and Mackinnon2014). It is possible to prevent the emergence of CVD risk in psychosis with the use of psychosocial interventions in the short term, though this is not sustained once the intervention is discontinued (Alvarez-Jimenez et al. Reference Alvarez-Jimenez, Martinez-Garcia, Perez-Iglesias, Ramirez, Vazquez-Barquero and Crespo-Facorro2010). Strategies do exist to manage established cardiometabolic risk factors (Lester et al. Reference Lester, Shiers, Rafi, Cooper and Holt2014), but despite this, a recent UK national audit reported screening rates for metabolic side effects of antipsychotic medication of only 11% (Barnes et al. Reference Barnes, Paton, Cavanagh, Hancock and Taylor2007). This is further compounded by the low treatment rates of identified cardiometabolic risk factors in psychosis (Nasrallah et al. Reference Nasrallah, Meyer, Goff, McEvoy, Davis, Stroup and Lieberman2006). The recent Schizophrenia Commission Report (UK) highlighted the disparity of care for individuals with psychosis and emphasized the need for improved physical health intervention (Schizophrenia Commission, 2012). Encouragingly, various UK medical Royal colleges have recently agreed joint management strategies for CVD risk in psychotic illnesses (Lester et al. Reference Lester, Shiers, Rafi, Cooper and Holt2014).
In this study we aimed:
-
(1) To determine the prevalence of cardiometabolic risks factors and establish the proportion of people with psychosis meeting the International Diabetes Federation (IDF) criteria for the MetS.
-
(2) To identify the key lifestyle behaviours associated with increased risk of the MetS.
-
(3) To investigate whether the presence of the MetS is associated with illness severity and degree of functional impairment.
Method
Study design
This study used baseline data from 450 randomly selected patients with established (multi-episode) psychosis recruited as part of the National Institute for Health Research-funded study: Improving Physical Health and Reducing Substance Use in Severe Mental Illness; a randomized controlled trial (IMPaCT RCT) (Gaughran et al. Reference Gaughran, Stahl, Ismail, Atakan, Lally, Gardner-Sood, Patel, David, Hopkins, Harries, Lowe, Orr, Arbuthnot, Murray, Greenwood and Smith2013).
Setting
The study took place in community mental health teams (CMHTs) in five Mental Health NHS Trusts, covering urban (Lambeth, Southwark, Lewisham, Croydon, Greenwich, Bexley, Bromley) and rural (Staffordshire, Somerset and Sussex) boroughs across England (Gaughran et al. Reference Gaughran, Stahl, Ismail, Atakan, Lally, Gardner-Sood, Patel, David, Hopkins, Harries, Lowe, Orr, Arbuthnot, Murray, Greenwood and Smith2013).
Subjects
Potential participants were identified through their care-coordinator. All care-coordinators in participating CMHTs were approached in a random sequence and invited to participate. Patients on each participating care coordinator's caseload, meeting the inclusion criteria, were likewise approached in a random order. These patients were entered into a random numbers generator to create a randomly ordered list from which to approach potential participants. Researchers then approached these patients sequentially to participate in the RCT. In situations where a patient did not wish to take part in the study or was uncontactable, the researcher selected another patient from the list in that random order.
The inclusion criteria were as follows: aged between 18 and 65 years old; a primary diagnosis of a psychotic illness [International Classification of Diseases (ICD)-10 diagnosis: F20–29, including schizophrenia, schizo-affective disorder, bipolar affective disorder (BPAD) and delusional disorder, F31.2, F32.3 and F33.3]. The exclusion criteria included: a primary diagnosis of intellectual disability (as defined by ICD-10 codes F70–F79 for intellectual disabilities); a first episode of psychosis (FEP); a primary substance misuse disorder (excluding cigarettes); a serious physical illness that could independently have an impact on metabolic measures; pregnant or up to 6 months postpartum; and receiving intensive input for a medical or terminal condition. Of patients screened, 39% (1043/2663) met the inclusion criteria, with the majority excluded for not meeting the diagnostic inclusion criterion (though the exact percentage excluded by each exclusion criterion is not known).
