Transcatheter perimembranous ventricular septal defect closure is one of the most challenging procedures in interventional cardiology. Although the transcatheter approach has been increasingly used as an alternative to surgery since the beginning of the 2000s, complications such as atrioventricular block and new aortic regurgitation development have limited uptake of the approach. Reference Butera, Carminati and Chessa1–Reference Holzer, de Giovanni and Walsh3 New devices with lower complication rates have been sought by interventional cardiologists. The Lifetech™ Konar-MF Occluder device (MFO) is one of these devices and is increasingly used by interventionalists. A limited number of studies regarding feasibility and early effectiveness of this device have been reported. Reference Tanidir, Baspinar and Saygi4–Reference Sadiq, Qureshi and Younas6 However, mid-term results have not been reported. Here, we present our centres’ results in order to investigate mid-term efficacy and safety of MFO device in transcatheter perimembraneous ventricular septal defect closure.
Patients and method
Patient population and selection
Between December 2017 and January 2021, we attempted transcatheter perimembraneous ventricular septal defect closure in 52 patients using MFO device. Hospital records and procedural details as well as echocardiographic follow-up results of the patients were reviewed.
We screened patients with perimembraneous ventricular septal defect with transthoracic echocardiographic examination. Left ventricular end-diastolic and end-systolic diameters and ventricular function were measured and recorded from the long-axis parasternal view using M mode. Patients who had left ventricular diameters + 2SD larger than their age and weight normals were considered as having indications for closure.
In transthoracic echocardiographic examination, parasternal short axis, apical five chamber, parasternal long axis, and subcostal coronal views were used to measure size of the defect. The left ventricular and right ventricular dimensions of the defect were recorded. The presence of aortic valve prolapse, aortic regurgitation, and ventricular septal aneurism was recorded A bowing or bulging to right ventricular side of the septum more than 5 mm was defined as ventricular septal aneurism. The presence of right-to-left shunt or bidirectional shunt flow or shunt flow velocity less than 3 m/s was considered a sign of pulmonary hypertension. Patients with inlet extension, pulmonary hypertension, aortic valve prolapse with more than mild aortic regurgitation, and other reasons for surgery, like subvalvular pulmonary stenosis or subaortic stenosis, were not eligible for the procedure and were referred for surgery. An informed consent form signed by the patient or parents was obtained. The local Ethical Committee of the University approved this retrospective study.
Brief device description
The MFO device is a self-expanding, double-disc device constructed using nitinol wires. It is double disc-designed device with different-shaped disks on each side connected with a cone-shaped waist. The waist of the device is flexible and radial strength is low (Fig 1). This design aims to reduce the risk of damaging conduction tissue. An important distinctive feature of this device is having screws at both of the disks that enable it to be used from both arterial and venous approaches. The sizes range from 5/3 mm to 14/12 mm, the length in total being 4 mm without stretching. Eight sizes are commercially available. The four largest models contain Polytetrafluoroethylene membranes, whereas others are bare metal. Delivery sheath sizes are between 4F and 7F.

Figure 1. Picture of the MFO Konar device. Screws on the both side of the device allows to use both arterial and venous approach.
Procedure
All procedures were performed under deep sedation without intubation and with the guidance of transthoracic echocardiography and monoplane angiography. Intravenous cefazolin prophylaxis 25 mg per kg was administrated before the procedure. Introducers were placed into the right femoral vein and left femoral artery. Heparin was administered at a dose of 50 units per kg. After haemodynamic measurements, left ventricle angiogram was performed in Left Anterior Oblique 40 and cranial 20 degrees in order to delineate VSventricular septal aneurism anatomy. We measured the diameters of the defect from the left ventricle side and right ventricular exit and also noted the existence of a ventricular septal aneurism and subaortic rim. Furthermore, particular attention was paid to the existence of multiple holes from the right ventricular side.
