Introduction
The Middle Triassic was an exceptional time period during which, after the end-Permian mass extinction, several global factors determined an acceleration in the recovery and extensive radiation of biota (Erwin, Reference Erwin1996). This key process in the evolution of life ended with the replacement of the “Palaeozoic Fauna” with the “Modern Fauna” (Sepkopski, Reference Sepkopski1984; Márquez-Aliaga, Reference Márquez-Aliaga2010; Escudero-Mozo et al., Reference Escudero-Mozo, Márquez-Aliaga, Goy, Martín-Chivelet, López-Gómez, Márquez, Arche, Plasencia, Pla, Marzo and Sánchez-Fernández2015). Between recognized biotic elements of the Middle Triassic, “mysidaceans” were one of the dominant components with a probably high abundance in some geographical areas (Feldmann et al., Reference Feldmann, Schweitzer, Hu, Huang, Zhou, Zhang, Wen, Xie and Maguire2015, Reference Feldmann, Schweitzer, Hu, Huang, Zhou, Zhang, Wen, Xie, F.R. Schram and Wade2017).
The orders Mysida, Stygiomysida, and Lophogastrida, earlier referred to as the “Mysidacea,” consist of ~1200 described species and 187 genera found at the present across all latitudes throughout the waters of the world, with the majority of species inhabiting coastal and open-ocean waters (Meland et al., Reference Meland, Mees, Porter and Wittmann2015). The “Mysidacea” refers to a paraphyletic group of the aforementioned taxa, including also the members of the extinct Carboniferous/Permian order Pygocephalomorpha, all considered as relatively primitive carapaced peracarids (Taylor et al., Reference Taylor, Schram and Shen2001).
During the Carboniferous, two lineages of the “Mysidacea,” the Pygocephalomorpha and the Lophogastrida, first appeared in the fossil record (Schram, Reference Schram1986; Taylor et al., Reference Taylor, Schram and Shen2001). The order Pygocephalomorpha are among the most common of eumalacostracan crustaceans preserved in the near-shore and brackish-water communities of the late Paleozoic (Schram, Reference Schram1986). Pygocephalomorpha might be related to recent Mysida or, more likely, recent Lophogastrida, but there has been much disagreement as to the relationships among these three orders (Schram, Reference Schram1986; Taylor et al., Reference Taylor, Shen and Schram1998). The order Lophogastrida is represented in the fossil record from the Carboniferous to the Jurassic with an apparent long persistence of body form, a trend already noted in other living members of the order Mysida (Taylor et al., Reference Taylor, Schram and Shen2001). No fossils are known in the order Stygiomysida, but the actual distribution of this family follows the path of the former Tethyan Sea (Pesce and Iliffe, Reference Pesce and Iliffe2002), which extended during the Mesozoic from today’s Caribbean eastwards to the Indian Ocean (Meland et al., Reference Meland, Mees, Porter and Wittmann2015).
As opposed to the fossil records of Lophogastrida and the species-rich fossil order Pygocephalomorpha, few fossil Mysida have been identified to date. Since the known Mysida have a soft body, it is assumed that they are generally poorly predisposed for fossilization and, consequently, their fossil record depends on exceptional conditions of conservation and fossilization (Secretan and Riou, Reference Secretan and Riou1986).
The earliest fossils attributed to the Mysida were the species Elder ungulatus Münster, Reference Münster1839, and Francocaris grimmi Broili, Reference Broili1917, both from the Jurassic Solnhofen limestones of Bavaria (Germany). However, Schram (Reference Schram1986, p. 124) and Taylor et al. (Reference Taylor, Schram and Shen2001, p. 310) did not agree with this attribution. Indeed, they said that these taxa are “too poorly understood to permit an unqualified assignment.”
The oldest “true” mysida fossils have been dated as far back as the Middle Jurassic (Callovian, 163–166 Myr) from the deposits of la Voulte-sur-Rhône, Ardeche, France. In that fossil site, Secretan and Riou (Reference Secretan and Riou1986) described Siriella antiqua and S. carinata, both with amazing similarity to extant forms.
The partia1 reconstruction of specimen LH 343 initially attributed to the family Eryonidae (order Decapoda) by Sanz et al. (Reference Sanz, Wenz, Yebenes, Estes, Martinez-Delclos, Jimenez-Fuentes, Diéguez, Buscalioni, Barbadillo and Via1988) from the Lower Cretaceous of las Hoyas fossil site (Cuenca, Spain) led Rabadà (Reference Rabadà1990) to include it in the Mysida, but the lack of more and better specimens leaves its affiliation doubtful. With a habitus apparently similar to fossils described by Rabadà (Reference Rabadà1990), De Angeli and Rossi (Reference De Angeli and Rossi2006) described Mysidopsis oligocenicus from the lower Oligocene of NE Italy, although its generic status is not clear due to a suboptimal state of preservation (Wittmann et al., Reference Wittmann, Schlacher and Ariani2014). In addition, fossil mysid statoliths have been described in Miocene deposits of the brackish Paratethys (Voicu, Reference Voicu1974, Reference Voicu1981; Ionesi and Pascariu, Reference Ionesi and Pascariu2011) and in the Aquitanian basin (SW France) (Steurbaut, Reference Steurbaut1989).
