INTRODUCTION
Mollusc and crab harvesting has received comparatively less attention than effects of fish harvesting on coastal biodiversity (Smil, Reference Smil2012). The effects of mollusc harvesting in coastal ecosystems have been scarcely considered until now (e.g. Beck et al., Reference Beck, Brumbaugh, Airoldi, Carranza, Coen, Crawford, Defeo, Edgar, Hancock, Kay, Lenihan, Luckenbach, Toropova, Zhang and Guo2011) and mollusc harvesting also has consequences for other species, such as predators, competitors, algae and parasites (Coleman et al., Reference Coleman, Underwood, Benedetti-Cecchi, Aberg, Arenas, Arrontes, Castro, Hartnoll, Jenkins, Paula, Della-Santina and Hawkins2006). The consequences are worldwide since a wide range of molluscs have been extensively harvested around the globe in intertidal and shallow subtidal areas, including clams, mussels, octopuses, squids, limpets and abalones (Hackney & Rippen, Reference Hackney, Rippen, Roy, Paine Carter, Flick and Lynn2000).
Abalones (Haliotis spp.) have been traditionally harvested in many coastal areas worldwide, e.g. Australia, New Zealand, Chile, South Africa and California, because of their commercial value (Leiva & Castilla, Reference Leiva and Castilla2001; Maynard et al., Reference Maynard, Hanna and Benzie2004; Li et al., Reference Li, Appleyard and Elliott2006). In recent decades, due to increasing harvesting effort the abalone stocks have dramatically decreased (Guzmán del Próo, Reference Guzmán del Próo, Shepherd, Tegner and and Guzmán del Próo1992; Morales-Bojórquez et al., Reference Morales-Bojórquez, Muciño-Díaz and Velez-Barajas2008), even to unsustainable levels underpinning that several species are currently of concern (Kashiwada & Taniguchi, Reference Kashiwada and Taniguchi2007; Micheli et al., Reference Micheli, Shelton, Bushinsky, Chiu, Haupt, Heiman, Kappel, Lynch, Martone, Dunbar and Watanabe2008). In Europe, the species Haliotis tuberculata Linnaeus, 1758, commonly known as the Ormer, has been traditionally considered a delicacy in the British Channel Islands and the adjacent Atlantic French coast (FAO, 1995). Official bans were imposed in the 1970s in an attempt to reverse the overexploitation of this mollusc (Berthou et al., Reference Berthou, Beurier, Buestel and Clavier1985). This species, as in all haliotids, has a short pelagic-larval dispersive stage before settlement (Courtois de ViÇose et al., Reference Courtois de Viçose, Viera, Bilbao and Izquierdo2007) and as a result their populations are highly patched (McShane, Reference McShane1996). Such reproductive behaviour may have implications on isolated populations, with limited or no connectivity with other abalone populations, for example in oceanic islands such as the Canary Islands.
A subspecies of the ormer (H. tuberculata coccinea Reeve, 1846) has been harvested in the Canary Islands since aboriginal times, because of its ready accessibility, high protein content and agreeable flavour (Nuñez et al., Reference Nuñez, Barquin and Brito1994). This situation also creates a high pressure on larger individuals, resulting in profound shifts in size classes of populations, with a lower representation of adult specimens (>35 mm shell size), together with a sharp reduction in abundances (Espino & Herrera, Reference Espino and Herrera2002). For these reasons, this subspecies was classified as an endangered species in the Catalogue of Threatened Species of the Canary Islands (BOC, 2001). This inclusion resulted in the closure of the abalone fishery in the whole archipelago. Unfortunately, no available information exists about the current state of conservation of this species, nor the effects of natural and anthropogenic stressors on size structure of populations throughout recent decades.
The main aim of the present study was to explore temporal size variations of Haliotis tuberculata coccinea individuals over the last 20 years (1994–2014) in Tenerife, one of the islands of the Canarian archipelago. We hypothesized that the mean size has decreased in more recent years due to several factors, such as harvesting, regardless of its inclusion as endangered species in the regional catalogue. Thus, we use size as a surrogate of the population status of this locally endangered gastropod.