A device that has skirt dimension 2–4 mm larger than the left ventricular dimension of the defect was selected for closure procedure in patients with ventricular septal aneurism. If the patient had no ventricular septal aneurism, we preferred a 2-mm larger than the defect LV size in order to avoid touching the aortic valve. However, device size selection should be individualised since each patient has different morphology and MFO provides different alternatives, as the device can be screwed from both sides.
It is a difficult decision to make regarding which side of the device is to be inserted in the left ventricle. In patients with ventricular septal aneurism, we placed the Amplatzer® Duct Occluder I similar disc at the LV side of the defect. We always tried to place the left ventricular disc into the aneurismal tissue by embedding as far away as possible from the aortic valve. If there is no ventricular septal aneurism, we preferred to place the conical shape disc at the LV side.
Femoral arterial (retrograde) approach
If the patient weight is enough to insert a sheath into the femoral artery to deliver the proper size device, we preferred to deploy the device from the arterial approach. In such cases, we crossed through the defect with a cut pigtail catheter (a pigtail catheter is cut according to engagement angle to the defect) with a hydrophilic 0.035’’ guidewire. Then we placed the wire in the distal pulmonary artery or superior vena cava and advanced the proper size SteerEase ® delivery sheath to the right ventricle (RV) apex. After checking the tip of the sheath with pressure control, echocardiographic examination visualisation, or hand injection, if it is at the safe position in RV, we advanced the screwed device delivery assembly through the sheath. We exposed the distal disk in the RV and pulled the system back to the defect. We used echocardiographic examination guidance for proper engagement of the device to the defect since there is a limited chance to control it by injection during the arterial approach. After we made sure that the device’s distal disk is placed in the proper position, we pulled back the sheath to expose the proximal disk of the device just beneath the aorta. In most cases, we pushed the delivery cable to the defect to keep it away from the aortic valve. After checking for aortic valve regurgitation, tricuspid valve regurgitation and residual shunt, we released the device, if satisfied. We observed that the movement of the device’s proximal disc was towards down and away from the aortic valve after deployment in many cases (Fig 2).

Figure 2. pmVSD closure procedure steps with venous (antegrade) approach: ( a ) Left ventricular injection in left anterior oblique 40 and cranial 20 degrees. ( b ) Partially exposing the device in the ascending aorta and checking the position of the aortic valve by performing a hand injection ( c ) lean on the devices’ left disc component to the defect and controlling by left ventricular injection. ( d ) Exposing of the right ventricular disk and just before releasing ( e ) control angiogram showing no residual shunt.
Femoral venous (antegrade) approach
In the case of a requirement for a larger sized device, a larger sheath needs to be inserted into the femoral artery to avoid arterial complications, so we had to make an arteriovenous loop. We crossed the defect from the left ventricular side and snared the 0.035’’ hydrophilic guidewire in the pulmonary artery, inferior vena cava, or superior vena cava and exteriorised from the femoral vein. The sheath was advanced from the femoral venous approach and the tip of the sheath was placed into the ascending aorta. The device was advanced into the tip of the sheath and the distal disk was exposed in the ascending aorta. Then the entire system was pulled back until the sheath and device assembly dropped below the aortic valve. Special attention was paid to the risk of trapping the device inside aortic cusps. Due to the MFO devices’ compact nature, we did not encounter this problem. We chose to expose the first disc in the ascending aorta instead of rerouting the long sheath to the LV apex, which takes time and could cause other problems, such as damage to the mitral valve. We performed a control angiogram to show the ventricular septal defect and device relation at that step. We pulled back the entire system up to completely lean the device on the defect. We exposed the second disc by pulling back the sheath. We made sure that the device was properly engaged with the defect and checked for residual shunt aortic and tricuspid valve regurgitation by echocardiographic examination. Then we released the device to turn a torque system counterclockwise rotation. We performed a control angiogram and echocardiographic examination after releasing the device (Fig 3).