Cartanyà (Reference Cartanyà1991) first described a crustacean found in the Triassic reservoir of the Pinetell (Montblanc, Spain) and identified it with certain reservations as a “mysidacean.” In this paper, a black and white photograph and a drawing with the trace of uropodal endopod statocysts are presented. A second “mysidacean” specimen was reported by Cartanyà (Reference Cartanyà1999b) without any reference to its morphology. Revision of the aforementioned fossil specimens allows us to re-describe in detail their morphological characteristics and ascribe them to a new genus and new species of the order Mysida.
This paper presents the results of this review as well as a discussion of the position of the new taxon between the different mysid families and subfamilies. The discovery of the fossil specimens from the Pinetell presented in this study extends the range of Mysida by ~70 Myr.
Stratigraphic and environmental settings
Marine conditions present in the Iberian region during the Triassic, especially during the late Anisian and the entire Ladinian, include a wide diversity of coastal and marine depositional environments (shores, lagoons, tidal flats, reefs, etc.) (López-Gómez et al., Reference López-Gómez, Arche and Pérez-López2002).
The Prades Mountains are located in the middle of the Catalonian Prelitoral Range, part of the Catalonian Coastal Ranges, and make up one of the most important physiographic units in the NE of the Iberian Peninsula. This unit is structured by a Paleozoic basement and a Mesozoic cover, which is composed for the most part of Triassic sediments (Cartanyà, Reference Cartanyà1999b). The main features of the European lithostratigraphic unit upper Muschelkalk in the eastern Prades Mountains are the presence of mound-reefs and of laminated dolomites in the Alcover unit filling the interreef depressions (Calvet and Tucker, Reference Calvet and Tucker1995; Tucker and Marshall, Reference Tucker and Marshall2004). The Alcover laminated dolomites unit is up to 70–80 m thick, and fills the inter-reef depressions with a sharp, undulating, and locally erosive unconformity. Stratigraphically it is dated as late Ladinian (middle Triassic) (Calvet et al., Reference Calvet, March and Pedrosa1987; Calvet and Tucker, Reference Calvet and Tucker1995), upper Muschelkalk, from 235–242 Myr (Lucas, Reference Lucas2010).
According to Esteban et al. (Reference Esteban, Calzada and Vía1977), the Alcover unit is composed of a clearly marine laminated dolomicrite. The uniformity of the lamination, its fine grain size, the absence of structures such as ripples, and presence of euhedral halite crystals, suggest a very calm and hypersaline depositional environment. Also, these authors suggested the following depositional sequence: (1) the Alcover unit was deposited in an anoxic basin, affected by sporadic currents (of density?) and evolving to hypersaline conditions; (2) it settled among preexisting depressions due to former bioherms; (3) the deposit was caused by decanting from muddy suspensions.
The Alcover unit has yielded an abundant Triassic fauna, traditionally considered as originating in a shallow, lagoonal basin within an extended reef complex (Fortuny et al., Reference Fortuny, Bolet, Sellés, Cartanyà and Galobart2011). The fossil assemblage is allochtonous and composed of floral and faunal groups such as land plants, jellyfishes, brachiopods, molluscs, arthropods, echinoderms, fishes, and reptiles (Villalta and Via, Reference Villalta and Via1966; Via and Villalta, Reference Via and Villalta1975; Beltan et al., Reference Beltan, Calzada, Via and Villalta1989; Carrasco, Reference Carrasco2012), but the reputation of these localities is based on the fossils being preserved in dolomicrite and their ichthyofaunal diversity (Cartanyà, Reference Cartanyà1999a, Reference Cartanyà1999b).
Via et al. (Reference Via, Villalta and Esteban-Cerdá1977) suggested a general parallel between sedimentological and paleobiological conditions of the Alcover unit and the well-known conditions of the Solnhofen-type limestones, based on several observations: the available paleontological collections show a clear predominance (79.6%) of nectonic organisms (e.g., fishes, reptiles, cephalopods, swimming decapods), and benthic organisms well represented by limulids, decapods, holothurians, crinoids, and brachiopods. In addition, there is a remarkable assemblage of semiterrestrial reptiles, insects, and several terrestrial plants. Algae, sponges, corals, bryozoans, gastropods, and echinoids are absent. The absence of burrowing organisms and the almost complete absence of fragmented or dislocated organisms suggest an allochtonous assemblage, passively transported by low-energy mechanisms, and an anoxic environment with strong salinity variations.
Materials and methods
Fossils were found in the El Pinetell outcrop of the Alcover unit (Fig. 1). A detailed description of this locality was presented by Cartanyà (Reference Cartanyà1993, Reference Cartanyà1994, Reference Cartanyà1999a, Reference Cartanyà1999b).