MATERIALS AND METHODS
This study was conducted at subtidal locations in Tenerife, the most populated island in the Canarian archipelago. A total of 15 locations (Armeñime, Bocacangrejo, Buenavista, Caleta de Interian, Caletillas, El Caleton, El Rio, Igueste, Los Gigantes, Medano, Puertito de Güímar, Punta del Hidalgo, Punta de Teno, Roque Bermejo and San Marcos) were sampled, corresponding to coastal towns throughout Tenerife (Figure 1). At each site, all abalones (Haliotis tuberculata coccinea) were recorded during 30 min of surveying by scuba. A minimum of three replicates were sampled at each locality. The sampling effort varied among field surveys (1994, 2002 and 2014), with different number of replicates within the same coastal location. Therefore, abalone abundance data were not utilized for comparative analysis. Each abalone was measured across the widest part of the shell to the nearest millimetre using calipers.

Fig. 1. Map of Tenerife (Canary Islands) showing sampling locations.
The shell size of Haliotis tuberculata coccinea is a surrogate of reproductive potential (Nuñez et al., Reference Nuñez, Barquin and Brito1994). The size of first maturity of this species is 30 mm, and >35 mm individuals are considered adults. Large-sized individuals (>50 mm) harbour the highest reproductive potential because fecundity increases exponentially with size (Nuñez et al., Reference Nuñez, Barquin and Brito1994).
The depth range of this species varied from the intertidal to 15 m depth, however, currently, H. tuberculata coccinea individuals are restricted to the shallow subtidal (1–6 m depth) because of the increase in barren areas of seabed in the Canaries (Espino & Herrera, Reference Espino and Herrera2002). This increase is underpinned by the high densities of the sea urchin Diadema africanum. The target species lives underneath stones and shows affinity for exposed and semi-exposed areas with round stones covered by coralline red algae that constitute its main food source (Espino & Herrera, Reference Espino and Herrera2002).
A comparative study, using individual sizes, was conducted using the current data collected in 2014 and previous data from 1994 (Nuñez et al., Reference Nuñez, Barquin and Brito1994) and 2002 (Espino & Herrera Reference Espino and Herrera2002). Spatial variability was also considered, grouping sampling locations together depending on their shore orientation and thus accessibility to the coast (North (<100 days accessible), East (200–250 days accessible) and Western (>300 days accessible)).
A multivariate comparison of the sizes of abalones was conducted using an analysis of variance (ANOVA). Despite the heterogeneity of the variances (Levene's test), the increase of type I error was avoided by reducing the P value to 0.01 level (Underwood, Reference Underwood1991). The factors ‘Time’ and ‘Orientation’ were included in the comparative study (1994–2002–2014). The Scheffé test was used for post-hoc procedures. All multivariate procedures were carried out using the SPSS 17.0 statistical package.
RESULTS
The mean individual size of abalones considering all surveys (1994, 2002 and 2014) is 28.85 ± 9.67 mm, typical of a non-adult specimen (<35 mm). Consistent differences were observed in size of individuals between 1994 and the remaining field surveys (2002 and 2014) (one-way ANOVA, F = 17.535, P << 0.0001) (Table 1). Post-hoc tests did not show differences between the sizes of individuals surveyed in 2002 and 2014 (Table 1). The mean individual size in 1994 was 33.51 ± 12.39 mm; <30 mm in 2002 (28.07 ± 9.21 mm) and 2014 (29.14 ± 8.41 mm) (Figure 2). However, the most abundant size class in 1994 was 21–25 mm (16.8%) whilst in 2002 was 31–35 mm (21.8%) and 26–30 mm (23%) in 2014.