Clinical and sociodemographic variables
Sociodemographic and clinical data including gender, age, ethnicity, diagnosis and self-reported duration of illness were collected. Diagnoses were based on ICD-10 diagnostic criteria and were extracted from the documented diagnosis in the clinical notes at the time of recruitment. Participants’ mental health status was measured using the Positive And Negative Syndrome Scale (PANSS; Kay et al. Reference Kay, Opler and Lindenmayer1989), the Global Assessment of Functioning (GAF; American Psychiatric Association, 2002) and the Montgomery–Åsberg Depression Rating Scale (MADRS; Montgomery & Åsberg, Reference Montgomery and Åsberg1979). Current alcohol, smoking and cannabis use was recorded using the following measures: the Alcohol Use Disorders Identification Test (AUDIT; Saunders et al. Reference Saunders, Aasland, Babor, DeLaFuente and Grant1993); the Nicotine Dependence Questionnaire (Fagerstrom, Reference Fagerstrom1978; Fagerstrom & Schneider, Reference Fagerstrom and Schneider1989); Timeline Follow-Back (Sobell & Sobell, Reference Sobell, Sobell, Litten and Allen1992); and a urine drug screen. Total scores were derived from individual scale items. If any individual items were missing, then the total score was treated as missing. Data for all measures were collected through face-to-face interviews conducted by trained research assistants.
Cardiometabolic risk factors
Height, weight and blood pressure (BP) were measured using standardized techniques. The BP values presented are the second BP measurements, taken after an interval of 5 min. Waist circumference was measured at the umbilicus with the patient standing. As both serum glucose and triglycerides are affected by recent food ingestion, we took fasting blood samples.
Obesity was defined using the World Health Organization reference standard [body mass index (BMI) ≥ 30 kg/m2]. Diabetes was diagnosed based on measured glycated haemoglobin (HbA1c) of ≥48 mmol/mol (≥6.5%) or fasting glucose ≥7.0 mmol/l or a prior diagnosis of diabetes. In addition, in keeping with recent guidance, an HbA1c of 42–46 mmol/mol (6–6.4%) was taken to indicate glucose dysregulation or a high risk of diabetes.
The IDF definitions of other cardiometabolic risk factors and of the MetS were used (see Table 1) (Alberti et al. Reference Alberti, Zimmet and Shaw2005, Reference Alberti, Zimmet and Shaw2006). However, we extended the definition of the MetS to include an HbA1c value ≥42 mmol/mol (≥6%), as this is consistent with a raised fasting plasma glucose ≥5.6 mmol/l (The International Expert Committee, 2009; Lester et al. Reference Lester, Shiers, Rafi, Cooper and Holt2014).
Table 1. Diagnostic criteria for the metabolic syndrome (International Diabetes Federation criteria) (Alberti et al. Reference Alberti, Zimmet and Shaw2006)
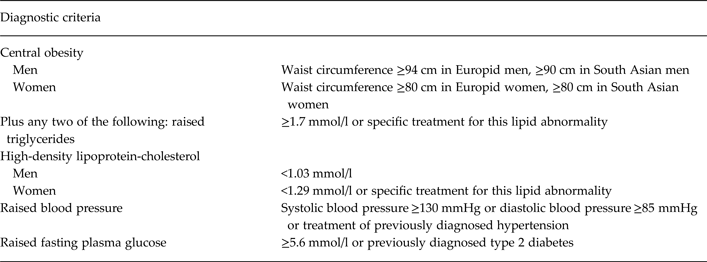
Additionally, we also determined if each patient met the adapted National Cholesterol Education Program (NCEP) Third Adult Treatment Panel (ATP-III) criteria (NCEP ATP-III criteria) (Grundy et al. Reference Grundy, Cleeman, Daniels, Donato, Eckel, Franklin, Gordon, Krauss, Savage, Smith, Spertus and Costa2005), to allow for comparison with selected studies. The ATP-III classification requires three of five criteria to be met for a diagnosis of the MetS and an abnormal waist circumference is not obligatory. In comparison with the IDF MetS criteria, the ATP-III criteria have a higher threshold for abnormal waist circumference, with 102 cm for males and 88 cm for females being the cut-offs. The other four criteria are comparable with the IDF criteria.
Hypertension was defined on the basis of a measured BP of either a systolic measure of 130 mmHg and/or a diastolic measure of 85 mmHg or existing treatment with antihypertensive medication.