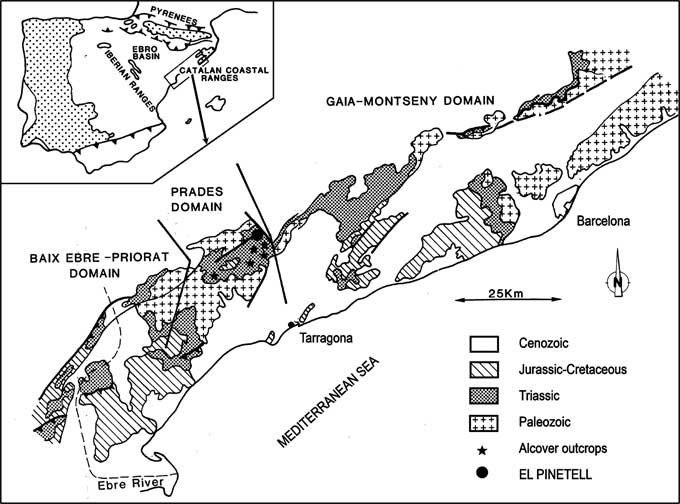
Figure 1 Locality map showing collection locality in the Alcover limestones unit of El Pinetell, in the district of Montblanc (Catalonia, Iberian Peninsula).
The analyzed individual, described as the holotype, was initially classified as an indeterminate mysidacean by Cartanyà (Reference Cartanyà1991, Reference Cartanyà1993, Reference Cartanyà1994). It is an epirelief in which the fossil protrudes from the surface. The main trace suggests that this animal was settled on the bottom with the ventral surface up. As a consequence of the fossilization process, many fine details of the appendages, such as setae or spines, have been loss. A second analyzed individual (paratype) is also an epirelief settled on the bottom in ventral position, showing the dorsal part of the body.
The fossil traces are relatively thin, so they require the use of different illuminations to observe different parts of the body (Figs. 2–5). Thus, the main appendages of the right ventral side of the holotype can be recognized (these are harder to see on the left side probably due to some kind of postmortem and/or fossilization torsion), as well as other main morphological traits of both analyzed specimens. The specimen sizes were determined from the measurement of the total body length (TL: from the apex of the rostrum to the posterior end of the telson).

Figure 2 Aviamysis pinetellensis n. gen. n. sp.: (1, 2) PI1, holotype female, photographs of the entire animal with two lateral illuminations, right side (1) and left side (2); (3, 4) PI47, male paratype, photographs of the entire animal with two lateral illuminations, right side (3) and left side (4). Scale bars=1 cm.

Figure 3 Aviamysis pinetellensis n. gen. n. sp.: (1, 2) PI1, holotype female, detailed photographs with two lateral illuminations, right side (1) and left side (2); Car=carapace, Abd=abdomen, Taf=tailfan, Ant=antennule, Aes=antennal scales, Lan=lanceolate-shaped median ventral projection arising from front of the labrum, Lab=labrum, Map=mandible palp, Mal=maxillule, Max=maxilla, Th1–Th8=thoracopods 1–8, Ost=oostegites, Uro=uropod, Sta=statocysts. Scale bars=5 mm.

Figure 4 Aviamysis pinetellensis n. gen. n. sp.: (1, 2) PI47, male paratype, detailed photographs with two lateral illuminations, right side (1) and left side (2); Ant=antennule, Aes=antennal scale, Ros=rostrum, Lan=lanceolate-shaped median ventral projection arising from front of the labrum, Eye=eyes, Car=carapace, Cs=cervical sulcus, Ls=spines of the carapace, Cap=posterolateral lobe of the carapace, T7–T8=thoracic somites 7 and 8, Abd=abdomen, 1–6=abdominal somites 1 to 6, Tel=telson, Cle=telson cleft, Uro=uropod, Sta=statocysts. Scale bars=5 mm.
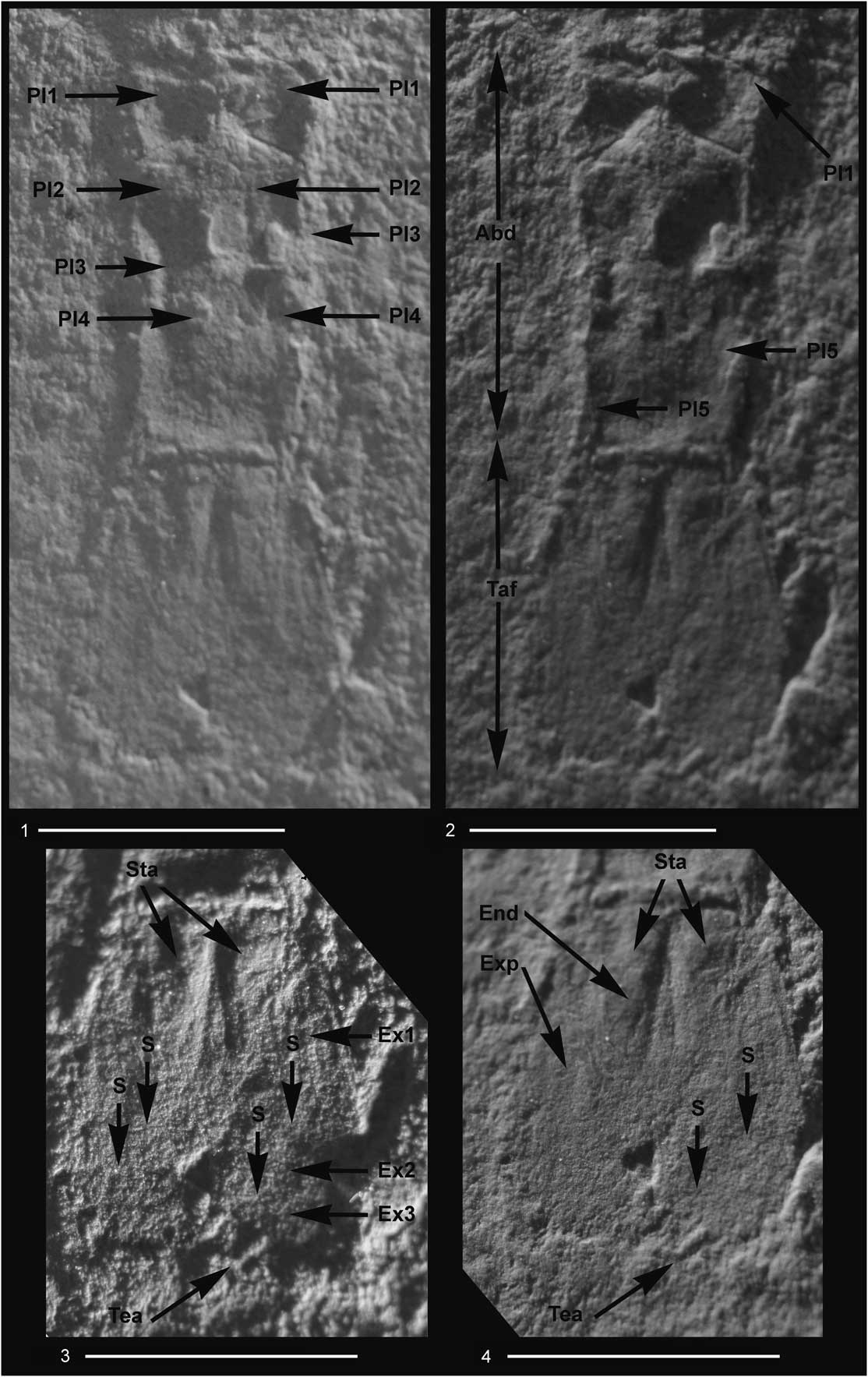