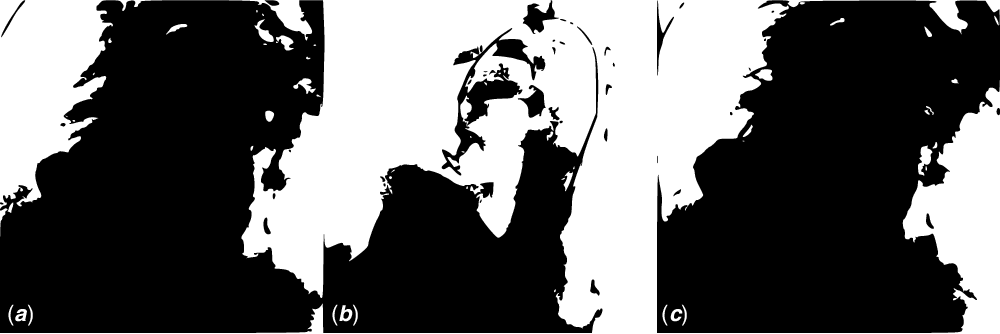
Figure 3. pmVSD closure procedure steps with the arterial (retrograde) approach: ( a ) Left ventricular injection in left anterior oblique 40 and cranial 20 degrees ( b ) exposing the device discs under transthoracic echocardiographic guidance ( c ) Control left ventricular angiogram shows no residual shunt.
Patient follow-up
Patients were discharged the day after the procedure. Acetylsalicylic acid treatment was started at 5 mg per kg for 6 months. The patients were followed up at 1st month, 6th month, and 1st year and yearly after then. Follow-up visits included physical examination, chest X ray, echocardiography, and ECG were performed. We reviewed the echocardiography for residual shunt, aortic regurgitation, and tricuspid regurgitation. Rhythm holter examination was performed in every case at the end of first year. A residual shunt smaller than 2 mm in colour doppler examination was considered haemodynamically insignificant. Reference Dodge-Khatami, Knirsch and Tomaske7
Statistical analysis
All data were expressed as a frequency or percentage for nominal variable, as median for categorical variables and mean ± standard deviation for continuous variables
Results
MFO device implantation was successfully performed in 98% of patients (n: 51). In one patient, the device was not released due to severe residual shunt and haemodynamic instability of the patient.
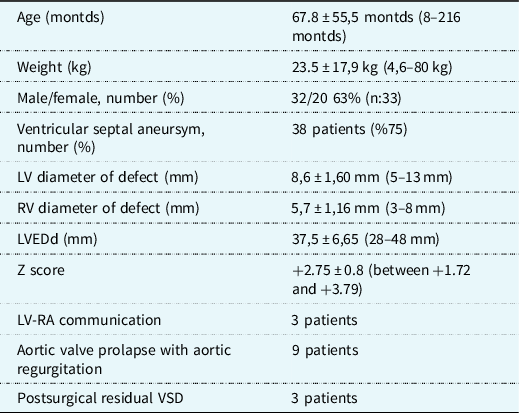
Thirty-two (63%) patients were male and 20 (37 %) female. Mean age was 67.8 ± 55.5 months (8–216 months), and weight was 23.5 ± 17.9 kg (4.6–80 kg).
In echocardiographic examination, 75% of the patients had VSA (n: 38). Aortic valve prolapse and aortic regurgitation were detected in 11 patients (21%). The mean LV diameter of the defect was 8.6 ± 1.60 mm. LV end-diastolic diameter was 37.5 ± 6.65 mm. In five patients (10%), more than one exit was detected at the right ventricular side of the defect.
Demographic and echocardiographic data of the patients are summarised in Table 1.
Table 1. Demographic and echocardiographic features of the patients. Mean ± standart deviation (range).
The mean diameter at the LV side of the defect was measured as 8.8 ± 2.74 mm by angiography (4.4–13.2 mm). The ratio of pulmonic to systemic flow (Qp/Qs) was calculated as 1.8 (1.5–2.1).