The Dietary Instrument for Nutrition Education (DINE; Roe et al. Reference Roe, Strong, Whiteside, Neil and Mant1994) was used to assess the dietary patterns of participants over the past week. Respondents were classified according to their saturated fat, unsaturated fat and fibre intake, based on self-reported consumption of various foods. The International Physical Activity Questionnaire was used to measure the intensity of physical activity (high, moderate, low) and the duration of physical activities over the previous week (Craig et al. Reference Craig, Marshall, Sjostrom, Bauman, Booth, Ainsworth, Pratt, Ekelund, Yngve, Sallis and Oja2003).
Statistical analysis
Statistical analyses were performed using the IBM Statistical Package for Social Sciences Statistics for Windows, version 20.0 (USA). Descriptive measures were used for the basic demographic and clinical variables as well as for variables relating to the evaluation of metabolic dysregulation. Student's t test for parametric data and the χ 2 test for categorical variables were employed. All statistical tests were two-sided and a p value ≤0.05 was considered statistically significant.
Ethical Standards
Ethical approval for this study was obtained from The Joint South London and Maudsley and The Institute of Psychiatry NHS Research Ethics Committee. Ethical approval was granted on 17 July 2009 (REC ref. no. 09/H080/41).
Results
The clinical characteristics of the study population are shown in Table 2. Mean age was 43.6 years (s.d. = 10.1) and mean duration of illness was 15.7 years (s.d. = 10.3); 57% (n = 257) were male. In respect of ethnicity, 55% (n = 239) were of Caucasian ethnicity, while 33% (n = 143) were of black African or black Caribbean ethnicity, with 7% (n = 27) of Asian ethnicity and 5% (n = 22) of mixed ethnicity.
Table 2. Clinical characteristics of the study population (n = 450)

s.d., Standard deviation; PANSS, Positive And Negative Syndrome Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; GAF, Global Assessment of Functioning.
Only 6% (n = 25) of the study population were not treated with antipsychotic medication. Of the participants, 30% (n = 127) were prescribed clozapine and 16% (n = 70) olanzapine. Of the participants, 75% (n = 321) were treated with second-generation (atypical) antipsychotics (including clozapine and olanzapine), 16% (n = 71) were treated with first-generation antipsychotics and 8% (n = 36) were treated with a combination of antipsychotics.
Cardiovascular risk factors (see Table 3)
Of the subjects, 48% (192/398) were obese (BMI > 30 kg/m2). Female patients had significantly higher BMIs than males [t = 2.329, degrees of freedom (df) = 426, p < 0.020].
Table 3. Cardiovascular risk factors and prevalence rates by gender

CI, Confidence interval; s.d., standard deviation; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; BP, blood pressure.
In all, 83% had abdominal obesity, with 95% (160/169) of females meeting the IDF criterion for central obesity, significantly higher than males (74%, n = 167/227) (χ 2 = 29.994, p < 0.0001). Of the participants, 89% (305/342) had evidence of dyslipidaemia, defined as the presence of at least one abnormal lipid parameter (high total cholesterol, low high-density lipoprotein-cholesterol, high triglyceride) (n = 233) or treatment with lipid-lowering drugs (n = 72). Sixty-six people out of the 330 (20%) for whom we had data regarding either previous diagnosis of diabetes mellitus (n = 38) and/or had relevant blood results (n = 28) met the criteria for type 2 diabetes mellitus. An additional 85/286 (30%) had evidence of glucose dysregulation on their blood tests. Hypertension was present in 214/398 (54%), of whom 62 were on anti-hypertensive therapy. Nearly half (49%, n = 195/398) were hypertensive on measurement. Of the subjects, 62% (268/432) smoked tobacco, smoking an average of 18.2 (s.d. = 11.6) cigarettes per day.
There was no significant difference between mean BMI (p = 0.241) and waist circumference (p = 0.437) when stratified by geographical location (by borough).
The prescription of either olanzapine or clozapine (n = 191) (which are the antipsychotic medications traditionally associated with the highest risk of metabolic dysfunction) was not associated with an increased rate of obesity (n = 80, 42%) compared with those not prescribed either dibenzodiazepine (n = 113, 54%) (χ 2 = 1.270, df = 1, p = 0.153). When clozapine alone (n = 117) was compared with other antipsychotics (excluding olanzapine), no excess in obesity was found on clozapine (47%, n = 55) versus other antipsychotics (52%, n = 99) (χ 2 = 0.675, p = 0.241). Also, there was no excess of other cardiometabolic risk factors [dyslipidaemia (p = 0.418), hypertension (p = 0.546) and diabetes (p = 0.463)].