Figure 5 Aviamysis pinetellensis n. gen. n. sp.: (1–4) PI1, holotype female, detailed photographs of the abdomen and telson with two lateral illuminations, right side (1, 3) and left side (2, 4); Abd=abdomen, Taf=tailfan, Pl1–Pl5=pleopods 1 to 5, Exp=uropod exopod, Ex1–Ex3=uropod exopod articles 1 to 3, S=sutures of the uropod exopod, End=uropod endopod, Sta=statocysts, Tea=telson apex. Scale bars=5 mm.
Nomenclature of higher crustacean taxa follows the classification proposed by Martin and Davis (Reference Martin and Davis2001) and higher Mysida taxa by Wittmann et al. (Reference Wittmann, Schlacher and Ariani2014). Article 30.1.1 of the International Code of Zoological Nomenclature (International Commission on Zoological Nomenclature, 1999) states that if the genus name is a compound word formed from two or more components, the gender is given by the final component. Thus Mysis should be treated as feminine (Holmquist, Reference Holmquist1958), and the new genus and species is named to accord with that gender.
Repositories and institutional abbreviations
Specimens examined in this study were collected in a field study during 1991 organized by the Centre d ‘Història Natural de la Conca de Barberà (CHNCB). Type material is deposited at the Centre d’Història Natural de la Conca de Barberà (Museu Comarcal de la Conca de Barberà), Montblanc (Catalonia, Spain). Holotype catalogue number: PI1. Paratype catalogue number: PI47.
Systematic paleontology
Class Malacostraca Latreille, Reference Latreille1802
Subclass Eumalacostraca Grobben, Reference Grobben1892
Superorder Peracarida Calman, Reference Calman1904
Order Mysida Boas, Reference Boas1883
Family Mysidae Haworth, Reference Haworth1825
Subfamily Boreomysinae Holt and Tattersall, Reference Holt and Tattersall1905
Genus Aviamysis new genus
Type species
Aviamysis pinetellensis n. gen. n. sp. by original designation and by monotypy.
Diagnosis
Relatively large sized mysid distinguished within the family Mysidae through the strong, lanceolate-shaped, median, ventral projection arising from front of the labrum; antennal scale triangular, with distal transverse suture; antennal peduncle with two flagella; first thoracopod forming a short maxilliped, without exopod; seven pair of oostegites; third to fourth thoracopods with endopod elongated and robust, much longer than the other thoracopods; uropod exopod 3-articulate; uropod endopod slender, with statocyst circular and without any trace of structures; telson cleft.
Etymology
The new genus name is given after the association with the Catalan-Latin name of the grandmother (“Avia”), due to its ancient nature, combined with the generic name Mysis to give Aviamysis. The name Aviamysis is defined as feminine.