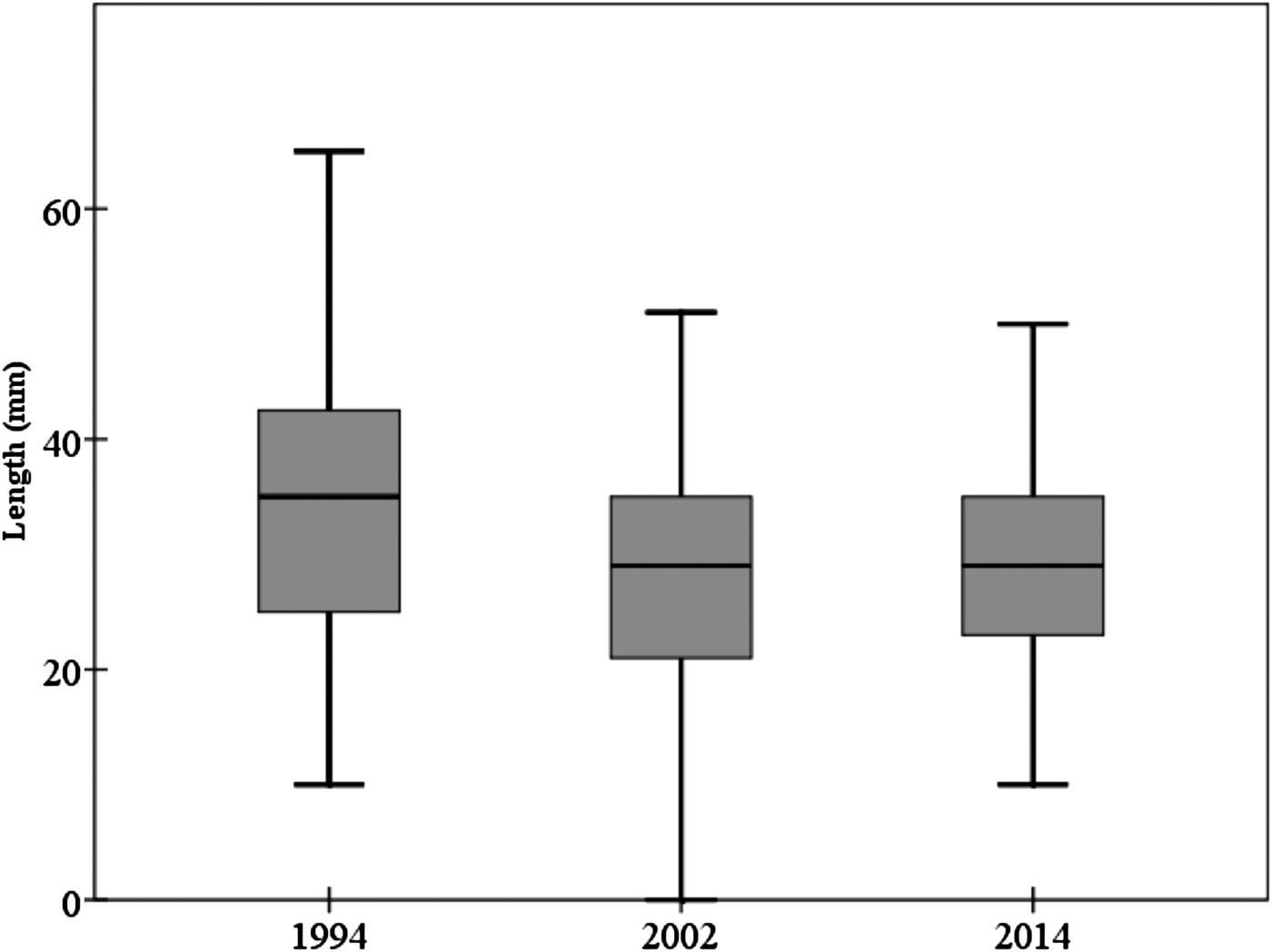
Fig. 2. Mean abalone size throughout the study period considering the three field surveys. Box-plot showing median (black line) and upper and lower data quartiles (box).
Table 1. Results of ANOVA testing for differences in abalone size throughout the study period (‘Time’, fixed factor) and at different shore locations (‘Orientation’, random factor).
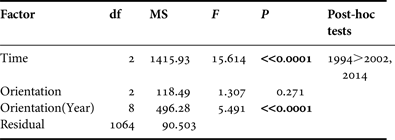
Significant differences (P < 0.01) are highlighted in bold.