Ethnicity
Those of white ethnicity had larger mean waist circumferences (mean = 108.5 cm, s.d. = 17.0) than those of black African or Caribbean ethnicity (mean = 104.4 cm, s.d. = 16.9) (t = 2.225, df = 345, p = 0.027). White patients had higher serum triglycerides than black African or Caribbean patients (mean difference = 0.89, p < 0.003). However, there were no significant differences in overall rates of dyslipidaemia (p = 0.545), hypertension (p = 0.101), diabetes (p = 0.298) and obesity (BMI > 30 kg/m2) (p = 0.140). By gender, females of black African or Caribbean ethnicity had significantly higher rates of obesity (n = 28/37) as measured by BMI than females of white ethnicity (n = 35/63) (χ 2 = 4.048, p = 0.035).
MetS
Of the 450 participants, there were 308 patients with complete laboratory and clinical measures to allow MetS status to be confirmed. In this group, the prevalence of IDF MetS was 56.8% (n = 175/308). The prevalence of the MetS as defined by the ATP-III criteria was 56.2% (n = 173/308). Those with IDF MetS were older (mean age = 44.68 years, s.d. = 9.8) than those without IDF MetS (42.12 years, s.d. = 10.4) (t = −2.28, p = 0.028). There was no difference in duration of illness (p = 0.358), or the use of clozapine (p = 0.282) or olanzapine (p = 0.303) in those with IDF MetS compared with those without. White patients had higher rates of IDF MetS (n = 114/171) than those of black African or Caribbean ethnicity (n = 47/101) (χ 2 = 10.654, p = 0.001), with this most pronounced in white males who had significantly higher rates of IDF MetS (n = 66/96) than males of black African or Caribbean heritage (n = 29/63) (χ 2 = 8.143, p = 0.004).
Of the 13 individuals who were not prescribed antipsychotics and who had complete measures to allow for MetS status to be confirmed, eight met the criteria for IDF MetS.
Psychopathology and cardiometabolic risk factors (see Table 4)
A consistent relationship was found between cardiometabolic risk factors and functional impairment as measured by GAF scores. Degree of psychosis as measured by the PANSS and subscale scores was not significantly associated with any individual cardiometabolic risk factor. The only individual cardiometabolic risk factor associated with depressive symptomatology (MADRS) was obesity (p = 0.032)
Table 4. Comparison of cardiovascular risk factors with clinical characteristics

Data are given as mean (standard deviation).
PANSS, Positive And Negative Syndrome Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; GAF, Global Assessment of Functioning.
* p < 0.05.
There was no relationship between duration of psychotic illness and any of obesity (BMI > 30 kg/m2) (t = 0.410, df = 329, p = 0.682), hypertension (t = −1.346, p = 0.179), dyslipidaemia (t = −0.144, p = 0.886) or presence of diabetes mellitus (t = −0.992, df = 251, p = 0.322).
Drug and alcohol use
Of the respondents, 28% (n = 68/291) had hazardous/harmful alcohol use (AUDIT score ≥ 8), while the alcohol intake of a further 4% (n = 12/291) indicated the need for diagnostic evaluation for alcohol dependence syndrome (AUDIT score ≥ 20).
Of the respondents, 18% (n = 76/422) were current cannabis users, with 52 people identified through the Timeline Follow-Back procedure and a further 24 identified through a cannabinoid-positive urinary drug screen.
Other substance use
Of the participants, 3% (n = 13/439) were cocaine or amphetamine users, 1.4% (n = 6) reported ongoing crack cocaine use and less than 1% (n = 3) reported using ecstasy.
Lifestyle measures
Of the participants, 23% (102/435) had a high saturated fat intake score on the DINE, 29.7% (129/435) had a medium fat intake, while 47% (204/435) had a low saturated fat intake; 51% of the participants (n = 210) had a low fibre intake, with only 25% (n = 102) reporting a high fibre intake.
Of the participants, 44% (n = 196) were engaging in low-intensity physical activity, 44% (n = 200) engaged in moderate-intensity physical activity, while 12% (n = 54) participated in high-intensity physical activity. Engaging in high levels of physical activity was associated with lower rates of obesity (χ 2 = 20.944, p < 0.0001) and dyslipidaemia (χ 2 = 17.537, p = 0.001) compared with low-intensity physical activity. Further, lower rates of the MetS were found in those with high-intensity physical activity (n = 15/41), compared with those engaging in low-intensity physical activity (n = 160/267) (χ 2 = 7.891, p = 0.004).