Aviamysis pinetellensis new species

Figure 6 Reconstructions of the habitus of Aviamysis pinetellensis n. gen. n. sp.: (1) PI1, holotype female, (2) PI47, male paratype; Ant=antennule, Apm=appendix masculine, Anp=antennal peduncle, Ae=antennal scale, Ros=rostrum, Eye=eye, Cs=cervical sulcus, Ls=spines of the carapace, Cap=posterolateral lobe of the carapace, T7–T8=thoracic somites 7–8, Lan=lanceolate-shaped median ventral projection arising from front of the labrum, Lab=labrum, Map=mandible palp, Mal=maxillule, Max=maxilla, Th1–Th8=thoracopods 1–8, D=dactylus, N=nail, A1–A6=abdominal somites 1–6, Pl1–Pl5=pleopods 1–5, Tel=telson, Cle=telson cleft, Exp=uropod exopod, End=uropod endopod, Sta=statocyst. Scale bars=5 mm.
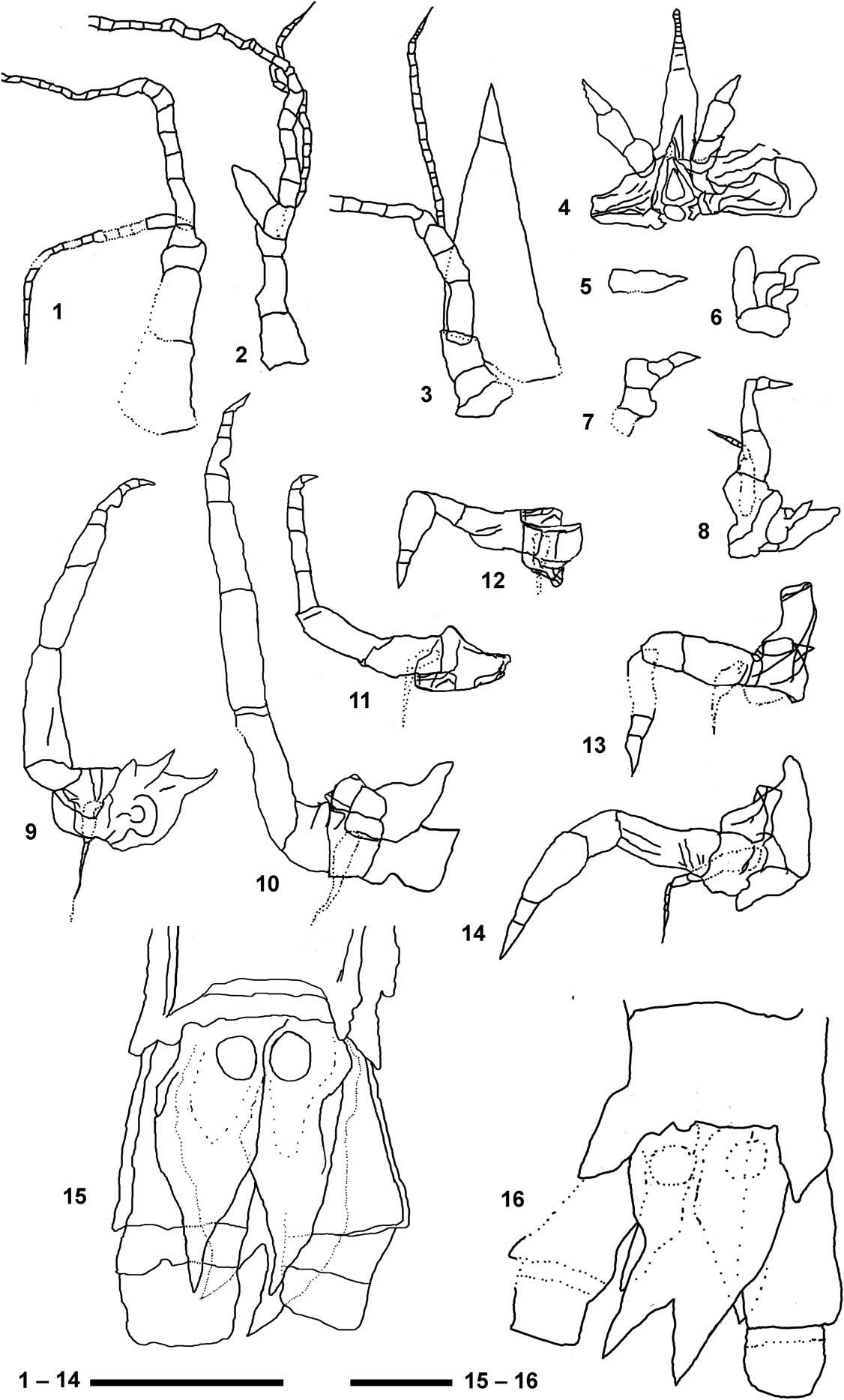
Figure 7 Detailed reconstructions of the main appendages of Aviamysis pinetellensis n. gen. n. sp.: (1, 3–15) PI1, holotype female, (2, 16) PI47, male paratype. (1) Female antennule; (2) male antennule; (3) antenna; (4) labrum, mandibles and the lanceolate-shaped median ventral projection arising from front of the labrum; (5) maxillule; (6) maxilla; (7) 1st thoracopod; (8) 2nd thoracopod; (9) 3rd thoracopod; (10) 4th thoracopod; (11) 5th thoracopod; (12) 6th thoracopod; (13) 7th thoracopod; (14) 8th thoracopod; (15) female telson and uropods in ventral view; (16) male telson and uropods in dorsal view. Scale bar 1–14=0.5 mm; scale bar 15, 16=0.2 mm.