Large-sized individuals (>50 mm) were rather scarce in all field surveys, ranging from 2.1% in 1994 to 0.37% in 2002. The adult individuals with a size >35 mm were not the dominant size of the abalone populations in the last two decades, ranging from 27.8% in 2002 to 38% in 2014 (Figure 3).

Fig. 3. Size classes of the abalone Haliotis tuberculata coccinea in the three field surveys.
If coastal orientation and accessibility is considered, no significant differences were found between northern, eastern and western abalone populations (F = 1.307, P = 0.271). However, these differences were consistent across all three field surveys (1994, 2002 and 2014) (F = 5.491, P < 0.0001). This variability was mainly explained by the higher sizes of abalones on the eastern (mean: 33 ± 13 mm) and northern (mean: 34 ± 12 mm) coasts of Tenerife in 1994 compared with the individuals surveyed in 2002 and 2014. In 2002 and 2014 no abalone populations in the different coasts of the island were characterized by a >35 mm size (adults) or even ‘pre-adult’ size (>30 mm), with the exception of western individuals in 2014 (mean: 31.5 ± 7.6 mm) (Figure 4).

Fig. 4. Mean (±SE) size of abalones considering coastal orientation in the field surveys.
DISCUSSION
Temporal trends showed a small, though significant, decrease in size of Haliotis tuberculata coccinea in the last 20 years. From 1994, the shell size of abalones has decreased 4–5 mm, resulting in populations characterized by non-adult mean sizes (<30 mm). Large-sized individuals, with the highest reproductive potential and the most value for illegal harvesters, were rather scarce (<2.5%) in all surveys.
These results are a priori surprising if we consider the inclusion of Haliotis tuberculata coccinea in the Regional Endangered Species Catalogue in 2001. However, several factors are currently affecting the recovery of this species in the Canary Islands, and particularly in Tenerife. Firstly, illegal harvesting still occurs in several areas because of lack of proper enforcement and the high demand on the black market (>30 € kg-). Secondly, the massive proliferation of the sea urchin Diadema africanum has underpinned a dramatic collapse of coastal ecosystem productivity in the Canary Islands (Tuya et al., Reference Tuya, Boyra, Sánchez-Jerez, Barberá and Haroun2004). This species has completely removed the algae from previously vegetated rocky reefs, resulting in barren seabeds and a depleted food source for abalone. No Haliotis individuals are found underneath stones on barren seabeds (Nuñez et al., Reference Nuñez, Barquin and Brito1994). Thus, suitable substrates for the colonization and settlement of abalones are limited to a narrow fringe from the lower intertidal to shallow subtidal (3–6 m deep), inaccessible to D. africanum due to wave exposure (Tuya et al., Reference Tuya, Boyra, Sánchez-Jerez, Barberá and Haroun2004). Overturning of rocks to collect fishing bait (e.g. crabs) in the intertidal also affects Haliotis populations due to consequential loss of algae on the rocks’ surface (Espino & Herrera, Reference Espino and Herrera2002). Another factor not usually considered is the increased sedimentation rate in coastal areas of Tenerife over the last two decades (Herrera & Riera, personal observation). This clearly reduces the number of suitable habitats for viable Haliotis populations.
Abalone harvesting in Tenerife has been banned since 2003 (fishing regulation no. 17/2003), due to the low abundances and predominance of small-size individuals. Populations of H. tuberculata coccinea probably still only exist in Tenerife because this species is no longer considered a priority-target for illegal harvesters over the last few years following the ban. Currently, other molluscs, such as limpets are the main priority for harvesters in the Canarian archipelago (Riera et al., Reference Riera, Perez, Alvarez, Simon, Diaz, Monterroso and Nuñez2016).