Total procedure and fluoroscopy time were 54.1 ± 37.02 min (14–120 min) and16.7 ± 13.7 min (4–59 min), respectively. The femoral venous approach was used in 27 patients (53 %) whilst no arteriovenous loop was established in the remaining of the patients. The most commonly used device size was 10 × 8 mm (n = 17), the smallest size was 6 × 4 (n = 2), and the largest size was 14 × 12 mm (n = 3). Device sizes and procedure details are depicted in Table 2
Table 2. Procedural details of the patients mean ± standart deviation (range).

On day 1 echocardiography, the residual ventricular septal defect rate was 31%. In the follow-up, at 6th months, only four patients had haemodynamically significant residual defects. In nine patients with baseline trace-minimal aortic regurgitation, there was no increase after the closure of the ventricular septal defect. In two patients, aortic regurgitation resolved after closure with the device. Follow-up results are presented in Table 3.
Table 3. Follow-up results.

We detected left ventricle to right atrial shunt in three patients that disappeared after closure. One patient had a non-obstructive subaortic ridge without aortic regurgitation.
Three of the patients had a previous history of transcatheter patent ductus arteriosus closure. We also closed residual defects after Tetralogy of Fallot surgical repair in three patients and one of those patients had residual pulmonary stenosis treated with balloon angioplasty at the same session. One patient had already undergone a transcatheter closure procedure with another device and the residual ventricular septal defect in this patient was closed with the MFO device.
The follow-up duration was: four patients were in the 18–24 month range, 28 patients were in the 25–36 month range, and 17 patients were in the 37–48 month range of follow-up. Only two patients have been followed up for more than 48 months. In the follow-up, no severe dysrhythmia was detected such as complete heart block. In one patient, a right bundle branch block pattern was detected in the 1st month of follow-up. This patient still has no further rhythm disturbance in the second year of his follow-up.
Discussion
Perimembranous ventricular septal defect have a varied morphology, including ventricular septal aneurism, subaortic rims, multiple exits from the RV side of the defect, and LV to right atrial shunt. This anatomical diversity is a major problem for closure device and defect shape matching, which is essential for the success of a transcatheter perimembranous ventricular septal defect closure procedure. Reference Knauth, Lock and Perry8,Reference Hijazi, Hakim and Haweleh9 The MFO has several advantages in terms of matching over other devices since it offers the cardiologist numerous geometrical closure variations. Thus, it solves the device and defect matching problem in the majority of perimembranous ventricular septal defects, even though their anatomies are dissimilar. In many cases, the use of an MFO in different variations makes transcatheter closure feasible.
Establishing the arteriovenous loop is one of the most challenging steps of the transcatheter perimembranous ventricular septal defect closure procedure. This not only prolongs the procedure and fluoroscopy time but may also cause complications, such as tricuspid chordea rupture, sticking of the device in the aortic valve cusps, and mitral valve damage. Reference Pekel, Ercan and Özpelit10 The Lifetech™ Konar-MF Occluder can be employed using both arterial and venous approaches. In particular, enabling the placement of the conical disc on the left ventricular side without arteriovenous looping is a non-negligible advantage considering that many cases have a conical shape VSA.
Additionally, the MFO is convenient for using small delivery sheaths, which facilitates an arterial approach. The smallest of the patients was 4.6 kg and 8 months old. Also, a large proportion of the procedures was performed using an arterial approach.
The AV block rate is very high in the transcatheter ventricular septal defect closure in previous reports, especially with the Amplatzer pm VSD Occluder. Reference Butera, Carminati and Chessa1,Reference Carminati, Butera and Chessa11 These serious complication ratios ended up with abandonment of the percutaneous ventricular septal defect closure in many clinics, where a surgical approach has been accepted as the standard therapy for perimembranous ventricular septal defect closure. Reference Santhanam, Yang and Chen12,Reference Nguyen, Phan and Dinh13
In this study, we did not encounter AV block in any of the patients during the follow-up period. Although the patient who had right bundle branch block did not progress to a further degree of AV block, he is still under close follow-up for this potential risk.