Discussion
This cohort of individuals with established psychosis had a high prevalence of both individual cardiometabolic risk factors and of the MetS. Rates of central obesity were alarmingly high at 83%, with almost all women (95%) exceeding the IDF threshold. Half the sample were obese, half had hypertriglyceridaemia and 54% were hypertensive. Of the study population, 50% had evidence of glucose dysregulation, including 20% of the sample with type 2 diabetes, which is much higher than the rate in the general UK population (International Diabetes Federation, 2013). This excess is amplified when an aged-matched comparison is used; the expected prevalence of diabetes in UK residents with an age range from 35 to 54 years would be 9.4% for men and 6.6% for women (Craig & Mindell, Reference Craig and Mindell2011).
Of our participants, 56.8% met criteria for the MetS, greatly exceeding the international figure of 34% described in a recent meta-analysis of the MetS in schizophrenia (Mitchell et al. Reference Mitchell, Vancampfort, Sweers, van Winkel, Yu and De Hert2013). This meta-analysis found a lack of homogeneity in the distribution of MetS prevalence across individual studies. Similar rates of cardiometabolic risk to what we found have been identified in a large Australian prevalence survey in psychosis, where rates of abdominal obesity greater than 80% were found, as well as comparable rates of the MetS, dyslipidaemia and hypertension (Galletly et al. Reference Galletly, Foley, Waterreus, Watts, Castle, McGrath, Mackinnon and Morgan2012). Our prevalence rates thus place this UK population at the higher end globally for cardiometabolic risk factors in psychosis. Given that UK general population studies have identified rates of the MetS of 25–34%, the almost doubled rate in psychosis is striking (Khunti et al. Reference Khunti, Taub, Tringham, Jarvis, Farooqi, Skinner and Davies2010; Langan et al. Reference Langan, Seminara, Shin, Troxel, Kimmel, Mehta, Margolis and Gelfand2012) and behoves us to investigate the antecedents and remedies. Consistent with general population studies, but in contrast to some cross-sectional studies in psychosis, we found no gender difference in the rates of the MetS (Ford, Reference Ford2005), although we did find higher rates of obesity (as defined by BMI) in females (61% v. 42%) (McEvoy et al. Reference McEvoy, Meyer, Goff, Nasrallah, Davis, Sullivan, Meltzer, Hsiao, Scott Stroup and Lieberman2005; Bobes et al. Reference Bobes, Arango, Aranda, Carmena, Garcia-Garcia and Rejas2007). There were ethnic differences in rates of the MetS, with those of white ethnicity showing higher rates compared with those of black African or Caribbean ethnicity, in keeping with previous work (McEvoy et al. Reference McEvoy, Meyer, Goff, Nasrallah, Davis, Sullivan, Meltzer, Hsiao, Scott Stroup and Lieberman2005; Keenan et al. Reference Keenan, Yu, Cooper, Appel, Guallar, Gennusa, Dickerson, Crum, Anderson, Campbell, Young and Daumit2013), results which have not been consistently demonstrated in the general population (Keenan et al. Reference Keenan, Yu, Cooper, Appel, Guallar, Gennusa, Dickerson, Crum, Anderson, Campbell, Young and Daumit2013).
Lifestyle choices amplify the potential consequences of abnormal metabolic parameters in this population: 62% were smokers, hugely in excess of the current smoking rates in the general UK population of 20% (Health & Social Care Information Centre, 2013). A recent 11-year follow-up of 517 people with schizophrenia showed an overall standardized mortality ratio of 2.80, smoking being a very strong predictor of death (relative risk of 4.66) (Dickerson et al. Reference Dickerson, Stallings, Origoni, Schroeder, Khushalani and Yolken2014). Smoking not only increases the risk of early death, but also complicates treatment, in that the hydrocarbons in cigarette smoke accelerate the metabolism of dibenzodiazepines, clozapine and olanzapine (Rostami-Hodjegan et al. Reference Rostami-Hodjegan, Amin, Spencer, Lennard, Tucker and Flanagan2004). Until recently, clinicians have shown little interest in supporting smoking cessation in psychosis patients. However, findings from a systematic review have demonstrated that people with schizophrenia want to stop smoking and that it is possible to help them to do so (Banham & Gilbody, Reference Banham and Gilbody2010) and it is logical that this should now be a priority public health measure.