Abalones have been overexploited in several areas worldwide (e.g. Leiva & Castilla, Reference Leiva and Castilla2001; Micheli et al., Reference Micheli, Shelton, Bushinsky, Chiu, Haupt, Heiman, Kappel, Lynch, Martone, Dunbar and Watanabe2008; Morales-Bojórquez et al., Reference Morales-Bojórquez, Muciño-Díaz and Velez-Barajas2008). A series of conservation actions have been developed to preserve populations, however, stocks of abalones have not experienced a substantial increase along the California coast (Gruenthal & Burton, Reference Gruenthal and Burton2005; Kashiwada & Taniguchi, Reference Kashiwada and Taniguchi2007). Marine protected areas (MPAs) have been shown to be an effective tool to enhance local population recovery of abalones (Micheli et al., Reference Micheli, Saenz-Arroyo, Greenley, Vazquez, Espinoza Montes, Rossetto and De Leo2012). Networks of marine reserves may work more effectively to ensure genetic flow among abalone populations (Rogers-Bennett et al., Reference Rogers-Bennett, Haaker, Karpov and Kushner2002), though their effects are spatially limited (Micheli et al., Reference Micheli, Saenz-Arroyo, Greenley, Vazquez, Espinoza Montes, Rossetto and De Leo2012).
Current genetic studies indicate that abalone populations are genetically different, e.g. the green abalone (Haliotis fulgens) (Gutierrez-Gonzalez et al., Reference Gutierrez-Gonzalez, Cruz, del Rio-Portilla and Perez-Enriquez2007). This phenomenon has profound implications in their conservation, since isolated populations may have higher probabilities to collapse (Miller et al., Reference Miller, Maynard and Mundy2009). A detailed genetic study involving populations of H. tuberculata coccinea from several islands of the Canarian archipelago is of utmost importance to establish the first steps of understanding small-scale variability and gene flow rates and patterns. Simultaneously, aquaculture trials with the study species (Haliotis tuberculata coccinea) have been conducted with successful results (Bilbao et al., Reference Bilbao, Tuset, Viera, Courtois de Vicose, Fernandez-Palacios, Haroun and Izquierdo2010; Courtois de Viçose et al., Reference Courtois de Viçose, Viera, Huchette and Izquierdo2012). These pilot studies were carried out for two main purposes: (i) production of juveniles for the shellfish market; (ii) restoration of natural coastal populations (Viera et al., Reference Viera, Courtois de Viçose, Fernandez-Palacios and Izquierdo2016). In several geographic areas aquaculture of abalones for the purposes of rearing juveniles for population replenishment may be one of the viable solutions to preserve isolated populations, with no connections with other abalone assemblages (Huchette & Clavier, Reference Huchette and Clavier2004; Bilbao et al., Reference Bilbao, Tuset, Viera, Courtois de Vicose, Fernandez-Palacios, Haroun and Izquierdo2010).
A network of no-take areas with a high connectivity among them is of utmost importance for the conservation of H. tuberculata coccinea in the Canary Islands and especially in Tenerife. These no-take areas should be accompanied by strict surveillance and enforcement to ensure no illegal harvesting occurs. Two marine protected areas, Anaga (NE of Tenerife) and Teno (NW of Tenerife) will be established in the near future (Pascual-Fernandez et al., Reference Pascual-Fernandez, Chinea-Mederos and De la Cruz-Modino2015). All-year round closed seasons are necessary in these areas in order to preserve pools of individuals in the island with a good representation of large-sized individuals (>50 mm). These actions must be implemented urgently, since the present results show the ineffectiveness of current conservation measures and fisheries enforcement in the Canarian archipelago.
ACKNOWLEDGEMENTS
We are grateful to the staff of CIMA SL for support during the fieldwork and encouragement throughout the study.
FINANCIAL SUPPORT
This study was funded by the European fisheries funding ‘Axis 4’ Sustainable development of Fishing areas’, through the Coastal Action Group within the framework of the project ‘Management and conservation of coastal shellfish resources in Tenerife’ (cod. 411NCAN00044). Fernando Espino and Oscar Tavio are also acknowledged for their support during 2002 field surveys that were funded by the Consejería de Medio Ambiente y Ordenación Territorial (Canarian Government) (SEGA, 2002).