Holotype
Holotype (PI1): one female, 38 mm TL. Bed of Alcover limestones unit (“Pedra d’Alcover”) from El Pinetell, in the district of Montblanc; 41°18'25''N, 01º07'53''E. Paratype (PI47): one male 29 mm TL, from the same locality and sampling data as the holotype.
Diagnosis
The same diagnostic characteristics used for the genus.
Occurrence
Alcover laminated dolomites unit, Middle Triassic, late Ladinian, upper Muschelkalk.
Description
Aviamysis pinetellensis n. gen. n. sp. was a relatively large mysid with an apparently robust general body form (Figs. 2–4). The reconstruction of the holotype individual (Figs. 3, 6.1), in which we interpret the presence of seven oostegites arising from the bases and coxae of the second to eighth thoracopods, as well as pleopods reduced to simple unjointed plates, indicates that the specimen was a female. Analysis of the paratype reveals characteristics of the dorsal part of the new taxon (notably the eyes and the carapace sculpture). The presence of an anteriorly produced ventral process on the distal end of the third segment of the antennule sympod of the paratype (interpreted as an appendix masculina) indicates that it was a male (Figs. 4, 6.2).
Carapace sculptured with spines, leaving the two posterior thoracic somites partially uncovered; anterior margin produced into a pointed rostrum not extending much farther than the ocular cornea; antero-lateral corners acutely produced, slightly projecting beyond anterior margin of carapace. Cervical sulcus marked, running across the dorsal surface. Four acute spines in the median cardial zone; eight smaller lateral spines on each carapace flank. Posterolateral lobes very sharp, extending to the middle second abdominal somite (Figs. 3, 4, 6.2).
Pleon shorter than the carapace in the female and subequal in length in the male, not tapering posteriorly, with the sixth somite longer than the other anterior abdominal somites (Figs. 3, 4, 5.1, 5.2, 6.2).
Eyes small, and globular, not broader than the eyestalk, laterally not extending beyond carapace limits (Figs. 4, 6.2).
Antennular sympod (Fig. 5.1, 6) not extending beyond antennal scale, with three segments. First article (precoxa) longer than wide; second article (coxa) half as long as broad, third (basis) article shortest; male third article slightly broader than long, supporting an appendix masculina (Figs. 5.1, 6.2, 7.1, 7.2). The sympod supports two flagella, with the outer flagellum three to four times longer than the inner one.
Sympod of the antenna closely fused with three discernible segments: praecoxa, coxa, and basis (Figs. 3, 4.1, 6, 7.3). Antennal scale triangular in shape, three times longer than maximum width, extending beyond antennular peduncle; with apical suture. Peduncle not extending beyond scale; composed of three segments, first article twice as long as broad; second and third articles sub-equal in length, both shorter than first one. The antennal peduncle supports two flagella, with the outer flagellum three to four times longer than the inner one.
Strong, lanceolate-shaped, median ventral projection arising from front of the labrum and extending beyond halfway point to the second segment (female) or to the third segment (male) of the antennular peduncle; anterior end rounded with apparent segmentation (Figs. 3.1, 6,1, 7.4).
Labrum pyriform, more or less symmetrical, half as wide as long (Fig. 3.1, 6.1, 7.4). Mandibles well developed (Figs. 3.1, 6.1, 7.4). Three-segmented palp, first article shortest; second article slightly longer than wide; third article about twice as long as broad. Third article of the right mandible with serrate outer margin. Gnathobasic processes of the left and right mandibles are not discernable; only the molar process is tenuously observed as a flat platelike structure provided with spines.
Maxillule difficult to define, without any trace of separation between the sympod and the lobes (Fig. 5.5). Maxilla exopod is seen as a plate attached to the outer side of the basis, with the outer margin markedly convex; two basal endites and endopod probably two-segmented (Figs. 3.2, 6,1, 7.5).
The first thoracopod differs considerably from the remaining thoracic appendages: modified as short maxilliped without exopod (Figs. 3.2, 6.1, 7.6). Endopod short and robust; apparently with four segments not clearly delineated, dactylus fused with nail to form a claw.
Female second to eighth thoracopods with oostegites (Fig. 3.2). Second thoracopod longer than first one, without epipodite and with exopod and oostegite (Figs. 3.2, 6.1). Endopod with preischium, ischium and merus fused, twice as long as broad; unsegmented carpopropodus three times as long as greatest width, tapering somewhat distally; dactylus short with pointed nail. Exopod shorter than endopod with basal plate and 6-segmented flagellum (Fig. 7.6).