Lack of exercise was common among the study population, with only 12% of individuals participating in high-intensity physical activity, consistent with previous reports (Faulkner et al. Reference Faulkner, Cohn and Remington2006), with high-intensity physical activity associated with lower rates of obesity and dyslipidaemia. As exercise is linked to greater longevity in the general population (Paffenbarger et al. Reference Paffenbarger, Hyde, Wing, Lee, Jung and Kampert1993; Manson et al. Reference Manson, Greenland, LaCroix, Stefanick, Mouton, Oberman, Perri, Sheps, Pettinger and Siscovick2002), it is likely that greater emphasis on engaging in physical activity among those with severe mental illnesses (SMI) would help reduce mortality risk (Vancampfort et al. Reference Vancampfort, Knapen, Probst, van Winkel, Deckx, Maurissen, Peuskens and De Hert2010). This will require addressing the individual barriers to regular physical activity and promoting it within the structure of community treatment programmes (Vancampfort et al. Reference Vancampfort, Knapen, Probst, Scheewe, Remans and De Hert2012) but will also entail addressing wider organizational barriers to regular physical activity and other health promotion programmes (O'Brien et al. Reference O'Brien, Gardner-Sood, Corlett, Ismail, Smith, Atakan, Greenwood, Joseph and Gaughran2014).
We did not identify any associations between individuals’ psychopathology scores on PANSS and their cardiometabolic risk. However, we did find associations between increased functional impairment and obesity, diabetes and hypertension and between higher depression scores and obesity. Of note, in the US Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study, the PANSS mean total score of 75.9 was higher than in IMPaCT (51.2) – with lower NCEP ATP-III MetS rates of 36% in CATIE compared with the ATP-III MetS rate of 56% in IMPaCT, meaning that our higher rates of cardiometabolic risk are not a result of illness severity (Meyer et al. Reference Meyer, Nasrallah, McEvoy, Goff, Davis, Chakos, Patel, Keefe, Stroup and Lieberman2005).
The rate of the MetS in the BPAD subgroup was 66%, which is higher than previously reported rates of 22–30% (Garcia-Portilla et al. Reference Garcia-Portilla, Saiz, Benabarre, Sierra, Perez, Rodriguez, Livianos, Torres and Bobes2008; van Winkel et al. Reference van Winkel, De Hert, Van Eyck, Hanssens, Wampers, Scheen and Peuskens2008). This may relate more to service bias than diagnostic factors. Patients with bipolar disorder requiring ongoing secondary care management in the UK may have a greater clinical need than those managed in primary care and as we have demonstrated an effect of global function on cardiometabolic risk, this is one potential explanation, although the numbers were too small to test this hypothesis.
Despite the mean duration of illness being 15.7 years, we found no effect of duration of illness on the prevalence of the MetS, in contrast to the meta-analysis conducted by Mitchell et al. (Reference Mitchell, Vancampfort, Sweers, van Winkel, Yu and De Hert2013). Even though our study excluded, for pragmatic reasons, those people registered with early intervention services, which manage younger people for up to 3 years from first presentation, we would have expected to find an effect.
A high proportion of this sample was prescribed dibenzodiazepines, which are associated with early emergence of cardiometabolic risk factors (Howes et al. Reference Howes, Gaughran, Amiel, Murray and Pilowsky2004; Nielsen et al. Reference Nielsen, Skadhede and Correll2010). However, we found no differences in cardiac risk factors between those on clozapine or olanzapine and those not. One potential explanation is that the cardiometabolic effect plateaus and becomes more evenly distributed over a more prolonged course of illness. However, as we do not have access to treatment histories, it may also be that this homogeneity across medications reflects the cumulative effects of medication changes over the years. This lack of difference is especially interesting in light of the evidence that patients with schizophrenia treated with clozapine have the least reduction in life expectancy of all patients with schizophrenia (Tiihonen et al. Reference Tiihonen, Lonnqvist, Wahlbeck, Klaukka, Niskanen, Tanskanen and Haukka2009; Hayes et al. Reference Hayes, Downs, Chang, Jackson, Shetty, Broadbent, Hotopf and Stewart2014).