A possible cause of AV block in the early stage after implantation is mechanical damage to the conduction tissue directly due to the effects of the device or manaeuvers of physicians during the procedure. Transcatheter closure of the perimembranous ventricular septal defect is becoming more common. This early AV block-free result may also be due to the soft delivery cable and mesh of the MFO device along with increasing experience. Lengthy AV block development processes have previously been identified, which are primarily related to the close proximity of the conduction tissue and the membranous area where the device is implanted. Reference Butera, Carminati and Chessa1 The conical shape of the MFO allows it to be implanted deep within the ventricular septal aneurism, away from the conduction tissue. Traditional double-disc devices have a time-linked mechanical damaging effect on the conduction tissue due to their clamping impact on the perimembranous septum. Unlike other devices, where AV block develops frequently, the MFO fills defects (the MFO wire is knitted to do this); it does not capture from both sides of the septum, which causes ventricular septum clamping. Because the radial stress force of the MFO is much lower than that of other double-disc devices, its harmful pressure on the conduction tissue is reduced. Reference Tanidir, Baspinar and Saygi4,Reference Sadiq, Qureshi and Younas6 Thus, the MFO metal mesh design does not cause the membranous septum to be squeezed, which is a major cause of an AV block.
Although its early closure rate is low compared to that of a standard double-disc device, the MFO device has a high complete closure rate over a long period of time. The vast majority of patients in this trial had a very significant ventricular septal defect and shunt ratio, which could explain the high residual shunt ratio shortly after the device was released. Another possible explanation for the early low closure rate is the device’s unique design, which aims to close the ventricular septal defect by filling and subsequent endothelisation rather than by the bilateral early physical closure effects of the disks. We may include the fact that the MFO device has a following 9 × 7 mm fabric patch, which efficiently closes the ventricular septal defect by blocking shunt flow throughout the device. A residual shunt is expected in patients with a device that is smaller than 9 × 7 mm. Despite the fact that none of these residual shunts are haemodynamically significant, they disappeared during follow-up.
One of the crucial drawbacks of transcatheter perimembraneous ventricular septal defect closure is aortic regurgitation, even though some studies have indicated the feasibility of transcatheter closure of perimembraneous ventricular septal defect with AR and AVP. Reference Chen, Li and Li14 Among our patients, nine had aortic valve prolapse and accompanying AR. We preferred to close these ventricular septal defects for two reasons. The first was that in the case of the patient with ventricular septal aneurism, the device could be implanted into the ventricular septal aneurism; AR is not a major problem for patients with AR accompanied by ventricular septal aneurism. Reference Guo, Li and Yu15 Secondly, the MFO device is useful in these situations because its soft structure does not lead to increasing AR, even if the left ventricular disk comes into contact with the aortic valve. Most (75%) of the patients had ventricular septal aneurism, so we did not observe AR in such cases as would otherwise be expected. However, the remaining patients, who had no ventricular septal aneurism, were at risk of AR. Therefore, we gave particular attention to placing the device as far away as possible from the aorta. We also did not use the oversized device in such cases.
During implantation, we always check the presence of the AR from different echocardiographic examination views. The AR and device position should be checked from the subcostal view since the regurgitation flow runs parallel to the ultrasound beams, making it easy to identify even small ARs. We do not release the device if we identify a new AR or an increase in an existing regurgitation. In such circumstances, we attempt to reposition the device and, in some cases, embed the distal component of the device into the defect. The device’s distal end is inserted into the defect rather than the left ventricle, and the aortic valve is not touched. Here, even though no AR was observed, we noticed if the device touched the aortic valves before releasing the device. It is necessary to be aware of the possibility of increased AR over time due to the damaging effect on the aortic valve of the device or its adherence to the aortic valve, which ends in fibrosis and inappropriate valve motion. In the follow-up, we were unable to identify any advancement of AR.