Third to fourth thoracopods with endopod elongated and robust, much longer than the other thoracopods (Figs. 3.1, 6.1, 7.9, 7.10). Endopod preischium difficult to define, ischium and merus subequal in length; carpopropodus 4-segmented, longer than merus; dactylus with pointed distal nail. Exopod apparently much shorter than endopod.
Fifth thoracopod with endopod about one half longer than the fourth (Figs. 3.1, 6.1, 7.11). Endopod preischium difficult to define, ischium and merus subequal in length; carpopropodus 4-segmented, longer than merus; dactylus with pointed distal nail. Exopod apparently much shorter than endopod.
Sixth to eighth thoracopods quite different from the rest, with preischium and ischium more or less fused, merus short, undivided carpopropodus more or less swollen anteriorly; dactylus with pointed distal nail (Figs. 3.1, 6.1, 7.12, 7.13, 7.14). Exopods apparently much shorter than endopods. Eighth thoracopod exopod with basal plate and 5-segmented flagellum (Fig. 7.14).
Female pleopods uniramous, reduced to unsegmented lobes (Figs. 5.1, 5.2, 6.1), increasing in length from first to fourth and fifth pairs; fifth pleopod extending to posterior end of posterior abdominal somite.
Uropod endopod slender, with statocyst, not extending to telson apex, outer margin sinuous (Figs. 3–6, 8.2). Statocyst trace apparently circular with a maximum diameter of ~1 mm, without any trace of structures (Figs. 5.3, 5.4, 6, 7.15, 7.16). Uropod exopod 3-articulate, longer and broader than endopod, extending slightly beyond telson apex; distal two articles about one third length of the basal article.
Telson short, about sub-equal in length to the last abdominal somite, twice as long as broad (Figs. 3–6, 7.15, 7.16); lateral margins converging distally; apex of telson bidentate; telson cleft to about one-third of its length.
Etymology
This species is named after “El Pinetell,” the bed of Alcover limestones unit (Catalonian) in which the species was found.
Remarks
The placement of Aviamysis n. gen. in the order Mysida family Mysidae, as defined by Wittmann et al. (Reference Wittmann, Schlacher and Ariani2014) seems beyond doubt. This assignment is supported by the presence of eight thoracopods without branchiae and with endopods divided in a number of segments, female with seven pair of oostegites, female with five rudimentary pleopods, and the presence of statocysts on the endopod of uropods. Its taxonomic situation among the diverse subfamilies included in the Family, however, is relatively ambiguous.
The family Mysidae is divided at present into ten subfamilies (Meland et al., Reference Meland, Mees, Porter and Wittmann2015), of which, Aviamysis has a clear affinity to the subfamily Boreomysinae. Boreomysinae are characterized by the following: a smooth outer margin on the antennal scale ending in non-articulate spine; seven pairs of oostegites; endopods of the third to the eighth thoracopods with carpus distinct and propodus usually divided into two or three subsegments; pleomeres without projecting pleural plates; females with pleopods reduced to unsegmented rods; exopod of uropods divided by a rudimentary, transverse articulation; statocyst containing a small, non-mineralized statolith; and telson with apical cleft (Tattersall and Tattersall, Reference Tattersall and Tattersall1951; Wittman et al., 2014). Two extant genera are included in this subfamily: Boreomysis Sars, Reference Sars1869, and Birsteiniamysis Tchindonova, Reference Tchindonova1981.
In accordance with this definition of the subfamily, the placement of Aviamysis in the Boreomysinae seems reasonable. In particular, different morphological characters traditionally regarded as plesiomorphic features between the order Mysida, as discussed by Schlacher et al. (Reference Schlacher, Wittmann and Ariani1992), are present on the subfamily Boreomysinae (e.g., subdivided exopod of uropods and seven pairs of oostegites). The remaining Mysidae taxa usually have two or three pairs of oostegites (Wittmann et al., Reference Wittmann, Schlacher and Ariani2014).
An additional shared character state in the basal taxa Petalophthalmidae, Boreomysinae, and Rhopalophthalminae (and also in the more derived Siriellinae) is the presence of a suture in the exopod of uropod. With reference to molecular phylogeny (Meland and Willassen, Reference Meland and Willassen2007), the divided exopod gains support as an ancestral state in Mysida evolution (Meland et al., Reference Meland, Mees, Porter and Wittmann2015).
The statocyst in Aviamysis n. gen. is apparently circular and without any trace of internal structure, such as statolith. Strong support for an ancestral status of Boreomysinae is also seen in the non-mineralized (organic) composition of the uropodal statoliths, a trait also found in the basal taxon Rhopalophthalminae. In both subfamilies, the nearly exclusively non-crystalline (organic) structure of the statoliths is plesiomorphic compares with the more complex mineralized statoliths found in all other subfamilies (Ariani et al., Reference Ariani, Wittmann and Franco1993; Wittmann et al., Reference Voicu1993).
It is noteworthy several features of Aviamysis, such as the presence of an antennal flagella, the strong lanceolate-shaped median ventral projection arising ventrally from front of the labrum and extending beyond it, similar to those of extant species such as Paramblyops brevirostris Tattersall, Reference Tattersall1955 or Schistomysis assimilis (Sars, Reference Sars1877), and the shape of the uropod exopod and the configuration of the thoracopods. Thus, assignment of Aviamysis to the subfamily Boreomysinae remains tentative.