Strengths of this study include a sample of randomly selected individuals with psychosis from diverse ethnic backgrounds and the mix of urban and rural locations adding to the generalizability of the study findings. Limitations of the study include the cross-sectional design, and the lack of a control group. We have not adjusted for the effect of socio-economic deprivation. The sample was recruited from across England, but predominantly from Greater London, including some boroughs with high levels of deprivation, though we identified no difference in metabolic variables by borough. A recent Scottish paper noted an excess of mortality in SMI linked to deprivation, although this effect was not evident for deaths due to cerebrovascular disease or CVD (Langan Martin et al. Reference Langan Martin, McLean, Park, Martin, Connolly, Mercer and Smith2014). The missing data from metabolic measures and clinical assessment scales may have introduced a selection bias into the study, but it is not obvious what effect such a bias would result in. Measures such as duration of illness were self-reported and thus are non-validated. We have no data on duration of antipsychotic treatment and previous antipsychotic treatments used over the longitudinal course of an individual's illness, both of which are confounding factors that could potentially make an impact on an individual's cardiometabolic risk factors. The exclusion of FEP patients from this study recognizes the different CVD profiles in the early stages of psychosis. Data on the evolution of CVD risk factors from first presentation are being gathered as part of a different project within IMPaCT. To have included FEP data within this study would have underestimated the extent of the problem which patients with established psychosis, their carers and their clinicians face.
Because of the design of the study, individuals with established psychosis cared for by their general practitioners were not included in the study population. This group is estimated to make up 30% of all patients with SMI in the UK (Reilly et al. Reference Reilly, Planner, Hann, Reeves, Nazareth and Lester2012). Our study population, compared with this primary care-only population, may be functionally or symptomatically more impaired; and antipsychotic prescribing patterns may be different – for instance, clozapine is rarely prescribed in primary care. Thus the study's findings may not necessarily generalize to those patients living in the community who share these same diagnoses but who are not managed in secondary care.
Conclusion
The results from this study are alarming and draw attention to the huge rates of modifiable cardiometabolic risk factors in people with established psychosis in the UK. The prevalence of the MetS in people with established psychosis in this UK sample is 56.8%. The main defining measure of the MetS, waist circumference, exceeded the IDF diagnostic cut-off in 83% of our patients, and in 95% of female patients. Although central obesity is known to be the best predictor of morbidity and mortality (Zimmet et al. Reference Zimmet, Alberti and Shaw2001), waist circumference has not to date been routinely measured in people with psychosis, although it has now been included in the latest National Institute of Health and Clinical Excellence (2014) guidelines for schizophrenia. Practice in in-patient psychiatric services has improved with the advent of incentivization and of shared guidelines for CVD risk management in psychosis such as the Lester Cardiometabolic Health Resource (Lester et al. Reference Lester, Shiers, Rafi, Cooper and Holt2014). However, serious shortcomings remain in the management of physical health care in community-based patients, as evidenced by the National Audit of Schizophrenia findings (NAS, 2012; Crawford et al. Reference Crawford, Jayakumar, Lemmey, Zalewska, Patel, Cooper and Shiers2014). Nevertheless, more tailored work is needed if we wish to improve life expectancy in psychosis, with these findings reinforcing the need for routine monitoring of waist circumference in psychosis and assertive management of cardiovascular risk in people with psychosis.
Acknowledgements
The National Institute for Health Research funds the IMPaCT programme at King's College London and the South London and Maudsley NHS Foundation Trust (ref. RP-PG-0606-1049). The funding source(s) had no role in the design of this study and did not have any role during its execution, analyses, interpretation of the data, or decision to submit results. We affirm that the paper is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
The study is registered with ISRCTN (International Standard Randomised Controlled Trial Number) as ISRCTN58667926.
Declaration of Interest
R.M.M. has received payment for lectures including service on speakers’ bureaus for BMS, Janssen and AZ. J.E. is an international medical manager at and employee of H. Lundbeck. F.G. has received honoraria for advisory work and lectures from Roche, BMS, Lundbeck and Sunovion, and has a family member with professional links to Lilly and GSK. The other authors have no financial relationships with any organizations that might have an interest in the study in the previous 3 years. There are no other relationships or activities that could appear to have influenced the study.