Tricuspid regurgitation can be seen after transcatheter pmVSD closure. This is mostly related to chordae tendinea tear due to AV loop during the procedure. Reference Xu, Liu and Bai16 In this context, arterial-side procedure attainability is an advantage for the MFO. On the other hand, tricuspid regurgitation is an undeniable complication for the MFO since the length of the device is excessive in some situations. As a result, a redundant component of the device may be left hanging in the RV and become entangled with the tricuspid valve. The presence of tricuspid regurgitation should be checked by the interventionalist before releasing the device. In preprocedural echocardiographic examination, it is very important to delineate the relation of ventricular septal aneurism with tricuspid valve chordea. Those cases with a ventricular septal aneurism constructed by tricuspid chordea are risky patients for Tricuspid regurgitation. We observed TR in two cases in this study, which is a very low rate. We paid special attention to avoiding this issue both before and during the procedure.
The value of transcatheter closure of LV to RA shunts is unclear; there are some limited reports in the literature. Reference Parvez and Das17,Reference Kerst, Moysich and Ho18 We believe that these shunts are suitable for transcatheter closure. We have successfully closed the transcatheter in three cases of perimembraneous ventricular septal defect with LV to RA shunt. In such cases, ventricular septal aneurism redirects the left ventricular flow to the right atrium, and closure of the defect results in the disappearance of LV-to-RA shunts.
Percutaneous closure of post-surgical residual shunts is well-defined for other devices with satisfying results and low complication ratios. Reference Zhang, Liang and Zheng19 We closed residual shunts after surgical repair of Tetralogy of Fallot in three cases, all performed using an arterial approach. The MFO is very useful for post-surgical residual shunts.
Device embolisation is an uncommon complication and can occur with any sort of device; it is related not to device selection but rather to the experience of the cardiologist and team. Reference Pillai, Rangasamy and Balasubramonian20,Reference Houeijeh, Godart and Jalal21 In this study, although all procedures were carried out under transthoracic echocardiographic guidance, we did not experience any device embolisation.
Infectious endocarditis is another exceedingly rare post-procedure complication of PmVSD, particularly in patients who have residual shunts or AR. Reference Tang, Zhou, Hua and Wang22 Despite the fact that the residual shunt rate in this trial was higher than that of other devices, none of the patients were hospitalised due to IE. This promising result can be interpreted as due to the presence of hemodynamically negligible residual shunts and their quick closure, notwithstanding the observed higher rate. Residual shunts vanished in 96% of patients after 6 months of follow-up, according to this interpretation.
Hemolysis is a reported complication, especially in patients who have undergone transcatheter closure using the coil. Reference Houeijeh, Godart and Jalal21,Reference Odemis, Saygi and Guzeltas23,Reference Solana-Gracia, Mendoza Soto and Carrasco Moreno Jé24 Despite the fact that we detected residual shunt in some of the reported cases in this study, it should not be considered a major complication risk since the mechanism of the haemolysis is quite different in those patients with perimembraneous ventricular septal defect closure using the coil. Therefore, the non-observation of haemolysis after MFO implantation in this study was an expected result.
The limitation of this study was that it was retrospective and performed by the same interventionalist in a centre where he is experienced with perimembraneous ventricular septal defect closure using other devices. The learning curve should be considered in the development of complications related to the device, such as tricuspid regurgitation, device embolisation, AV block, and AR.
Conclusion
This study describes the mid-term results of MFO in transcatheter closure perimembraneous ventricular septal defect. It showed that MFO is a safe and effective device in the transcatheter treatment of perimembraneous ventricular septal defect’s not only short term but also in the mid-term follow-up.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Confict of interest
None.
Ethical standards
Informed consent form was obtained from all the patients or the parents. Ethical committee approval was achieved from the Koc University Faculty of Medicine.