A morphological peculiarity of Aviamysis concerns the shape of the antenna. This appendix includes a sympod with three discernible segments (praecoxa, coxa, and basis), an antennal scale and a peduncle composed of three segments that supports two flagella. This structure can be considered as unique within the order Mysida because, in general, the antennal endopod takes on the form of a multi-segmented flagellum (Tattersall and Tattersall, Reference Tattersall and Tattersall1951; Meland et al., Reference Meland, Mees, Porter and Wittmann2015). A second peculiarity is derived from the 3-segmented uropodal exopod in Aviamysis: in Mysida, the uropodal exopod is divided by no more than two distal or proximal articulations. Finally, the configuration of thoracopods in Aviamysis differs from those seen in living Boreomysinae (Tattersall and Tattersall, Reference Tattersall and Tattersall1951). In Aviamysis, the thoracopods are grouped in at least two different shape groups (third to fourth thoracopods with endopod elongated and robust, sixth to eighth with shorter endopods and undivided carpopropodus). By comparison, in Boreomysinae the endopods of the third–eighth thoracopods are all similar in shape, with the carpus distinct and propodus usually divided into two or three subsegments.
The formalization of a new subfamily based on the description of only two fossil individuals seems very hazardous, so we included it (with reservation) in the Boreomysinae. However, given the scale of time elapsed between the age of Aviamysis and the first fossils of previously known Mysida (~70 Myr), it is not impossible that this taxon will someday define a new subfamily. To do this, however, discovery and descriptions of new Mysida fossils from the Triassic will be necessary.
Discussion
Taylor et al. (Reference Taylor, Shen and Schram1998) provided a large-scale phylogenetic analysis including recent “mysidacean” and lophogastrid taxa plus fossil pygocephalomorphs. True fossils of the order Mysida have been dated as far back as Middle Jurassic time (Callovian, 163–166 Myr). These animals were well developed, very similar in appearance to the mysids of today, which led Secretan and Riou (Reference Secretan and Riou1986) to suggest that the evolutionary stage reached by the Jurassic has not appreciably changed in the intervening time period. As suggested by Taylor et al. (Reference Taylor, Schram and Shen2001), the Mysida origin was sometime before the Jurassic, although the question of where and when cannot yet be answered. The description of the fossil specimens of El Pinetell presented in this study strengthens the evidence for the suggestion of Taylor et al. (Reference Taylor, Schram and Shen2001), as well as greatly extends time of the origin of this crustacean order. Moreover, the general appearance of Aviamysis n. gen. provides greater morphological diversity to the first fossil forms and, consequently, to the evolutionary history of the order Mysida.
The mode of life of Aviamysis pinetellensis n. gen. n. sp. in the Middle Triassic marine environment of the Western Tethys is an open question. Marine conditions prevailing in the Iberian region during the Triassic, especially during the whole Ladinian, included a wide diversity of coastal and depositional environments (shores, lagoons, tidal flats, reefs, etc.). Assemblage information from the Alcover unit suggests that this Triassic mysid lived in a marine shallow environment or lagoonal basin within an extended reef complex.
Some features of Aviamysis pinetellensis n. gen. n. sp. suggest that this mysid could have had an epibenthic lifestyle: (1) the general body form was apparently robust; (2) the shape of the thoracopod exopods, apparently much shorter than the endopods; and (3) the short abdomen. The relatively robust thoracopod endopods also suggest a good capacity for benthic locomotion.
Schram (Reference Schram1974) proposed that the radiation of the Peracarida took place in the Permo-Triassic, which is the period when the first recognisable peracarid fossils appeared (Schram, Reference Schram1986). In his view, the dominant Palaeozoic peracarids were the Pygocephalomorpha, which were epibenthic. At the end of the Permian, primitive peracarids occupied refugia or went extinct and were replaced by advanced peracarids adapted to benthic strategies (Poore, Reference Poore2005). In this sense, comparing the zoogeographic distribution of extant genera with that of the fossil forms, Udrescu (Reference Udrescu1984) also hypothesized that primitive Lophogastrida were littoral-benthic forms. In contrast with the more recent interpretations of the Triassic Lophogastrida pelagic lifestyle (Feldmann et al., Reference Feldmann, Schweitzer, Hu, Huang, Zhou, Zhang, Wen, Xie and Maguire2015, Reference Feldmann, Schweitzer, Hu, Huang, Zhou, Zhang, Wen, Xie, F.R. Schram and Wade2017), data provided by the new fossil taxon described here seem to corroborate that the first Triassic representatives of the order Mysida probably had an epibenthic lifestyle and lived in a coastal benthic environment, from which they were diversified to colonize a broad range of aquatic ecosystems on Earth.
Acknowledgments
We wish to express our gratitude to F. Aguilar for providing us with the nice photographs of the holotype. Special thanks to the referees (C.E. Schweitzer and an anonymous reviewer) for improving the manuscript.









